Abstract
Post-translational modifications play a key role in tau protein aggregation and related neurodegeneration. Because hyperphosphorylation alone does not necessarily cause tau aggregation, other post-translational modifications have been recently explored. Tau acetylation promotes aggregation and inhibits tau’s ability to stabilize microtubules. Recent studies have shown co-localization of acetylated and phosphorylated tau in AD and some 4R tauopathies. We developed a novel monoclonal antibody against acetylated tau at lysine residue 274, which recognizes both 3R and 4R tau, and used immunohistochemistry and immunofluorescence to probe 22 cases, including AD and another eight familial or sporadic tauopathies. Acetylated tau was identified in all tauopathies except argyrophilic grain disease (AGD). AGD is an age-associated, common but atypical 4R tauopathy, not always associated with clinical progression. Pathologically, AGD is characterized by neuropil grains, pre-neurofibrillary tangles, and oligodendroglial coiled bodies, all recognized by phospho-tau antibodies. The lack of acetylated tau in these inclusions suggests that AGD represents a distinctive tauopathy. Our data converge with previous findings to raise the hypothesis that AGD could play a protective role against the spread of AD-related tau pathology. Tau acetylation as a key modification for the propagation tau toxicity deserves further investigation.
Keywords: tau, pathology, autopsy, acetylation, immunohistochemistry, human
INTRODUCTION
Tau proteins are expressed primarily in the central nervous system and help to promote microtubule (MT) stability and neuronal survival. Six tau isoforms exist, range in size from 352 to 441 amino acid residues, and contain either three (3R-tau) or four (4R-tau) repeats [16]. These repeats constitute the MT-binding domains [26].
Specific neurodegenerative disorders such as Alzheimer’s disease (AD), Pick’s disease, corticobasal degeneration, progressive supranuclear palsy and chronic traumatic encephalopathy are classified as tauopathies due to abnormal neuronal and glial tau aggregations [15]. These aggregates are generally considered to be toxic. In AD, for instance, the progression of tau neurofibrillary cytoskeletal changes, rather than the β-amyloid burden, is associated with increasing clinical severity [3, 11, 14]. While AD is associated with both 3R- and 4R-tau pathology, most tauopathies have primarily 4R-tau inclusions; Pick’s disease remains the one tauopathy associated with predominantly 3R-tau inclusions [7].
Post-translational tau modifications may be one mechanism underlying tau aggregation [2], and tau hyperphosphorylation at multiple serine and threonine residues has been identified as the most consistent change across the tauopathies, including AD [19]. Nevertheless, it remains uncertain whether tau hyperphosphorylation alone is enough to produce disease because tau hyperphosphorylation is not always accompanied by abnormal tau aggregation [6, 27]. Recently, tau acetylation has been proposed as a mechanism that contributes to tau aggregation and accumulation in tauopathies. Using acetylated tau specific antibodies, we have shown that tau acetylation is elevated in human AD brain specimens and in rodent AD model systems [29]. Under cell-free conditions, tau acetylation promotes aggregation and inhibits tau’s ability to stabilize microtubules [10]. Tau acetylation could further contribute to tau accumulation via inhibiting tau polyubiquitination and degradation [29]. Neuropathologically, acetylated tau has been detected in the inclusions and tangles in AD and in the 4R tauopathies corticobasal degeneration and progressive supranuclear palsy using a polyclonal antibody that recognizes only acetylated 4R-tau isoforms [22]. Still, it remains unknown whether tau acetylation occurs across the full spectrum of 3R and 4R tauopathies or whether acetylation at other lysine residues occurs in pathological tau inclusions.
We developed a novel monoclonal antibody specific for acetylated-tau at lysine residue 274, which, along with other positively charged lysine residues in the microtubule-binding domain, was shown to be involved in tau MT-binding [18]. This antibody recognizes both 3R and 4R tau, allowing us to examine tauopathies involving either isoform. To characterize acetylation at lysine residue 274 in human tissue from patients with AD and other tauopathies, we optimized this antibody for immunohistochemistry and double label immunofluorescence. Here, we show that tau acetylation at lysine residue 274 characterizes all major human tauopathies except for the 4R tauopathy, argyrophilic grain disease (AGD).
METHODS
Case Selection and Neuropathological Methods
Cases were selected from the Neurodegenerative Disease Brain Bank (NDBB) at the University of California, San Francisco (UCSF). The NDBB receives brain and spinal cord donations from patients enrolled in UCSF Memory and Aging Center longitudinal clinical research programs. The fresh brains were dissected into ~1 cm coronal slabs, which were alternately fixed (in 10% neutral buffered formalin for 72 hours) or rapidly frozen, providing tissues preserved with both methods bilaterally for every cut surface. A secondary diagnostic dissection (24 regions) was used to capture dementia-related structures. Blocks were embedded in paraffin wax, cut into 8 micron-thick sections, and stained with chemical stains and immunohistochemistry to facilitate diagnosis. Cases were selected by taking into consideration the neuropathological diagnosis only. We selected two cases (when available) of each available tauopathy, starting with the most recent case. The third AD case was fixed in formalin for 3 weeks and was used for antibody optimization. We selected all AGD cases available in the NDBB, provided that they lacked a comorbid moderate or severe tauopathy that would confound interpretation of the findings
Monoclonal antibody generation and characterization
Rabbit monoclonal antibodies against acetylated tau were generated by Epiotomics (Mountain View, CA, USA). Synthetic tau peptide that contains ac-K274 was used to immunize three rabbits. After splenectomy and hybridoma fusion, we screened the supernatants of dozens of clones for the specificity against ac-K274 using western blot analyses. Briefly, HEK293T cells were co-transfected with expression plasmid encoding p300 (pcDNA3.1-p300) with that encoding human tau (pcDNA3-hTau) or hTauK274R (pcDNA3-hTauK274R). Hybridoma clone MAb 359 was selected since its supernatant specifically recognized hTau acetylated at K274 by p300. No immunoreactivity was observed in cells transfected with hTau alone or co-transfected with hTauK274R and p300.
