Abstract
Background
Systematic data on discontinuation of statins in routine practice of medicine are limited.
Objective
To investigate reasons for statin discontinuation and the role of statin-related events (clinical events / symptoms thought to have been caused by statins) in routine care settings.
Design
A retrospective cohort study
Setting
Practices affiliated with one of two academic hospitals.
Patients
Adults who received a statin prescription between 01/01/2000 and 12/31/2008.
Measurements
Information on reasons for statin discontinuations was obtained from a combination of structured electronic medical record (EMR) entries and analysis of electronic provider notes by validated software.
Results
Statins were discontinued at least temporarily for 57,292 out of 107,835 patients. Statin-related events were documented for 18,778 (17.4%) patients. Statins were discontinued at least temporarily by 11,124 of these patients, 6,579 (59.1%) of whom were rechallenged with a statin over the subsequent 12 months. Most patients who were rechallenged (92.2%) were still taking a statin 12 months after the statin-related event. Among the 2,721 patients who were rechallenged with the same statin to which they had a statin-related event, 1,295 (47.6%) were on the same statin 12 months later, including 996 on the same or higher dose.
Limitation
Statin discontinuations and statin-related events were assessed in practices affiliated with two academic medical centers. Utilization of secondary data could have led to missing or misinterpreted data as a result of incomplete documentation. Natural language processing tools used to compensate for the low (30%) proportion of reasons for statin discontinuation documented in structured EMR fields are not perfectly accurate.
Conclusion
Statin-related events are commonly reported and often lead to their discontinuation. However, most patients who are rechallenged can tolerate statins long-term. This suggests that many of the statin-related events may have other etiologies, are tolerable or may be specific to individual statins rather than the entire drug class.
Hypercholesterolemia is one of the most common chronic conditions and is strongly associated with cardiovascular disease, including cardiovascular mortality (1-3). Hydroxy methylglutaryl coenzyme A reductase inhibitors (a.k.a. statins) decrease mortality in patients with hypercholesterolemia (4-10) and are the most commonly used medications for treating hypercholesterolemia in the U.S (11).
Despite their well documented benefits, statins are commonly discontinued (12-15). Statin discontinuation has been linked to increased risk of cardiovascular events and death in patients with coronary artery disease (16-19). Nevertheless the reasons why statins are stopped are only starting to be explored (20-22). Adverse reactions to statins feature prominently in these reports. At the same time, in randomized, placebo controlled clinical trials statins are associated with only a slight increase in adverse reactions and no increase in discontinuation of treatment as compared to placebo, with a total incidence of adverse reactions around 5-10% (9, 23, 24). It is not known whether this difference is real or reflects misattribution of the patients’ symptoms. In particular, it is not clear whether these symptoms are reproducible when rechallenged with a different or even the same statin. Consequently it is possible that statins may be discontinued inappropriately or unnecessarily, representing a major barrier to this potentially lifesaving therapy (13).
This lack of information reflects the challenges of studying epidemiology of reported adverse reactions to statins in routine care settings. Prominent among them is the fact that these adverse reactions are frequently documented only in narrative documents (25). To address this gap in knowledge, we used a validated natural language processing software (25) in an electronic medical record system (EMR) to analyze statin discontinuation in a large cohort of patients with a particular focus on statin-related clinical events that may be interpreted as adverse reactions by patients or their clinicians.
METHODS
DESIGN
We conducted a retrospective cohort study to evaluate the reasons for discontinuation of statins in routine care. We also analyzed prevalence of statin-related events and long-term (> 12 months) statin discontinuation after a statin-related event.
STUDY COHORT
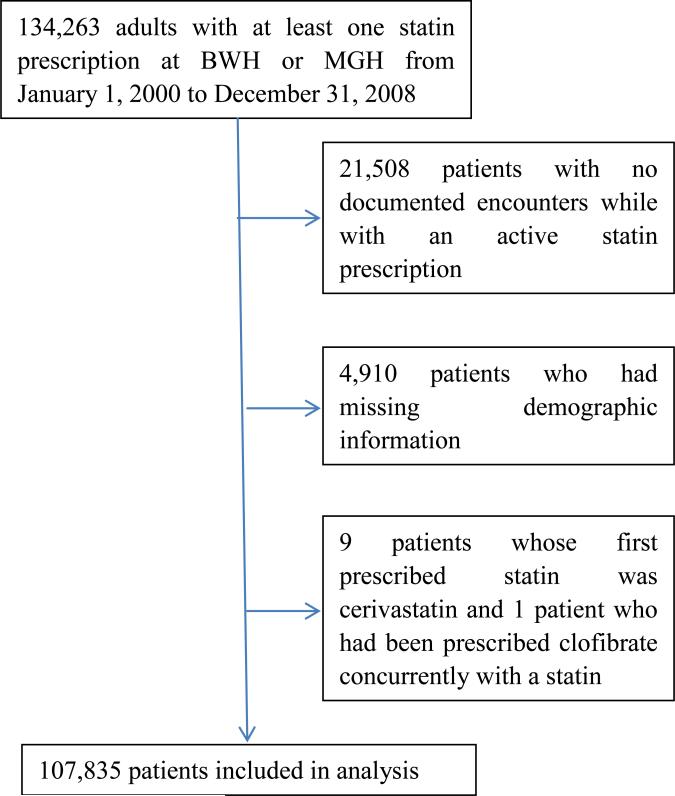
Adult patients who have had at least one statin prescription in an EMR at the Brigham and Women's Hospital (BWH) or Massachusetts General Hospital (MGH), both in Boston, MA, between January 1, 2000 and December 31, 2008 were studied. Patients without demographic information or no encounters during the study period were excluded. We also excluded patients taking cerivastatin or clofibrate because the number of these patients was too small to allow meaningful analytical conclusions.
