Abstract
Background
More frequent patient-provider encounters may lead to faster A1c, blood pressure and LDL control and improve outcomes but there are no guidelines for how frequently patients with diabetes should be seen.
Methods
This retrospective cohort study analyzed 26,496 patients with diabetes and elevated A1c, blood pressure, and/or LDL cholesterol treated by primary care physicians at two teaching hospitals between 1/1/2000 and 1/1/2009. Relationship between provider encounter (defined as a note in medical record) frequency and time to A1c, blood pressure and LDL control was assessed.
Results
Comparing patients who had encounters with their physicians between 1-2 weeks vs. 3-6 months, median time to A1c < 7.0% was 4.4 vs. 24.9 months (not on insulin) and 10.1 vs. 52.8 months (on insulin); median time to blood pressure < 130/85 mm Hg was 1.3 vs. 13.9 months; and median time to LDL < 100 mg/dL was 5.1 vs. 36.9 months, respectively (p < 0.0001 for all). In multivariable analysis, doubling the time between physician encounters led to a 35%, 17%, 87%, and 27% increase in median time to A1c (off and on insulin), blood pressure, and LDL targets, respectively (p < 0.0001 for all). Time to control decreased progressively as encounter frequency increased up to once every two weeks for most targets, consistent with pharmacodynamics of respective medication classes.
Conclusions
Biweekly primary care provider encounters are associated with fastest achievement of A1c, blood pressure, and LDL targets for patients with diabetes.
Diabetes is increasingly common in the U.S. and worldwide1,2. Elevated blood glucose, blood pressure (BP), and LDL cholesterol are associated with increased risk for micro- and macrovascular complications and their reduction decreases the risk3-8. Nevertheless, most patients with diabetes do not have A1c, BP, and LDL under control9,10.
A number of studies have shown that patients who see their physicians more frequently have better outcomes11-13. Current guidelines for treatment of diabetes do not include recommendations for how frequently the patients should be seen14. Recommended intervals for medication adjustments and testing range from every 2-3 days (for insulin) to every 3 months (for measuring A1c)14-16; however, benefits of more frequent provider encounters may not be limited to treatment intensification and testing.
We therefore performed a retrospective study of over 26,000 patients with diabetes and hyperglycemia, hypertension, and/or hyperlipidemia who received care in a primary care setting to test the hypothesis that higher encounter frequency is associated with better diabetes control.
MATERIALS AND METHODS
Design
We conducted a retrospective cohort study to determine the optimal frequency of provider-patient encounters for patients with diabetes. We evaluated the relationship between the mean encounter frequency and time to A1c, BP, and LDL control. We also conducted a secondary analysis to examine the relationship between encounter frequency and the rate of decrease in A1c, BP, and LDL.
Study Cohort
Patients with diabetes mellitus seen by primary care physicians affiliated with the Brigham and Women's Hospital (BWH) and Massachusetts General Hospital (MGH) for at least two years between 1/1/2000 and 1/1/2009 were studied. Patients were included in the analysis if they were at least 18 years old, had a documented diagnosis of diabetes mellitus or hemoglobin A1c ≥ 7.0%, and at least one instance of A1c, BP, or LDL above treatment target. Patients with missing zip codes were excluded to enable adjustment for median income by zip code.
To capture both face-to-face and remote interactions between patients and providers, we defined any note in the electronic medical record (EMR) as an encounter. We utilized treatment goals recommended at the beginning of the study period: A1c < 7.0%17, BP < 130/85 mm Hg17,18, and LDL < 100 mg/dL17.
This study was approved by the Partners HealthCare System institutional review board, and the requirement for written informed consent was waived.
Study Measurements
A single uncontrolled period served as the unit of analysis. We conducted four analyses: one for each of the three treatment targets (A1c, BP, and LDL) and a combined analysis that integrated all three. For analyses of individual treatment targets, an uncontrolled period started on the day when the relevant measurement (A1c, BP, or LDL for hyperglycemic, hypertensive, and hyperlipidemic periods, respectively) was noted to be above the treatment target for the first time. The period ended on the first subsequent date when the measurement fell below the target. Each patient could contribute multiple periods, if measures fluctuated above and below target levels during the nine-year study period. A combined uncontrolled period started on the first date when any of the three measures was above the treatment target and ended on the first subsequent date when all of the measures were below their targets. Last known value was carried forward if all measurements were not available on the same date.
The lowest measurement on a given date was used in the analysis. Lowest BP was defined as the BP measurement with the lowest mean arterial pressure (MAP). Transient elevations were defined as periods that contained only a single elevated measurement that subsequently normalized without any treatment intensification, and were excluded from the analysis. Uncontrolled periods without at least one annual encounter with a BWH/MGH primary care physician were excluded from the analysis to exclude patients not actively treated in these practices. Periods without any medication information available in the EMR were excluded to enable inclusion of insulin treatment as a confounder variable in the analysis. Periods that contained more than one encounter with an endocrinologist were excluded to focus the analysis on the primary care setting. Finally, hyperglycemic and hyperlipidemic periods where rate of change of A1c and LDL, respectively, was greater than three standard deviations from the mean were excluded to eliminate likely measurement errors from the analysis.