Antibody optimization for immunohistochemistry
We conducted a series of assays to optimize MAb 359 immunoperoxidase and immunofluorescence protocols for human postmortem brain tissue. Different primary antibody concentrations, antigen retrieval methods, incubation times and detection methods were tested. For these experiments we made use of serial sections of AD and control tissue from inferior temporal cortex. Furthermore, to test MAb 359 specificity against acetylated tau at position 274, an antigen competition experiment was performed. MAb 359 was pre-incubated with the acetylated-tau peptide used as the immunogen for antibody generation. An immunohistochemistry experiment was run in duplicate. In the primary antibody step, one slide had MAb 359 pre-incubated with acetylated tau peptide and the other slide had antibody (MAb 359) not pre-incubated with peptide. All other parameters of the experiment remained constant. We tested tissues fixed in formalin for either 72 hours or for extended periods (i.e. long-term fixation). In each reaction, we included slides in which the primary antibody was replaced by saline buffer as negative controls. Comparable immunoperoxidase results could be achieved on both short and long fixed tissue, although we noted weaker immunofluorescent signal in tissue fixed for over 72 hours.
Immunohistochemistry
In Experiment #1, materials from patients with all available tauopathies and AD (Table 1) were submitted to the protocol described below, in a single run. In Experiment #2, seven additional cases with AGD were selected and submitted to the same protocol, including three in which AGD was widespread and considered the primary diagnosis and four with a primary diagnosis of frontotemporal lobar degeneration with TDP-43 immunoreactive inclusions (FTLD-TDP) who had co-morbid AGD. In Experiment #3, all 21 short-fixed cases were again submitted to immunofluorescence using the same protocol.
Table 1.
Cases used in this study
| Case # |
Disease | Gender | Age at onset (y) |
Age at death (y) |
PMI (h) |
|---|---|---|---|---|---|
| 1 | AD | F | 80 | 91 | 5.5 |
| 2 | AD | F | 48 | 56 | 4.9 |
| 3 | AD a | F | 54 | 65 | 10.9 |
| 4 | AGD b (widespread) | F | 83 | 86 | 7.8 |
| 5 | AGD b (widespread) | M | 73 | 82 | 7.6 |
| 6 | AGD b (widespread) | M | 65 | 77 | 4.9 |
| 7 | Atypical 4R tauopathy | M | 57 | 65 | 10.0 |
| 8 | CTE | M | 47 | 50 | 9.2 |
| 9 | CBD | M | 66 | 72 | 36 |
| 10 | CBD | M | 61 | 68 | 7.4 |
| 11 | FTDP-17 | M | 47 | 60 | 15.8 |
| 12 | FTLD-TDP + AGD b (limbic) | M | 55 | 58 | 7.5 |
| 13 | FTLD-TDP + AGD (limbic) | M | 65 | 68 | 7.5 |
| 14 | FTLD-TDP + AGD b (limbic) | F | 57 | 65 | 8.5 |
| 15 | FTLD-TDP + AGD b (limbic) | F | 62 | 76 | 17.1 |
| 16 | FTLD-TDP + AGD b (limbic) | F | 64 | 72 | 6.1 |
| 17 | FTLD-TDP + AGD (limbic) | M | 53 | 65 | 14.3 |
| 18 | Pick's | M | 60 | 70 | 10.2 |
| 19 | Pick's | F | 62 | 74 | 8.5 |
| 20 | PSP | F | 60 | 69 | 32.7 |
| 21 | PSP | M | 63 | 69 | 8.3 |
| 22 | WMT-GGI | F | 72 | 80 | 6.4 |
AD: Alzheimer’s disease; AGD: Argyrophilic grain disease; CBD: corticobasal degeneration; CTE: Chronic traumatic encephalopathy; FTDP-17: frontotemporal dementia with Parkinsonism linked to chromosome 17; FTLD-TDP: frontotemporal lobar degeneration with TDP inclusions; PSP: progressive supranuclear palsy; WMT-GGI: white matter tauopathy with globular glial inclusions.
Case formalin fixed for 3 weeks.