For each patient we defined study entry as the date of the first note or the date of the first statin prescription during the study period. This study was approved by the Partners HealthCare System institutional review board, and the requirement for written informed consent was waived.
STUDY MEASUREMENTS
An individual patient served as the unit of analysis. Information on statin-related events (clinical events / symptoms documented by healthcare providers as having been caused by a statin) was obtained from a combination of structured EMR data and computational processing of narrative electronic provider notes as previously described. The software utilizes a sophisticated language model of documentation of clinical events related to medications that includes over 1,200 rules. These rules recognize clinical events that are etiologically linked in the text to a set of specific medications (e.g. “Lipitor”) or classes (e.g. “statins”). The software does not identify medication discontinuations and does not compare results based upon text processing with drug dispensing information within the EMR. While the software can recognize documentation of clinical events related to any set of medications, it was specifically validated for statins against a set of 242 randomly selected electronic notes that were manually rated by two reviewers who were not involved in the design of the software. The reviewers ratings were subsequently reconciled to create a “gold standard” rating to which the software output was then compared. The software achieved sensitivity of at least 86.5% and specificity of at least 91.9% for identification of documented statin-related events (25).
Only the first statin-related event during the study period was considered for each patient to avoid intra-patient correlations. Statin discontinuations were identified either based on an explicit discontinuation in the EMR or as absence of statin prescriptions for at least 12 months. EMR used in the study practices requires a declaration of the reason for discontinuation when a medication is explicitly stopped (e.g. “No longer necessary”, “Adverse reaction”, etc.). Long-term statin discontinuation was defined as having no active statin prescription at 12 months after the statin-related event or absence of any statin prescriptions for at least 12 months after the statin-related event. Statin rechallenge was defined as any documentation of a statin being started after statin discontinuation during the 12 months after the statin-related event. Statin-related events were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) – terminology that Food and Drug Administration (FDA) requires pharmaceutical companies to use when reporting adverse events to medications.
Patient age was calculated at study entry. The highest equivalent statin dose was defined as the maximum statin dose during the initial study phase divided by the dose of that particular statin that decreases LDL by 30-40%, (i.e. atorvastatin 10 mg, fluvastatin 80 mg, lovastatin 40 mg, pravastatin 40 mg, rosuvastatin 5 mg, simvastatin 20 mg) (4, 26, 27). Diagnoses of coronary artery disease (CAD) and diabetes mellitus (DM) were established based on the administrative data. In order to ensure specificity of the diagnosis, rhabdomyolysis was defined as a combination of documentation of rhabdomyolysis in the EMR plus creatine kinase (CK) elevation of at least 10 × ULN. Likely allergic reaction was defined as one of the following statin-related events: angioedema, drug sensitivity, erythema multiforme, eye swelling, face edema, itching, lip swelling, edema mouth, pharyngeal edema, rash, swelling, swelling face, swollen tongue, urticaria or wheezing.
Demographic information, medication and laboratory data were obtained from the EMR at Partners HealthCare, an integrated health care delivery network in eastern Massachusetts that includes BWH and MGH. There weren't any changes to these systems over the 8 years of this study. The Partners HealthCare EMR contains all medication prescription and laboratory records starting in at least 2000, and earlier for many patients.
STATISTICAL ANALYSIS
Summary statistics were conducted by using frequencies and proportions for categorical data and means (SDs), medians, and ranges for continuous variables.
All the analyses were performed using SAS, version 9.2 (SAS Institute Inc, Cary, North Carolina).
RESULTS
We identified 134,263 adults with at least one statin prescription at BWH or MGH from January 1, 2000 to December 31, 2008. Of these, 107,835 patients were included in the study (Figure 1). Atorvastatin was the most common statin taken by the study patients followed by simvastatin; 5% of patients were taking fibrates at the same time (Table 1); 38.7% of patients had CAD or diabetes.
Figure 1.
Selection of Study Patients.
Abbreviations: BWH, Brigham and Women's Hospital; MGH, Massachusetts General Hospital.
Table 1.
