Rosacea is generally categorized into 4 distinct clinical subtypes: erythematotelangiectatic, papulopustular, phymatous, and ocular.1 Granulomatous rosacea, rosacea fulminans, and perioral dermatitis have been described as additional variants.2 Herein we describe 14 patients with rosacea and prominent neurologic symptoms, who represent another distinct subset of rosacea meriting a unique approach to management.
Methods
Patients with prominent neurologic symptoms in addition to classic features of rosacea were identified during routine appointments at a major teaching hospital. Details regarding medical history, disease symptoms and triggers, and response to treatments were obtained via clinic visits and telephone interviews. The study was approved by the institutional review board of the University of California, San Francisco.
Results
Twelve of the 14 patients were women, and 12 were white. Mean age at disease onset was 38 years. Prominent symptoms included burning or stinging pain (100% [14 of 14]), erythema (100% [14 of 14]), and flushing (93% [13 of 14]), sometimes accompanied by facial edema (50% [7 of 14]), telangiectasias (50% [7 of 14]), pruritus (43% [6 of 14]), and papules (36% [5 of 14]). Important symptom triggers included heat (93% [13 of 14]), sunlight (93%[13 of 14]), hot showers (79% [11 of 14]), stress (71% [10 of 14]), exercise (64% [9 of 14]), and alcohol consumption (57% [8 of 14]). Use of makeup (50% [7 of 14]), eating spicy foods (43% [6 of 14]), touching skin (36% [5 of 14]), drinking hot beverages (29% [4 of 14]), cold weather (21% [3 of 13]), and humidity (14% [2 of 13]) were less reliable triggers. Notably, 71% of patients experienced relief from cooling via fans or cold compresses or ice applied to the face or held in the mouth (10 of 14). Figure 1 depicts typical examination findings in these patients.
Figure 1.
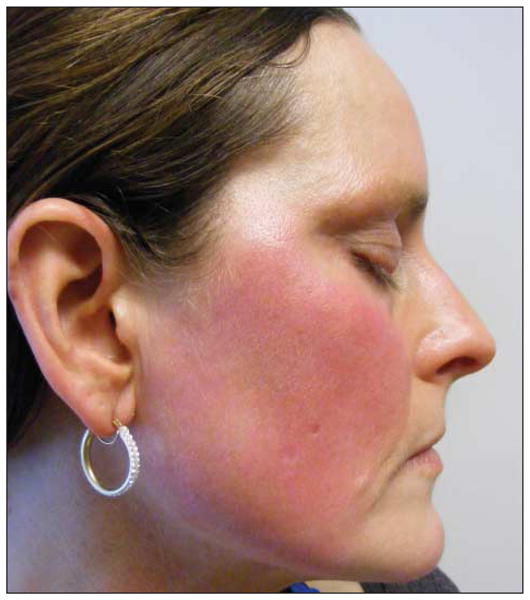
Facial erythema is seen in most patients at baseline and uniformly during flares. Inflammatory papules and pustules and rhinophymatous change are unusual in this subset of patients.
A notably high percentage of patients had neurologic (43% [6 of 14]) or neuropsychiatric (50% [7 of 14]) conditions, including complex regional pain syndrome, essential tremor, depression, and obsessive-compulsive disorder. Neurovascular disorders, including headaches (71% [10 of 14]) and Raynaud phenomenon (29% [4 of 14]), as well as rheumatologic disorders (36% [5 of 14]), including lupus, rheumatoid arthritis, fibromyalgia, mixed connective tissue disease, and psoriatic arthritis, were also common.
Many patients had tried the following treatments with limited success: topical metronidazole (0 of 12 were helped), topical steroids (1 of 8), and oral antibiotics, usually tetracyclines (4 of 8). Most patients benefitted from neurologically focused treatments (9 of 12), including gabapentin (5 of 11), duloxetine (4 of 6), pregabalin (1 of 4), tricyclic antidepressants (2 of 3), and memantine (2 of 2). Topically formulated neuroleptic agents, including doxepin, glycopyrrolate, amitriptyline, capsaicin, and ketamine, were occasionally effective (3 of 7). Hydroxychloroquine (3 of 5), an antimalarial agent with neuromodulatory effects, and vasoactive agents including β-blockers and alpha-1 adrenergic receptors (2 of 5) were each effective in a subset of patients. Comorbidities, demographic data, and individual treatment responses are summarized in the Table.