Cases added for the second experiment
All immunohistochemical studies were performed on eight microns-thick sections cut from paraffin blocks and mounted on glass slides. Hippocampus and inferior temporal gyrus were examined in all patients, and we added midbrain for PSP and middle frontal gyrus in widespread AGD. For each case, we stained three slides: (1) immunoperoxidase against phospho-tau (CP-13, gift of Peter Davies, NY), (2) immunoperoxidase against MAb 359, and (3) double label immunofluorescence against CP-13 and MAb 359. CP-13 is a monoclonal antibody against phosphorylated tau, pS202/T205, which recognizes phosphorylated 3R- and 4R-tau. All tauopathies show CP-13-positive inclusions. For both antibodies, immunoperoxidase staining was performed using an avidin-biotin complex detection system (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) with 3,3 diaminobenzidine as the chromogen. Slides were pretreated for antigen retrieval by immersion in citrate pH 6.0 in an autoclave at 121° C for 5 min. The primary antibodies, CP-13 (1:250) and MAb 359 (1:250) were incubated overnight at 4°C and species-specific biotinylated secondary antibodies were incubated for one hour at room temperature. Sections were submitted to immunohistochemistry for MAb359 in a single batch and were overexposed to DAB to ensure that all tau pathology, especially that seen in AGD, would be detected if acetylated. This approach resulted in a higher than usual background but increased confidence in our negative AGD findings without affecting the interpretability of the results.
For double-label immunofluorescence, after antigen retrieval in the autoclave, the sections were treated to block autofluorescence using a 0.1% Sudan Black solution. Both primary antibodies were diluted together and incubated overnight at 4°C, followed by a mixture of species-specific fluorescent secondary antibody (DyLight 488 Horse Anti-Mouse and DyLight 549 Goat Anti-Rabbit IgG Antibodies, Vector Laboratories, Burlingame, CA) for one hour and coverslipped with Vectashield hardest mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Slides were analyzed and photographed immediately following the reactions. We included sections from FTLD-TDP (without AGD) cases in each batch as negative controls. In addition, AD sections were incubated with the secondary antibodies alone to rule out nonspecific staining.
Tissue analyses
We first assessed the slides for tau-positive inclusions, then compared inclusion burden, shape and localization between CP-13 and MAb 359 immunostaining. For the double immunofluorescence labeled slides, we characterized the cell types in which CP-13 and MAb 359 signal overlapped and whether CP-13-only or MAb 359-only inclusions were present.
Western blot
Human brain tissues were homogenized and sonicated at 4°C in 10 fold (W:V) RIPA buffer containing 50mM Tris, pH 8.0, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitor cocktail , 1 mM phenylmethyl sulfonyl fluoride , phosphatase inhibitor cocktail II and III , and HDAC inhibitors including 5 mM nicotinamide and 1 uM trichostatin A (R0278, Sigma, St. Louis, MO)). Protein concentration were determined by Bradford assay and equal amount of protein was resolved by 4–12% SDS-PAGE (Invitrogen, Carlsbad, CA) and electro-transferred onto nitrocellulose membranes (Amersham, NJ). After blocking by 5% non-fat milk for 1hr at room temperature, membranes were incubated with rabbit anti-ac-Tau (MAb 359, 1:500) or mouse anti-Tau5 (Fisher, 1:50,000), mouse anti-GAPDH (Millipore, Bedford, MA; 1:100,000) overnight at 4°C and then incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Millipore, Bedford, MA; 1:2,000) or goat anti-mouse IgG (Millipore, Bedford, MA; 1:2,000) antibodies. Bands were visualized by enhanced chemiluminescence.
RESULTS
Monoclonal antibody MAb 359 is highly specific against tau acetylated at lysine 274
A monoclonal antibody was generated with a synthetic tau peptide spanning amino acids 264–287 in the microtubule-binding domain. The synthetic peptide contains acetylated lysines at positions 274, which was identified to be acetylated in our previous studies [29] (Fig. 1a). After screening supernatants from hybridoma clones and subclones, MAb 359 was identified as a highly specific monoclonal antibody recognizing acetylated lysine at position 274. In HEK293 cells transfected with tau, expression of acetyltransferase p300 markedly elevated a signal detected with MAb 359 with modest increase of total-tau detected with Tau 5 antibody, suggesting that MAb 359 selectively recognized p300-induced acetylated tau. Alternative splicing of exon 10 of the tau gene results in the presence or absence of the second microtubule-binding repeat (R2), resulting in 4R or 3R tau [17]. MAb 359 recognized acetylation of both 4R and 3R tau because lysine 274 is located in the first microtubule-binding repeat (R1) (Fig. 1b). To confirm the specificity of MAb 359 for lysine 274, we transfected tau with lysine 274 mutated to arginine (K274R) in HEK293 cells. Mutation of lysine 274 completely abolished the signal detected with MAb 359 in the presence of p300 (Fig. 1c). As shown in online resource 1, the reaction using the pre-incubated antibody resulted in a completely negative reaction, whereas the non-preincubated reaction was positive, strongly supporting that MAb 359 detects acetylated-tau specifically in immunohistochemical analyses. These results suggest that MAb 359 specifically recognizes tau protein with acetylated lysine 274.
Figure 1.
(a) Location and sequence of antigen used to generate an antibody targeting acetylated tau at lysine 274. (b) MAb 359 recognizes both 4R and 3R tau. HEK293 cells were transfected with either 4R or 3R tau in the presence or absence of p300 overexpression. MAb 359 selectively recognized p300-induced acetylation in both 4R and 3R tau. Total tau level was detected with Tau5 antibody. (c) MAb 359 is specific for ac-K274. HEK293 cells were transfected with WT or K274R tau in the presence or absence of p300 overexpression. Mutation of K274 abolished p300-induced immunoreactivity of MAb 359.