Patient Characteristics
| Variable | All patients studied | Patients with no statin-related events | Patients with statin-related event; no discontinuation | Patients with statin-related event; discontinuation |
|---|---|---|---|---|
| Study patients, n | 107,835 | 89,057 | 7,654 | 11,124 |
| Age, mean (SD), years | 61.1 (13.1) | 61.2 (13.3) | 61.2 (12.2) | 60.8 (12.4) |
| Women, n (%) | 53,911 (50.0) | 43,175 (48.5) | 4,270 (55.8) | 6466 (58.1) |
| Race/Ethnicity n (%) | ||||
| Caucasian | 79,336 (73.6) | 65,157 (73.2) | 5,803 (75.8) | 8,376 (75.3) |
| African-American | 5,127 (4.8) | 3,991 (4.5) | 460 (6.0) | 676 (6.1) |
| Hispanic | 5,718 (5.3) | 4,783 (5.4) | 383 (5.0) | 552 (5.0) |
| Asian | 1,825 (1.7) | 1,528(1.7) | 126 (1.7) | 171 (1.5) |
| Other1 | 15,829 (14.7) | 13,598 (15.3) | 882 (11.5) | 1,349 (12.1) |
| Health insurance, n (%) | ||||
| Government | 54,021 (50.1) | 44,358 (49.8) | 4,005 (52.3) | 5,658 (50.9) |
| Non-government | 53,814 (49.9) | 44,699 (50.2) | 3,649 (47.7) | 5,466 (49.1) |
| Median of median income by zip code, mean (SD), $1,000s | 55.0 (22.4) | 55.0 (22.5) | 55.0 (22.7) | 55.0 (22.0) |
| History of CAD, % | 20.1 | 20.4 | 19.1 | 1,992 (17.9) |
| History of DM, % | 26.4 | 27.4 | 21.1 | 2,441 (21.9) |
| Concomitant fibrate use, % | ||||
| Fenofibrate | 2,806 (2.6) | 2,294 (2.6) | 209 (2.7) | 303 (2.7) |
| Gemfibrozil | 2,630 (2.4) | 2,081 (2.3) | 211 (2.8) | 338 (3.0) |
| None | 102,399 (95.0) | 84,682 (95.1) | 7,234 (94.5) | 10,483 (94.2) |
| First statin prescribed during the study period, n(%) | ||||
| Atorvastatin | 55,895 (51.8) | 46,464 (52.2) | 3,860 (50.4) | 5,571 (50.1) |
| Fluvastatin | 1,430 (1.3) | 1,010 (1.1) | 164 (2.1) | 256 (2.3) |
| Lovastatin | 3,979 (3.7) | 3,296 (3.7) | 285 (3.7) | 398 (3.6) |
| Pravastatin | 6,813 (6.3) | 4,842 (5.4) | 862 (11.3) | 1,109 (10.0) |
| Rosuvastatin | 4,155 (3.9) | 3,016 (3.4) | 463 (6.1) | 676 (6.1) |
| Simvastatin | 35,563 (33.0) | 30,429 (34.2) | 2,020 (26.4) | 3,114 (28.0) |
| Highest relative equivalent statin dose, mean (SD) | 2.1 (1.8) | 2.1 (1.8) | 2.3 (2.0) | 2.1 (1.9) |
| Number of patients with highest relative equivalent statin dose unavailable (%) | 177 (0.16) | 162 (0.18) | 10 (0.13) | 5 (0.05) |
Includes unknown
Abbreviations: CAD, coronary artery disease; DM, diabetes mellitus.
Documented Reasons for Statin Discontinuation
More than one half of study patients (57,292 / 53.1%) had their statin discontinued at least once and 39,568 (69.1%) of these had a reason for discontinuation recorded in structured EMR data (Table 3). The default reason for discontinuation in our EMR (“No longer necessary”) was the most common reason for patients with a statin-related event and second most common for patients without one. Adverse reaction was selected as the reason for discontinuation for 2,233 patients. Other common reasons included decision support prompts about duplicate medications in the same class (these commonly trigger when one statin is being switched to another) and insurance / financial reasons.
Table 3.