Table 1.
Table Patient Characteristics
| Patient No./Sex/ Age at Onset, y/Symptom Duration, y | Pertinent Medical History | Previous Ineffective Therapies | Therapies With at Least Partial Efficacy |
|---|---|---|---|
| 1/F/38/9 | Depression, chronic pain, cervical stenosis, fibromyalgia, migraines, essential tremor, Raynaud phenomenon | Topical steroids, topical clindamycin | Gabapentin, combination cream of ketamine, 15%/amitriptyline, 5%/clonidine, 0.2%/ketoprofen, 10% |
| 2/F/45/15 | Depression, migraines | Topical capsaicin, pregabalin, tramadol | Gabapentin, duloxetine |
| 3/F/25/10 | Acne vulgaris, migraines, allergic contact dermatitis | Oral tetracyclines, topical metronidazole, nadolol, gabapentin, amitriptyline | Amoxicillin, desonide for itch |
| 4/F/50/10 | Carpal tunnel syndrome, autoimmune thyroiditis, chronic pain, neurogenic itch, spondylolisthesis | NA | Gabapentin, duloxetine |
| 5/F/36/8 | Complex regional pain syndrome, positive ANA without other known autoimmune disease | Oral antibiotics, topical steroids | Pregabalin, duloxetine |
| 6/F/29/8 | Mixed connective tissue disease, Raynaud phenomenon, depression, bulimia | Topical metronidazole, hydroxychloroquine | Gabapentin, minocycline |
| 7/M/37/13 | Scrotal dysesthesia, psoriasis, C3–6 degenerative joint disease | Topical metronidazole | Minocycline, doxycycline |
| 8/F/41/17 | Rheumatoid arthritis | Topical metronidazole, topical steroids, oral antibiotics, β-blockers, gabapentin, hydroxychloroquine, amitriptyline | Nortriptyline |
| 9/M/15/10 | Psoriatic arthritis, irritable bowel syndrome, obsessive compulsive disorder | Topical metronidazole, topical steroids, gabapentin, topiramate, botulinum toxin type A, laser therapy, thoracic sympathectomy, clonidine, propranolol | Topical glycopyrrolate, 3%; prazosin, memantine NMDA-receptor antagonist |
| 10/F/53/5 | Oral lichen planus, headaches | Topical sulfa | Amoxicillin |
| 11/F/32/12 | Headaches | Topical metronidazole, topical steroids, gabapentin, pregabalin | Topical capsaicin, duloxetine |
| 12/F/60/2 | Complex regional pain syndrome, Raynaud phenomenon, depression, esophageal dysmotility, positive ANA without other known autoimmune disease | Topical metronidazole, topical steroids, topical doxepin | Gabapentin, hydroxychloroquine |
| 13/F/37/8 | Anxiety, depression, headaches, Raynaud phenomenon | Topical sulfasalazine, topical metronidazole, azelaic acid, doxycycline, gabapentin, intense pulsed light, topical amitriptyline, topical ketamine | Titanium dioxide, hydroxychloroquine, amitriptyline, propranolol |
| 14/F/22/9 | Systemic lupus erythematosus, Raynaud phenomenon | Topical metronidazole, oral tetracyclines, gabapentin, pregabalin, β-blockers, vascular laser therapy, topical doxepin, topical ketamine | Hydroxychloroquine, memantine |
Abbreviations: ANA, antinuclear antibody; NA, not applicable; NMDA, N-methyl-D-aspartic acid.
Comment
We propose that this group of patients with strikingly prominent neurologic symptoms represents an underrecognized subgroup of rosacea that we term neurogenic rosacea. By highlighting and formally naming this subgroup, we hope to increase awareness and recognition of these patients and aid the practicing dermatologist in their therapeutic management.
The cause of rosacea is complex, poorly understood, and likely multifactorial, with significant proposed contributions from dysfunctional cutaneous vasculature and innate immunity.3 We believe neuronal dysregulation is equally integral to rosacea pathogenesis. It may contribute to disease via various mechanisms, such as vaso-motor instability, release of proinflammatory neuro-peptides, and neuronal injury leading to perceived dysesthesias.4,5 In an individual patient, the relative importance of each of the mechanisms may differ, influencing both the spectrum of clinical symptoms and the optimal treatment strategy.