All tauopathies except argyrophilic grain disease show tau acetylation at position 274
Inclusions found in all human tauopathies except AGD stained positively with CP-13 and MAb 359 antibodies (Figs. 2 – 4). Specifically, MAb 359 immunoreactivity was absent in CP-13-positive grains, pre-neurofibrillary tangles and coiled bodies in all nine AGD cases (Figs. 5 and 6). However, AD-related neurofibrillary tangles were MAb 359-positive in the same AGD cases (Fig. 5d and Fig. 6 g – i). MAb 359 positivity was found across all cell types staining positively with CP-13, such as neurons, astroglia and oligodendrocytes. Tau-positive Pick bodies and Pick’s disease glial cytoplasmic inclusions were acetylated (Fig. 2 i–l and online resource 2). AD neuritic plaques also showed positivity for MAb 359 (Fig. 2c and 4a). Although some gray and white matter threads contained acetylated tau positive for MAb 359, most threads were only positive for CP-13 (Fig. 2 and online resource 3). The results were consistent across all experimental runs.
Figure 2.
Abnormal tau pathology in inferior temporal cortex sections of tauopathy cases immunostained with CP-13 (a, c, e, g, i, k, m, o) or MAb 359 (b ,d ,f, h, j, l, n, p). a–d Alzheimer’s disease (case 3). Arrowheads and arrows show neuritic plaques and neurofibrillary tangles, respectively. e–h White matter tauopathy with globular glial inclusions (case 22). g, h show the white matter of case 22 at high magnification. Note that the glial globolar inclusions are positive for phospho-tau and acetylated tau. i–l Atypical 4R tauopathy (case 7), and m–p chronic traumatic encephalopathy (case 8). In both cases, the tau inclusions are phosphorylated and acetylated.
Scale bars represent 1 mm in a, b, e, f, i, j, m, n and 100 µm in c, d, g, h, k, l, o, p.
Figure 4.
High magnification of acetylated-tau inclusions located in inferior temporal cortex (a–c and e–i) and midbrain (d), after immunostaining with MAb 359. a neuritic plaque (Alzheimer’s disease - case 3). b neurofibrillary tangle (Alzheimer’s disease - case 3). c astrocytic plaque (corticobasal degeneration - case 9). d tufted astrocyte in periaqueductal gray matter (progressive supranuclear palsy – case 21 ). e Pick body (Pick’s disease – case 18). f neuronal cytoplasmicinclusion (chronic traumatic encephalopathy- case 8). g neuronal cytoplasmicinclusion (FTLP-17 – case 11). h globular glial inclusions (white matter tauopathy with globular glial inclusion - case 22). i glial inclusion (atypical tauopathy- case 7). Scale bars represent 10 µm
Figure 5.
Hippocampal sections of a case of argyrophilic grain disease (case 5) after immunostaining with CP-13 (a, c, e) and MAb 359 (b, d, f) antibodies. a– b dentate gyrus. Only phosphorylated tau immunoreactivity (CP-13) is seen (arrowheads). c – d CA1 sector. Grains are observed only in the section immunostained with CP-13 (ph-tau). Almost all AGD cases have overlapping AD and AGD pathology to varying degrees. The arrowhead shows an Alzheimer’s disease type neurofibrillary tangle. e –f CA2 sector. AGD is characterized by pre-neurofibrillary tangles with a perinuclear halo as seen in e. These pretangles are not acetylated at position 274. Scale bars represent 100 µm.
Figure 6.
Hippocampal formation sections of a case of argyrophilic grain disease (case 5) after immunofluorescence staining with CP-13 (a, d, g, j) and MAb 359 (b, e,h,k) antibodies. a– c dentate gyrus. Only phosphorylated tau immunoreactivity (CP-13) is seen (arrowheads and green fluorescent signal). d – f CA1 sector. Grains are observed only in the section immunostained with CP-13 (ph-tau). g – i CA2 sector. AGD is characterized by pre-neurofibrillary tangles with a perinuclear halo as seen in k and m. These pretangles are not acetylated at position 274. The arrowheads show an Alzheimer’s disease type neurofibrillary tangle. j – l white matter adjacent to entorhinal cortex. As oppose to corticobasal degeneration and other tauopathies, white matter pathology, including coiled bodies (arrows) are not tau-acetylated in AGD. Scale bars represent 100 µm.
Tau acetylation proved similar in widespread and limbic AGD
Widespread AGD represents a rare condition and has been proposed as a different entity than limbic AGD. However, we found no differences concerning acetylation between three widespread and six limbic AGD cases.