Reasons for Statin Discontinuation
| Reason for discontinuation | Patients with a statin-related event Number (%) N=18,778 | Patients without a statin-related event Number (%) N=89,057 |
|---|---|---|
| Discontinued explicitly1 | 8,698 (46.3) | 30,870 (34.7) |
| No longer necessary2 | 2452 (13.1) | 6,942 (7.8) |
| Ineffective | 208 (1.1) | 878 (1.0) |
| Adverse reaction | 2,233 (11.9) | 0 (0) |
| Rejected by patient | 627 (3.3) | 1,127 (1.3) |
| Too expensive | 137 (0.7) | 2,413 (2.7) |
| Change requested by insurance | 325 (1.7) | 4,863 (5.5) |
| Erroneous entry | 59 (0.3) | 477 (5.4) |
| Inadequately covered by insurance | 79 (0.4) | 1,333 (1.5) |
| Other | 2578 (13.7) | 12,837 (14.4) |
| Therapeutic duplication warning3 | 1,407 (7.5) | 9,443 (10.6) |
| Switch to another drug | 74 (0.4) | 1,041 (1.2) |
| No prescription for one year4 | 2,426 (12.9) | 15,298 (17.2) |
| Total | 11,124 (59.2) | 46,168 (51.8) |
Patients for whom the reason for discontinuation was explicitly recorded in the EMR
Default reason for medication discontinuation in the EMR used at the study sites
Therapeutic duplication warning is a discontinuation as a result of a decision support prompt that alerts the user that the patient is already taking a medication in the same class as the one being added to the medication list. User is then prompted to discontinue either the new or the old medication
Patients who did not have a prescription for statin for at least one year were assumed to have had their statin discontinued
Statin-related events
Of all study patients, 18,778 (17.4%) had a statin-related event documented during the study period. Less than a third (30.0%) of these patients had documentation of a statin-related event in structured EMR data. Myalgia / myopathy was the most common category of statin-related events, affecting 27.0% of patients who had any statin-related event documented and 4.7% of all patients in the study. Other common statin-related events included other musculoskeletal and connective tissue disorders, general disorders and administration site conditions, hepatobiliary disorders, unspecified drug intolerance, gastrointestinal disorders, and nervous system disorders (Table 2). Only 0.006% of patients had rhabdomyolysis. Memory problems were reported for 0.06% of study patients.
Table 2.
Frequencies of statin-related events among study patients
| Statin-related event category | Patients (%) |
|---|---|
| Myalgia or myopathy | 5075 (4.7) |
| Rhabdomyolysis | 7 (0.006) |
| CK 3-10 ULN | 992 (0.9) |
| Musculoskeletal and connective tissue disorders other than myalgia or myopathy | 2742 (2.5) |
| Muscle spasms | 882 (0.8) |
| Pain in extremity | 537 (0.5) |
| Arthralgia | 356 (0.3) |
| Other | 967 (0.9) |
| General disorders and administration site conditions | 2493 (2.3) |
| Pain | 1222 (1.1) |
| Fatigue | 313 (0.3) |
| Asthenia | 208 (0.2) |
| Other | 750 (0.7) |
| Hepatobiliary disorders | 2308 (2.1) |
| Drug intolerant | 1827 (1.7) |
| Gastrointestinal disorders | 1681 (1.6) |
| Nervous system disorders | 564 (0.5) |
| Memory problems | 70 (0.06) |
| Immune system disorders | 399 (0.4) |
| Vascular disorders | 399 (0.4) |
| Psychiatric disorders | 333 (0.3) |
| Unknown | 246 (0.2) |
| Cardiac disorders | 133 (0.1) |
| Injury, poisoning and procedural complications | 112 (0.1) |
| Skin and subcutaneous tissue disorders | 107 (0.1) |
| Reproductive system and breast disorders | 99 (0.09) |
| Respiratory, thoracic and mediastinal disorders | 82 (0.08) |
| Drug ineffective | 50 (0.05) |
| Ear and labyrinth disorders | 35 (0.03) |
| Blood and lymphatic system disorders | 34 (0.03) |