We propose the diagnostic and treatment algorithm illustrated in Figure 2 as a guide for clinicians in managing a patient with suspected neurogenic rosacea. Patients with prominent vasomotor symptoms, defined clinically by flushing and telangiectasias, may respond to vasoactive medications, including β-blockers, alpha-1 adrenergic blockers, and calcium channel blockers. In addition, laser- and light-based therapies seem to be more effective in this subset of patients.
Figure 2.
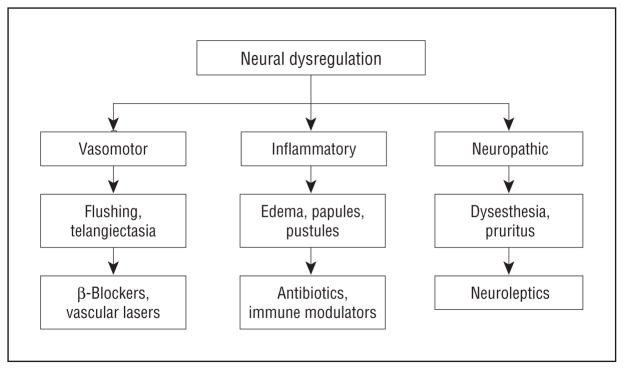
Proposed diagnostic and treatment algorithm for a patient with rosacea based on clinical-pathologic correlation. In any given patient, more than 1 pathway and treatment method might be important, but identifying individual components can help guide management.
Patients with inflammatory features such as papules, pustules, or edema may respond, if symptoms are mild, to traditional topical therapies such as metronidazole, azelaic acid, or sulfur. Systemic antibiotics and antimalarial agents used for their anti-inflammatory effect may be useful for nonresponders.
Finally, patients with dysesthesia out of proportion to flushing or inflammation can be difficult to treat and require a unique approach first used to treat disorders such as complex regional pain syndrome and neuropathic itch. In our experience, neuroleptic agents (eg, gab-apentin, pregabalin), tricyclic antidepressants, and pain-modifying antidepressants (eg, duloxetine) are the most effective. N-methyl-D-aspartic acid receptor antagonists (eg, memantine), systemic antibiotics, and other topically formulated medications (eg, ketamine, glycopyr-rolate, capsaicin) may be helpful in certain cases. Because of the associated heightened sensitivity to heat and sunlight, laser- and light-based interventions should be used with caution.
Because our understanding of this enigmatic subclass of rosacea is extremely limited, further research is clearly needed to better describe the underlying patho-physiologic characteristics and to identify additional effective treatment methods.
Footnotes
Financial Disclosure: Dr Steinhoff serves as a consultant for and holds a research grant and patent with Galderma. Dr Berger is a consultant for Prescription Solutions and serves as a nonsalaried principal investigator in other research studies involving GlaxoSmithKline, Clinsys Clinical Research Inc, Merz Pharmaceuticals, and Pharmanet.
Author Contributions: Drs Scharschmidt, Wang, and Berger had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Scharschmidt and Yost contributed equally to this work. Study concept and design: Scharschmidt, Yost, Wang, and Berger. Acquisition of data: Scharschmidt, Yost, Truong, and Steinhoff. Analysis and interpretation of data: Scharschmidt, Yost, Steinhoff, and Wang. Drafting of the manuscript: Scharschmidt, Truong, and Steinhoff. Critical revision of the manuscript for important intellectual content: Steinhoff, Wang, and Berger. Statistical analysis: Yost. Administrative, technical, and material support: Scharschmidt, Truong, and Steinhoff. Study supervision: Scharschmidt, Steinhoff, Wang, and Berger.
Additional Contributions: Elaine Yu, BA, Susan McGuire, BA, and Chris Walker, JD, provided technical assistance and support.
References
- 1.Wilkin J, Dahl M, Detmar M, et al. National Rosacea Society Expert Committee. Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50(6):907–912. doi: 10.1016/j.jaad.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 2.Crawford GH, Pelle MT, James WD. Rosacea: I. Etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51(3):327–341. doi: 10.1016/j.jaad.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55(2):77–81. doi: 10.1016/j.jdermsci.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K, Berger T. Dermatological neurological interactions. In: Aminoff MJ, editor. Neurology and General Medicine. 4. Philadelphia, PA: Churchill Livingstone; 2008. [Google Scholar]
- 5.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86(4):1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]