Western blot analyses
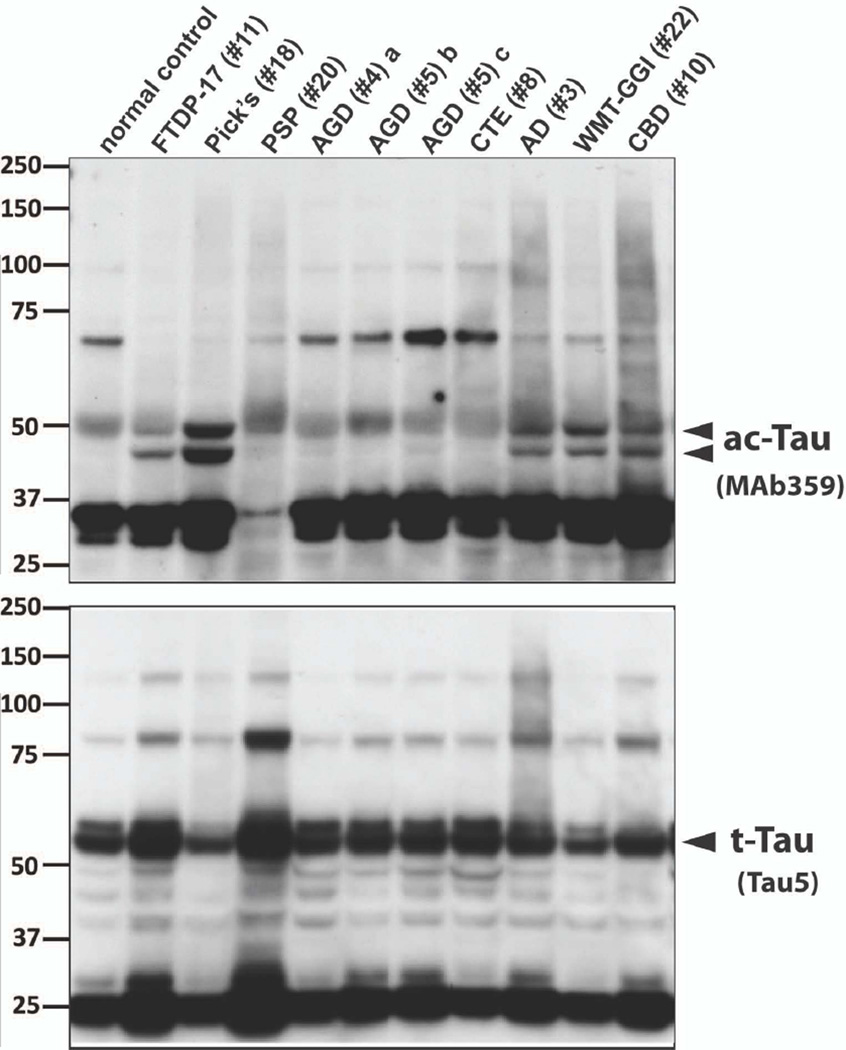
Robust ac-K27 bands were observed in lysates from AD, Pick’s disease, FTDP-17, CBD , and white matter tauopathies brains. In contrast, trace to no signal was observed in brain lysates from AGD and other tauopathies (Figure 7).
Figure 7.
Western blot analyses of ac-K274 in brain lysates from brains of human patients. Frozen tissues from specific regions of human tauopathy brains were lysed with RIPA buffer containing HDAC and phosphatase inhibitors. Levels of ac-K274 were probed with MAb 359; total tau was probed with Tau-5. Anti-GAPDH was used to ensure that equal amount of protein was loaded (60 µg protein/lane). The lysates were extracted from inferior temporal cortex, unless otherwise specified.
Footnote: AD: Alzheimer’s disease; AGD: Argyrophilic grain disease; CBD: corticobasal degeneration; CTE: Chronic traumatic encephalopathy; FTDP-17: frontotemporal dementia with parkinsonism linked to chromosome 17; PSP: progressive supranuclear palsy; WMT-GGI: white matter tauopathy with globular glial inclusions/ globular glial tauopathy. a insula. b precentral gyrus. c posterior cingulate gyrus
DISCUSSION
This study produced two major findings using a newly developed monoclonal antibody specific for tau acetylated at lysine 274. First, tau acetylation is present in AD and seven subtypes of FTLD-tau but not in AGD, a common but atypical 4R tauopathy not always associated with clinical progression. Second, tau acetylation is present in Pick’s disease, white matter tauopathy with globular glial inclusions (WMT-GGI), also known as globular glial tauopathy [1, 25], chronic traumatic encephalopathy,and unclassifiable 4-R tauopathies, expanding the diversity of tauopathies in which tau acetylation has been demonstrated.
Because tau hyperphosphorylation alone does not necessarily cause tau aggregation or cellular dysfunction [6, 27], recent research has begun to investigate other post-translational modifications. Tau acetylation at lysine residues has been demonstrated to enhance the deleterious effects of phosphorylation and is increased in AD and other tauopathies [10, 29]. In the only human neuropathological reports to date, Irwin et al [22] and Cohen et al [10], from the same group at the University of Pennsylvania, characterized tau acetylation at position 280 in tauopathies using immunohistochemistry. This residue is located in the second tau microtubule-binding repeat; therefore, the antibody only recognizes 4R-tau isoforms. These authors found that acetylated tau inclusions displayed a similar distribution and pathological burden to hyperphosphorylated tau in all pathological tau inclusions except neuropil threads, which showed stronger evidence of phosphorylation than acetylation. Our findings align with these reports for all diseases examined in common across studies except for AGD and add additional 3-R and 4-R tauopathies to the list of entities showing pathological tau acetylation.
Using immunohistochemistry, we identified AGD as the lone tauopathy in which tau acetylation at K274 is absent. These findings were confirmed in three experiments and proved independent of disease stage. Our findings differ from those of Cohen et al. [10], who reported ac-tau immunoreactivity in AGD; the data provided leave some uncertainty, however, regarding whether the tau acetylation observed at position 280 represented characteristic AGD pathologies or comorbid AD. Using western blot analyses, we observed little to no ac-K274 signal in brain lysates from AGD cases. In contrast, strong signals were observed in lysates from AD, Pick’s disease, FTDP-17, CBD, and white matter tauopathy brains lysates, in agreement with the abundance of ac-tau inclusions observed by immunohistochemistry in those cases. We were unsuccessful, however, in detecting ac-tau signal in lysates from a few other tauopathy cases, including PSP. Tissue homogenates lack cellular resolution and signal can be diluted in cases of low inclusion burden, leading to false negative results. Immunohistochemical analysis, which offers cellular and subcellular resolution, provides a more sensitive approach and detected acetylated tau in all tauopathies studied here apart from AGD.