| Renal and urinary disorders | 23 (0.02) |
| Eye disorders | 20 (0.02) |
| Metabolism and nutrition disorders | 16 (0.01) |
Number of patients with statin-related events among all 107,835 patients included in the study. Statin-related events were classified according to the Medical Dictionary for Regulatory Activities (MedDRA).
Abbreviations: CK, creatine kinase; ULN, upper limit of normal.
Statin Discontinuation after a Statin-related event
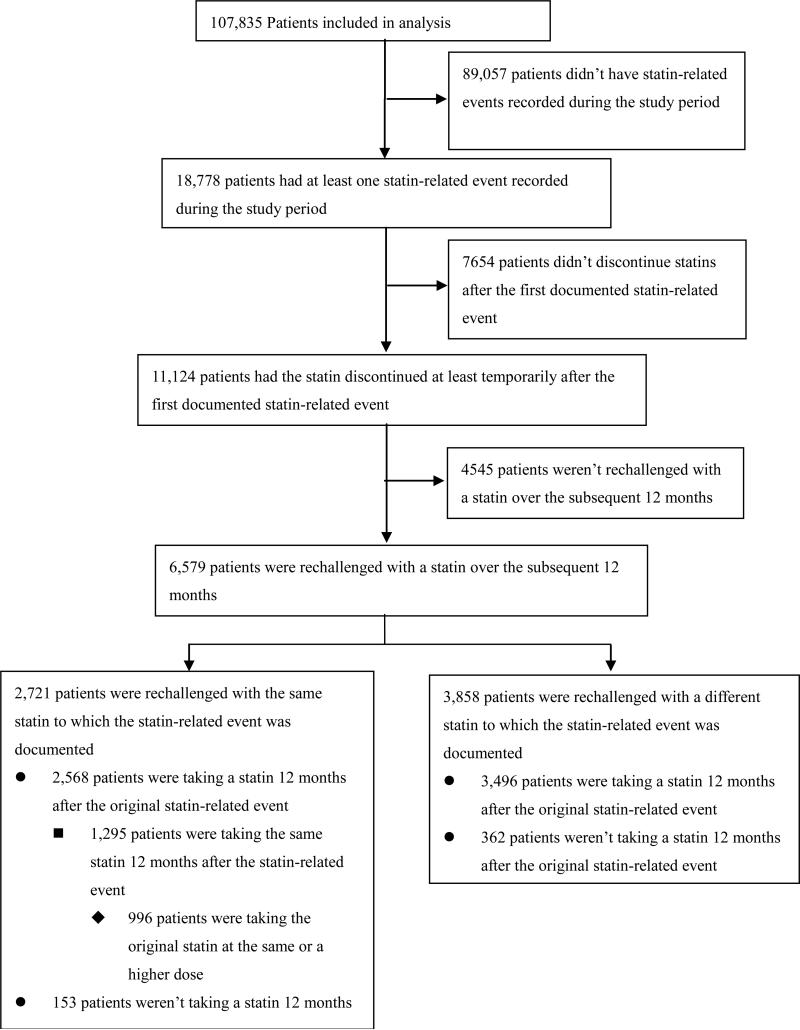
Among study patients who ever had a statin-related event documented, 11,124 patients (59.2%) had the statin discontinued at least temporarily (Figure 2). More than half (6,579/11,124) of the patients who had the statin discontinued were rechallenged with a statin over the subsequent 12 months. Over 90% (6,064/6,579) of the patients who were rechallenged were taking a statin 12 months after the original statin-related event. On average, patients were rechallenged with 1.2 unique statins over 12 months after the statin-related event. More than 40% (2,721/6,579) were rechallenged with the same statin to which the statin-related event was documented. Nearly a half (1,295/2,721) of these patients were taking the same statin 12 months after the statin-related event and more than one third (996/2,721) were taking the original statin at the same or a higher dose.
Figure 2.
Statin Discontinuation by patients with statin-related events.
Among patients with no CK elevation > 3 ULN, 4,854 patients (26.6%) discontinued all statins for at least one year after the statin-related event. In contrast, 40.3% (150) patients with CK elevation between 3 to 10 times the ULN discontinued all statins for at least one year. Over 90% (140) of these 150 patients discontinued statins long-term without rechallenge during the study period. On the other hand, of the 122 patients who had CK elevation > 3 ULN, stopped the statin at least temporarily but were subsequently rechallenged, only 10 (8.2%) discontinued statins long-term. Over one fifth (23.4%) out of 560 patients who had a possible allergic reaction discontinued the statin without rechallenge.
Among 3,858 patients who had a statin-related event, had the original statin discontinued and were then rechallenged with another statin, a second statin-related event was subsequently documented for 510 (13.2%) individuals. Only 381 (9.9%) of these patients had myalgia / myopathy severe enough to warrant discontinuation of the rechallenge statin, and none had rhabdomyolysis.
Among 8,698 study patients who had a documented statin-related event and had a statin discontinued explicitly on a specific date (the other 2,426 patients did not have any statin prescriptions for > 12 months, implying a discontinuation), 5,572 (64.0%) did not have any other medications discontinued on the same date. The mean number of non-statin medications discontinued on the same date for this patient group was 0.79. This finding is consistent with the majority of statin discontinuations being due to the statin-related events rather than, for example, general illness prompting discontinuation of multiple medications.
Statin Discontinuation in Patients without a Statin-Related Event
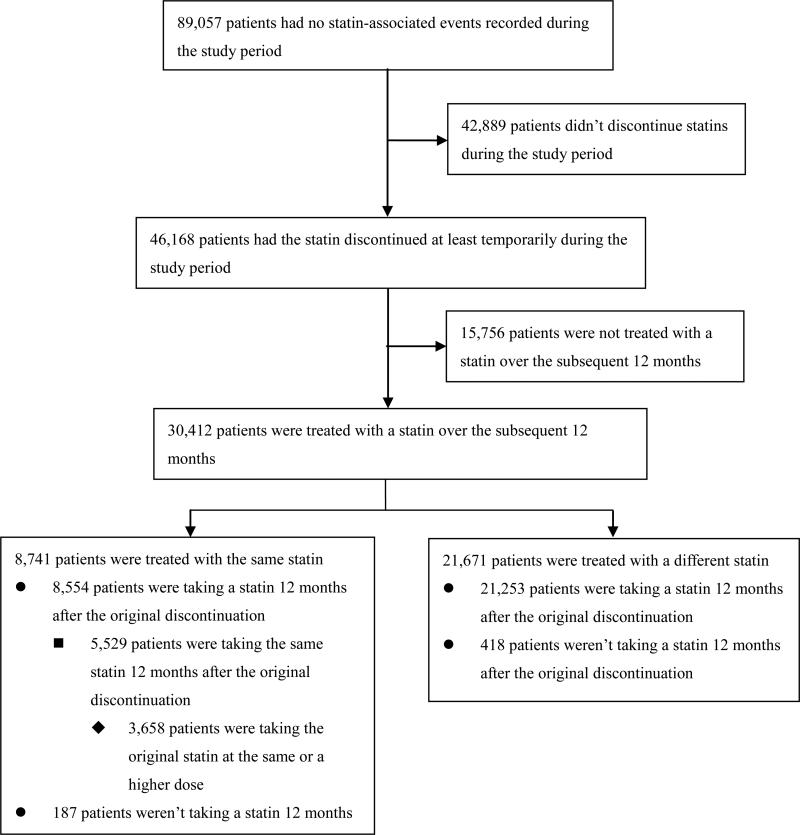
Out of 46,168 patients without a statin-related event who discontinued a statin during the study period, nearly two thirds (30,412 / 65.9%) had another statin prescription over the subsequent 12 months (Figure 3). Most of these (21,671) were for a different statin. Overall, over 98% of patients without a statin-related event who had a statin discontinued and then restarted, were still on a statin 12 months later.