The nine AGD cases we studied spanned disease stages, and in three, AGD was considered the patient’s primary neuropathological diagnosis. AGD is a late-onset 4R tauopathy, first described in the 1980’s as a distinctive degenerative disease characterized by argyrophilic grains confined to limbic structures and affecting a subset of patients with adult-onset dementia [4]. AGD’s true prevalence remains unknown because there are no distinctive antemortem clinical features that could be used in epidemiological studies. Based on clinicopathological studies, AGD is considered age-related with prevalence varying from 11% to 43% of subjects over 65 years of age [13, 33]. Neuropathologically, AGD is characterized by argyrophilic and 4R-tau immunoreactive grains in neuronal processes, pre-neurofibrillary tangles and oligodendroglial coiled bodies, and only recently has it been recognized as an independent pathological entity [13]. Pre-neurofibrillary tangles are best recognized within the CA2 sector of the hippocampus and are characterized by a diffuse cytoplasmic tau immunostaining and perinuclear halos. All AGD-related pathomorphologies are readily recognized by phospho-tau antibodies. Biochemically, AGD’s tau signature is distinct from AD and Pick’s disease, but similar to CBD and PSP [35]. On the other hand, selective alteration of mitochondrial subunits exclusive to AGD has been identified recently, reinforcing the concept that AGD may represent a distinct tauopathy [21]. AGD changes are usually restricted to limbic areas [13], but in some cases the changes spread beyond this region. In the rare patients in whom AGD reaches frontal and insular regions, behavioral variant frontotemporal dementia is a typical presentation [8, 20], but up to 30% of AGD cases are diagnosed in individuals without any cognitive or behavioral impairment [24]. Additionally, AGD is often encountered in patients with long lasting, non-progressive amnestic mild cognitive impairment [23, 30]. AGD is almost always found in combination with mild to moderate AD neurofibrillary pathology and less often with other neurodegenerative diseases. This overlap with AD could be explained by the high prevalence of both diseases in the elderly. On the other hand, this overlap and the benign course associated with AGD could suggest that AGD may serve as a protective mechanism against the spread of AD. While investigating phosphorylation-regulating kinases at specific tau sites in AGD, Ferrer et al found that AGD inclusions show elevated levels of GSK-3-P [12]. Elevated GSK-3-P has the capacity to inhibit apoptosis in certain experimental models [9] and may prevent abnormal tau-containing cells from dying. Individuals with AD and AGD show milder cognitive deficits [31] and neuronal loss [35] than those with AD alone. On the other hand, conflicting data suggests that AGD lowers the threshold for cognitive deficits in the presence of moderate amounts of AD-type pathology [32, 34]. Whether the absence of tau acetylation in AGD supports a protective or deleterious role remains uncertain, but it does distinguish AGD from other proven pathogenic tauopathies. The mechanisms leading AGD to spread beyond the limbic areas are unknown, and this diffuse AGD form has been proposed as a separate entity from the more usual limbic AGD [28]. In our study, we found that both variants lack tau acetylation, suggesting some degree of shared mechanisms.
In the current study, we identified lack of ac-K274 staining as a key pathological signature that differentiates AGD from other tauopathies. Just as different tau phosphorylation sites are known to affect tau dysfunction differently, acetylation at different lysine sites on tau could lead to different outcomes. Some lysine sites may be acetylated under non-pathological conditions. Whether other acetylysines on tau could serve also serve as a pathological signature remains to be determined. Finally, our study design did not allow us to evaluate the timing of tau acetylation at residue 274 compared to tau phosphorylation. Most patients represented late stages of disease, and the majority of inclusions were both acetylated and phosphorylated. A study surveying a broader disease severity spectrum would add important information to this emerging topic. Although the antigen competition assay result supports the specificity of the immunohistochemistry reactions, we cannot fully exclude the possibility that antigen cross reactivity produced positive signals detected by immunohistochemistry but not in Western blot analyses. We suspect that antibodies developed specifically for Western blot analysis would allow us to further confirm tau acetylation in tauopathies showing positive ac-tau immunohistochemistry.
Tau acetylation was observed in all cell types commonly affected by tau hyperphosphorylation. However, axons and distal dendrites were rarely acetylated at position 274, consistent with previous observations on acetylated tau at position 280 [10, 22]. In AD, tau phosphorylation is first observed in axons and distal dendrites [5] and later in neuronal somata. Considering that tau phosphorylation is widely observed in cognitively normal individuals beginning in the second decade of life [5], we speculate that acetylation may represent a turning point, triggered by phosphorylation and other events in the neuronal somata, that accelerates tau toxicity. This hypothesis may help to explain why AGD is most often associated with a non-progressive medial temporal lobe amnestic syndrome.
CONCLUSION
Using a novel monoclonal specific antibody for acetylated tau, we demonstrated that tau inclusions in AD and the majority of 3R and 4R tauopathies are acetylated at lysine 274, which is involved in tau microtubule binding. AGD represents the only tauopathy lacking this post-translational modification, supporting it as a distinct tauopathy. Our new data converge with previous findings suggesting that AGD could play a protective role against the spread of AD, a hypothesis for exploration in future studies. Tau acetylation as a key step towards the propagation of tau toxicity deserves further investigation.
Supplementary Material
Online resource 1
Antigen competition assay. a inferior temporal cortex of an AD case (case # 3) after immunohistochemistry with MAb 359. Note the plaques and tangles in dark brown. b higher magnification of (a). The plaques and tangles can be visualized in detail. c a parallel slide to (a) after immunohistochemistry with MAb 359 pre-incubated with the antigen used for its generation. All the other reaction steps were the same as in (a). Note that the reaction was negative, supporting MAb 359 specificity against tau acetylated at position 274. d higher magnification of (b). Scale bars represent 500 µm in a and c, and 100 µm in b and d.
Online resource 2
Inferior temporal cortex of Pick’s disease (case 18) after immunofluorescence with CP-13 (a) and MAb 359 (b) antibodies. Pick bodies are both phosphorylated (a) and acetylated (b). Most of the inclusions have both changes and the processes are mainly phosphorylated only (c). Scale bar represents 50 µm
Online resource 3
Across all tauopathies, neuronal processes were rarely ac-tau positive. This figure shows parallel sections of the hippocampal dentate gyrus granule cell (top of the figure) and molecular layers (bottom of the figure) in Alzheimer’s disease (case 3) immunostained with (a) CP-13 and (b) MAb 359. Note the strong phospho-tau positivity and absent ac-tau positivity in the external portion of the molecular layer. The dentate gyrus molecular layer contains abundant axons and dendrites. This figure demonstrates the remarkable discrepancy between phospho-tau and ac-tau changes in neuronal processes. The arrow in b shows an acetylated tau positive neurofibrillary tangle as an internal positive control.
Figure 3.
Abnormal tau pathology in brain sections of tauopathy cases immunostained with CP-13 (a, c, e, g, i, k) or MAb 359 (b ,d ,f, h, j, l). a– d Corticobasal degeneration (case 10) – inferior temporal cortex. Star symbols indicate the white matter. c, d show the white matter at high magnification. e – h Progressive supranuclear palsy (case 20) –midbrain. Arrowheads show globose tangles in the periaqueductal gray matter. i – l Pick’s disease (case 18) – inferior temporal cortex. Arrowheads show Pick bodies in layer II. Scale bars represent 1 mm in a, b, e, f, i, j and 100 µm in c, d, g, h, k, l.
ACKOWLEDGMENTS
We thank Jian Yang, Norbert Lee, and Stephanie Gaus for technical assistance, and our patients and their families for their invaluable contributions to neurodegenerative disease research.
Study funding: National Institute of Health (NIH) P50 AG023501 to BLM and WWS, Tau Consortium (to LG and WWS), NIH R01AG030207 (to LG), NIH R01AG040311 to LTG, the John Douglas French Alzheimer’s Disease Foundation (to LTG and WWS), the Consortium for Frontotemporal Dementia Research (to WWS).
REFERENCES
- 1.Ahmed Z, Doherty KM, Silveira-Moriyama L, Bandopadhyay R, Lashley T, Mamais A, et al. Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4-repeat tauopathies. Acta Neuropathol. 2011;122:415–428. doi: 10.1007/s00401-011-0857-4. [DOI] [PubMed] [Google Scholar]
- 2.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 3.Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Braak E. Cortical and subcortical argyrophilic grains characterize a disease associated with adult onset dementia. Neuropathol Appl Neurobiol. 1989;15:13–26. doi: 10.1111/j.1365-2990.1989.tb01146.x. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 6.Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain ResRev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 7.Buee L, Delacourte A. Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick's disease. Brain Pathol. 1999;9:681–693. doi: 10.1111/j.1750-3639.1999.tb00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 10.Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, et al. The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun. 2011;2:252. doi: 10.1038/ncomms1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duyckaerts C. Looking for the link between plaques and tangles. Neurobiol Aging. 2004;25:735–739. doi: 10.1016/j.neurobiolaging.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer I, Barrachina M, Tolnay M, Rey MJ, Vidal N, Carmona M, et al. Phosphorylated protein kinases associated with neuronal and glial tau deposits in argyrophilic grain disease. Brain Pathol. 2003;13:62–78. doi: 10.1111/j.1750-3639.2003.tb00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain. 2008;131:1416–1432. doi: 10.1093/brain/awm305. [DOI] [PubMed] [Google Scholar]
- 14.Giannakopoulos P, Gold G, Kovari E, von Gunten A, Imhof A, Bouras C, et al. Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experience. Acta Neuropathol. 2007;113:1–12. doi: 10.1007/s00401-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 15.Goedert M. The significance of tau and alpha-synuclein inclusions in neurodegenerative diseases. Curr Opin Genet Dev. 2001;11:343–351. doi: 10.1016/s0959-437x(00)00200-8. [DOI] [PubMed] [Google Scholar]