Figure 3.
Statin discontinuation by patients with no statin-related events
DISCUSSION
Consistent with previously published studies, we found that statins are commonly discontinued in routine care settings – nearly one in five patients in our cohort stopped all statins for at least 12 months. Temporary discontinuations were even more common, although many of these – particularly those for which “therapeutic duplication warning” was given as the reason – represented a change to a different statin rather than complete discontinuation of statin therapy.
Reasons for discontinuations could not always be directly ascertained. While the EMR utilized by the study practices prompts providers to declare a specific reason when a medication is discontinued, anecdotally the default value of “No longer necessary” is frequently chosen irrespective of the actual reason. Additionally, for a large number of patients discontinuations were inferred from the absence of prescriptions for at least 12 months, without an explicit declaration of the reason.
Discontinuations, and particularly long-term discontinuations, were more common among patients who had documented statin-related events, corroborating the findings of patient surveys (20-22). While the causative link between the statin-related event and discontinuation could not always be directly established, many discontinuations that did not have “Adverse reaction” declared as the reason could have been due to statin-related events. These include the default reason “No longer necessary”, free-text “Other” entries, changes to a different statin and discontinuations without a stated reason, among others. Overall, as many as 87% of statin discontinuations among patients with documented statin-related events could have been due to these events.
The rate of reported statin-related events to statins was nearly 18% - substantially higher than the 5-10% rate usually described in randomized placebo-controlled clinical trials (24). This finding of a ≈20% rate of statin-related events is consistent with previously published observational studies (28-31). Similar to both clinical trials and observational studies (23, 32), musculoskeletal symptoms predominated - accounting for 40% of statin-related events. Overt rhabdomyolysis was found in only 0.006% of the study patients, also consistent with previous reports that statin-induced myopathy is a rare event (33). On the other hand, memory loss, highlighted in the recent FDA changes to the statin labels, was reported by only 0.06% of the study patients.
The discrepancy between clinical trials and observations of routine care has been attributed to a number of factors including patient selection in randomized trials, which may exclude older subjects, enroll insufficient numbers of women or suffer from a selection bias based on the kind of individuals willing to participate in trials (31). Patients who have multiple comorbidities, take other medications which may affect the metabolism of statins, have adverse effects during the run-in phase, have a history of statin-related adverse events, or are considered to be at high risk for such events are also generally excluded (31). In clinical practice, however, such patients might well be prescribed statins. Finally, some of the statin-related events reported by the patients may not be due to statins, as evidenced by the high rates of adverse reactions reported in the placebo group in clinical trials (24).
We found that the majority - over 90% - of patients who were rechallenged with a statin after a statin-related event, were ultimately able to tolerate one. Few of the rechallenged patients had another statin-related event and serious reactions, such as rhabdomyolysis, were quite rare. Patients who were rechallenged were not selected at random and were less likely to have CK elevations. However, among the small number of patients documented to have CK elevations who were rechallenged with a statin, most were able to continue statin therapy long term. Our results were therefore supportive of the hypothesis that many of the statin-related events to statins reported in observational studies may not be related to these medications etiologically while others may be either mild enough to be tolerable or not reproducible with other statins.
Discontinuation of statins, particularly in high-risk patients, is associated with increased risk of cardiovascular events and may even affect overall mortality (27, 34). Therefore guidelines generally advocate a conservative approach to stopping statin therapy. Discontinuation due to tolerable myalgias not accompanied by over 10-fold CK elevations is not recommended, and in the event the statin is discontinued, most guidelines suggest rechallenging with the same or a different statin (35, 36). Our findings support the guidelines. In keeping with previously published studies in much smaller cohorts (37-43), we found that many patients who were rechallenged after a statin-related event were able to tolerate a statin long-term, and that mild CK elevations were not predictive of long-term statin tolerance.
It is unlikely that a randomized clinical trial would or could be designed to include all patients who will be prescribed statins in routine clinical settings. This unique large study of statin-related events in routine care settings was made possible by a combination of two technologies – electronic medical records (44) and computational analysis of electronic text (a.k.a. natural language processing) (45, 46). We have previously shown that many statin-related events are documented only in narrative provider notes (25). In our study, only 30.0% had a statin-related event recorded in a structured format. To overcome this barrier, this study employed a specially designed tool capable of processing up to 40 notes / second, enabling analysis of millions of documents. In the future similar technologies could be employed both for retrospective and prospective monitoring for adverse drug reactions.