- 16.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 17.Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 1989;8:393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goode BL, Denis PE, Panda D, Radeke MJ, Miller HP, Wilson L, et al. Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol Biol Cell. 1997;8:353–365. doi: 10.1091/mbc.8.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanger DP, Betts JC, Loviny TL, Blackstock WP, Anderton BH. New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer's disease brain using nanoelectrospray mass spectrometry. J Neurochem. 1998;71:2465–2476. doi: 10.1046/j.1471-4159.1998.71062465.x. [DOI] [PubMed] [Google Scholar]
- 20.Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 21.Ilieva EV, Kichev A, Naudi A, Ferrer I, Pamplona R, Portero-Otin M. Mitochondrial dysfunction and oxidative and endoplasmic reticulum stress in argyrophilic grain disease. J Neuropathol Exp Neurol. 2011;70:253–263. doi: 10.1097/NEN.0b013e31820f8765. [DOI] [PubMed] [Google Scholar]
- 22.Irwin DJ, Cohen TJ, Grossman M, Arnold SE, Xie SX, Lee VM, et al. Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies. Brain. 2012;135:807–818. doi: 10.1093/brain/aws013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jicha GA, Petersen RC, Knopman DS, Boeve BF, Smith GE, Geda YE, et al. Argyrophilic grain disease in demented subjects presenting initially with amnestic mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65:602–609. doi: 10.1097/01.jnen.0000225312.11858.57. [DOI] [PubMed] [Google Scholar]
- 24.Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs GG, Majtenyi K, Spina S, Murrell JR, Gelpi E, Hoftberger R, et al. White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol. 2008;67:963–975. doi: 10.1097/NEN.0b013e318187a80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee G, Neve RL, Kosik KS. The microtubule binding domain of tau protein. Neuron. 1989;2:1615–1624. doi: 10.1016/0896-6273(89)90050-0. [DOI] [PubMed] [Google Scholar]
- 27.Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurage CA, Sergeant N, Schraen-Maschke S, Lebert F, Ruchoux MM, Sablonniere B, et al. Diffuse form of argyrophilic grain disease: a new variant of four-repeat tauopathy different from limbic argyrophilic grain disease. Acta Neuropathol. 2003;106:575–583. doi: 10.1007/s00401-003-0762-6. [DOI] [PubMed] [Google Scholar]
- 29.Min SW, Cho SH, Zhou Y, Schroeder S, Haroutunian V, Seeley WW, et al. Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron. 2010;67:953–966. doi: 10.1016/j.neuron.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 31.Steuerwald GM, Baumann TP, Taylor KI, Mittag M, Adams H, Tolnay M, et al. Clinical characteristics of dementia associated with argyrophilic grain disease. Dement Geriatr Cogn Disord. 2007;24:229–234. doi: 10.1159/000107085. [DOI] [PubMed] [Google Scholar]
- 32.Thal DR, Schultz C, Botez G, Del Tredici K, Mrak RE, Griffin WS, et al. The impact of argyrophilic grain disease on the development of dementia and its relationship to concurrent Alzheimer's disease-related pathology. Neuropathol Appl Neurobiol. 2005;31:270–279. doi: 10.1111/j.1365-2990.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 33.Tolnay M, Clavaguera F. Argyrophilic grain disease: a late-onset dementia with distinctive features among tauopathies. Neuropathology. 2004;24:269–283. doi: 10.1111/j.1440-1789.2004.00591.x. [DOI] [PubMed] [Google Scholar]
- 34.Tolnay M, Schwietert M, Monsch AU, Staehelin HB, Langui D, Probst A. Argyrophilic grain disease: distribution of grains in patients with and without dementia. Acta Neuropathol. 1997;94:353–358. doi: 10.1007/s004010050718. [DOI] [PubMed] [Google Scholar]
- 35.Tolnay M, Sergeant N, Ghestem A, Chalbot S, De Vos RA, Jansen Steur EN, et al. Argyrophilic grain disease and Alzheimer's disease are distinguished by their different distribution of tau protein isoforms. Acta Neuropathol. 2002;104:425–434. doi: 10.1007/s00401-002-0591-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online resource 1
Antigen competition assay. a inferior temporal cortex of an AD case (case # 3) after immunohistochemistry with MAb 359. Note the plaques and tangles in dark brown. b higher magnification of (a). The plaques and tangles can be visualized in detail. c a parallel slide to (a) after immunohistochemistry with MAb 359 pre-incubated with the antigen used for its generation. All the other reaction steps were the same as in (a). Note that the reaction was negative, supporting MAb 359 specificity against tau acetylated at position 274. d higher magnification of (b). Scale bars represent 500 µm in a and c, and 100 µm in b and d.
Online resource 2
Inferior temporal cortex of Pick’s disease (case 18) after immunofluorescence with CP-13 (a) and MAb 359 (b) antibodies. Pick bodies are both phosphorylated (a) and acetylated (b). Most of the inclusions have both changes and the processes are mainly phosphorylated only (c). Scale bar represents 50 µm
Online resource 3
Across all tauopathies, neuronal processes were rarely ac-tau positive. This figure shows parallel sections of the hippocampal dentate gyrus granule cell (top of the figure) and molecular layers (bottom of the figure) in Alzheimer’s disease (case 3) immunostained with (a) CP-13 and (b) MAb 359. Note the strong phospho-tau positivity and absent ac-tau positivity in the external portion of the molecular layer. The dentate gyrus molecular layer contains abundant axons and dendrites. This figure demonstrates the remarkable discrepancy between phospho-tau and ac-tau changes in neuronal processes. The arrow in b shows an acetylated tau positive neurofibrillary tangle as an internal positive control.