Our study had several limitations. Our analysis was retrospective in nature and therefore could establish only associations rather than causal relationships. Our study population was patients from two primarily academic hospitals in eastern Massachusetts, so the results may not be generalizable to patients in other settings. Data used in the study were collected in the course of routine care and therefore not systematic. The exact nature of statin-related events could not always be identified if documentation was incomplete. Many patients did not have laboratory data available, which may have led to underestimation of the frequency of some of the statin-related events. Accuracy of the natural language processing algorithm while high, was not perfect; if the errors of the algorithm were distributed unequally with respect to the predictor or outcome variables, it could have biased the results of our analysis. We analyzed only the first reported statin-related event to avoid intra-patient correlations, but this may not represent all the statin-related events the patients might have had. Further studies are needed to confirm these findings in a prospective fashion and extend them to other medication classes.
Our findings indicate that patients who had statin-related clinical events may frequently be able to tolerate statins in the long term. Permanent cessation of statin therapy under these circumstances could lead to many preventable cardiovascular events and deaths. Providers should consider rechallenging patients who report statin-related events to identify those who can continue taking them.
ACKNOWLEDGEMENTS
We would like to thank Dr. Robert Dluhy for this thoughtful review of the manuscript.
ROLE OF THE FUNDING SOURCE
This study was supported in part by grants from National Library of Medicine (5RC1LM010460), Diabetes Action Research and Education Foundation and Chinese National Key Program of Clinical Science. The funding sources had no role in the design, data collection, analysis, interpretation, implementation, or conduct of the study; drafting, revision, or approval of the manuscript; or the decision to submit the manuscript for publication.
REFERENCES
- 1.Ford ES, Li C, Pearson WS, Zhao G, Mokdad AH. Trends in hypercholesterolemia, treatment and control among United States adults. Int J Cardiol. 2010;140(2):226–35. doi: 10.1016/j.ijcard.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Zaninotto P, Head J, Stamatakis E, Wardle H, Mindell J. Trends in obesity among adults in England from 1993 to 2004 by age and social class and projections of prevalence to 2012. J Epidemiol Community Health. 2009;63(2):140–6. doi: 10.1136/jech.2008.077305. [DOI] [PubMed] [Google Scholar]
- 3.Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). Jama. 1986;256(20):2823–8. [PubMed] [Google Scholar]
- 4.Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, et al. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR* Trial). Am J Cardiol. 2003;92(2):152–60. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 5.Taylor F, Ward K, Moore TH, Burke M, Davey Smith G, Casas JP, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;(1):CD004816. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–9. [PubMed] [Google Scholar]
- 7.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 9.The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. The Post Coronary Artery Bypass Graft Trial Investigators. N Engl J Med. 1997;336(3):153–62. doi: 10.1056/NEJM199701163360301. [DOI] [PubMed] [Google Scholar]
- 10.Cholesterol Treatment Trialists’ Ctt C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012 doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337–44. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 12.Avorn J, Monette J, Lacour A, Bohn RL, Monane M, Mogun H, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279(18):1458–62. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 13.Ellis JJ, Erickson SR, Stevenson JG, Bernstein SJ, Stiles RA, Fendrick AM. Suboptimal statin adherence and discontinuation in primary and secondary prevention populations. J Gen Intern Med. 2004;19(6):638–45. doi: 10.1111/j.1525-1497.2004.30516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–61. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 15.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288(4):462–7. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 16.Heeschen C, Hamm CW, Laufs U, Snapinn S, Bohm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105(12):1446–52. doi: 10.1161/01.cir.0000012530.68333.c8. [DOI] [PubMed] [Google Scholar]
- 17.Wei L, Wang J, Thompson P, Wong S, Struthers AD, MacDonald TM. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow up study. Heart. 2002;88(3):229–33. doi: 10.1136/heart.88.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–86. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 19.Ho PM, Magid DJ, Shetterly SM, Olson KL, Maddox TM, Peterson PN, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772–9. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 20.McGinnis B, Olson KL, Magid D, Bayliss E, Korner EJ, Brand DW, et al. Factors related to adherence to statin therapy. Ann Pharmacother. 2007;41(11):1805–11. doi: 10.1345/aph.1K209. [DOI] [PubMed] [Google Scholar]
- 21.Garavalia L, Garavalia B, Spertus JA, Decker C. Exploring patients’ reasons for discontinuance of heart medications. J Cardiovasc Nurs. 2009;24(5):371–9. doi: 10.1097/JCN.0b013e3181ae7b2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung V, Sinclair F, Wang H, Dailey D, Hsu J, Shaber R. Patients’ perspectives on nonadherence to statin therapy: a focus-group study. Perm J. 2010;14(1):4–10. doi: 10.7812/tpp/09-090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370(9601):1781–90. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 24.Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114(25):2788–97. doi: 10.1161/CIRCULATIONAHA.106.624890. [DOI] [PubMed] [Google Scholar]
- 25.Skentzos S, Shubina M, Plutzky J, Turchin A. Structured vs. Unstructured: Factors Affecting Adverse Drug Reaction Documentation in an EMR Repository. AMIA Proceedings. 2011:1270–79. [PMC free article] [PubMed] [Google Scholar]
- 26.Brown AS, Bakker-Arkema RG, Yellen L, Henley RW, Jr., Guthrie R, Campbell CF, et al. Treating patients with documented atherosclerosis to National Cholesterol Education Program-recommended low-density-lipoprotein cholesterol goals with atorvastatin, fluvastatin, lovastatin and simvastatin. J Am Coll Cardiol. 1998;32(3):665–72. doi: 10.1016/s0735-1097(98)00300-3. [DOI] [PubMed] [Google Scholar]
- 27.Gomez Sandoval YH, Braganza MV, Daskalopoulou SS. Statin discontinuation in high-risk patients: a systematic review of the evidence. Curr Pharm Des. 2011;17(33):3669–89. doi: 10.2174/138161211798220891. [DOI] [PubMed] [Google Scholar]
- 28.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–14. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 29.Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23(8):1182–6. doi: 10.1007/s11606-008-0636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78(6):393–403. doi: 10.3949/ccjm.78a.10073. [DOI] [PubMed] [Google Scholar]
- 31.Maningat P, Breslow JL. Needed: pragmatic clinical trials for statin-intolerant patients. N Engl J Med. 2011;365(24):2250–1. doi: 10.1056/NEJMp1112023. [DOI] [PubMed] [Google Scholar]
- 32.Pasternak RC, Smith SC, Jr., Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Circulation. 2002;106(8):1024–8. doi: 10.1161/01.cir.0000032466.44170.44. [DOI] [PubMed] [Google Scholar]
- 33.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C–60C. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Tziomalos K, Athyros VG, Mikhailidis DP. Statin discontinuation: an underestimated risk? Curr Med Res Opin. 2008;24(11):3059–62. doi: 10.1185/03007990802469102. [DOI] [PubMed] [Google Scholar]
- 35.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 36.Pasternak RC, Smith SC, Jr., Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Stroke. 2002;33(9):2337–41. doi: 10.1161/01.str.0000034125.94759.41. [DOI] [PubMed] [Google Scholar]
- 37.Hansen KE, Hildebrand JP, Ferguson EE, Stein JH. Outcomes in 45 patients with statin-associated myopathy. Arch Intern Med. 2005;165(22):2671–6. doi: 10.1001/archinte.165.22.2671. [DOI] [PubMed] [Google Scholar]
- 38.Glueck CJ, Aregawi D, Agloria M, Khalil Q, Winiarska M, Munjal J, et al. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther. 2006;28(6):933–42. doi: 10.1016/j.clinthera.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Backes JM, Venero CV, Gibson CA, Ruisinger JF, Howard PA, Thompson PD, et al. Effectiveness and tolerability of every-other-day rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother. 2008;42(3):341–6. doi: 10.1345/aph.1K604. [DOI] [PubMed] [Google Scholar]
- 40.Athyros VG, Tziomalos K, Kakafika AI, Koumaras H, Karagiannis A, Mikhailidis DP. Effectiveness of ezetimibe alone or in combination with twice a week Atorvastatin (10 mg) for statin intolerant high-risk patients. Am J Cardiol. 2008;101(4):483–5. doi: 10.1016/j.amjcard.2007.09.096. [DOI] [PubMed] [Google Scholar]
- 41.Ruisinger JF, Backes JM, Gibson CA, Moriarty PM. Once-a-week rosuvastatin (2.5 to 20 mg) in patients with a previous statin intolerance. Am J Cardiol. 2009;103(3):393–4. doi: 10.1016/j.amjcard.2008.09.095. [DOI] [PubMed] [Google Scholar]
- 42.Arca M, Pigna G. Treating statin-intolerant patients. Diabetes Metab Syndr Obes. 2011;4:155–66. doi: 10.2147/DMSO.S11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaier O, Lishner M, Elis A. Managing statin-induced muscle toxicity in a lipid clinic. J Clin Pharm Ther. 2011;36(3):336–41. doi: 10.1111/j.1365-2710.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 44.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501–4. doi: 10.1056/NEJMp1006114. [DOI] [PubMed] [Google Scholar]
- 45.Hripcsak G, Friedman C, Alderson PO, DuMouchel W, Johnson SB, Clayton PD. Unlocking clinical data from narrative reports: a study of natural language processing. Ann Intern Med. 1995;122(9):681–8. doi: 10.7326/0003-4819-122-9-199505010-00007. [DOI] [PubMed] [Google Scholar]
- 46.Brown SH, Elkin PL, Rosenbloom ST, Fielstein E, Speroff T. eQuality for all: Extending automated quality measurement of free text clinical narratives. AMIA Annu Symp Proc. 2008:71–5. [PMC free article] [PubMed] [Google Scholar]