Time to normalization for A1c, BP, and LDL during the respective uncontrolled periods was the length of the uncontrolled period. Mean encounter interval was determined by dividing the period length by the number of encounters with primary care physicians during that period. In our analyses we categorized encounter intervals as ≤ 1 week, > 1 week and ≤ 2 weeks, > 2 weeks and ≤ 3 weeks, > 3 weeks and ≤ 1 month, > 1 month and ≤ 2 months, > 2 months and ≤ 3 months, and > 3 months. Treatment intensification was defined as initiation of a new or an increase in the dose of an existing medication19. Treatment intensification rate was defined as the number of unique dates per month on which at least one medication in the relevant class was intensified. Medication change was conservatively classified as intensification as previously described20 because there is no reliable method to estimate relative medication potency for individual patients. Drug cessations were not captured in this analysis. Average rate of change for A1c, systolic BP (SBP), diastolic BP (DBP), and LDL was calculated by subtracting the final value from the initial value and dividing by the period length. The patient's primary care physician was defined as the physician in a primary care practice who had the most encounters with the patient during the uncontrolled period.
Demographic information, BP measurements, and medication and laboratory data were obtained from the EMR at Partners HealthCare - an integrated healthcare delivery network in eastern Massachusetts that includes BWH and MGH. Partners HealthCare EMR contains all medication prescription and laboratory records starting in at least 2000 and earlier for many patients. Blood pressure was obtained from a combination of structured vital signs records in the EMR and computational processing of narrative electronic provider notes as previously described21. Medication intensification was abstracted from a combination of structured medication records and computational analysis of electronic provider notes as previously validated22.
Statistical Analysis
Summary statistics were conducted by using frequencies and proportions for categorical data and using means, standard deviations, medians, and ranges for continuous variables. Log-rank test was utilized to compare times to A1c, BP, and LDL normalization between different encounter intervals.
Marginal Cox proportional-hazards models for clustered data23 were constructed to estimate the association between time to normalization and encounter interval, while accounting for repeated events within individual patients, and adjusting for demographic confounders (age, sex, race, primary language, health insurance, and median income by zip code), patients’ Charlson comorbidity index (CCI), as well as treatment intensification, A1c and LDL measurement rates, and maximum A1c, SBP, DBP, and LDL (where appropriate). In order to more clearly present our findings as a direct effect of encounter interval on time to normalization, we reanalyzed our data using Weibull regression models. We confirmed the equivalence of the Weibull regression models and the marginal Cox regression models by comparing Cox-Snell residuals between these models using paired t-test, and graphically with Nelson-Aalen plots.
To rule out ascertainment bias stemming from increased A1c, BP and LDL measurement opportunities for patients with more frequent encounters, a sensitivity analysis was conducted at the patient-level to compare the probability of target achievement at the end of two years from the first elevated A1c, BP, or LDL measurement with the frequency of patient-physician encounters. The logistic regression model adjusted for demographic confounders, CCI, treatment intensification rates, maximum A1c, BP, and/or LDL measures and rates of A1c and LDL measurements, where appropriate, and adjusted for clustering within providers.
To determine the relationship between the encounter interval and the rate of A1c, BP, and LDL change, we constructed hierarchical multivariable mixed linear regression models with random intercepts to account for clustering within individual physicians and repeated measurements with compound symmetry structure within patients24. The models also included patient age, sex, race, primary language, income, health insurance, treatment intensification rate during the uncontrolled period, and insulin usage for hyperglycemic and combined periods. P-values were obtained using type III test, and were adjusted for multiple hypothesis testing using the Simes-Hochberg method25,26.
All analyses were performed with SAS statistical software, version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
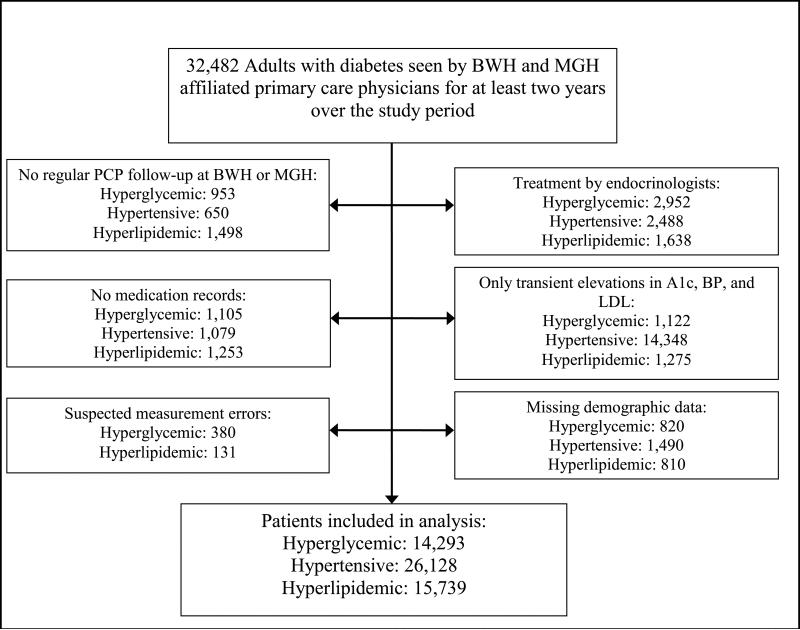
We have identified 32,482 adults with diabetes who were regularly seen by BWH or MGH primary care physicians and had experienced at least one hyperglycemic, hypertensive or hyperlipidemic period (Figure 1). We excluded 7,332 hyperglycemic, 20,055 hypertensive, and 6,605 hyperlipidemic patients who were treated by endocrinologists, had no medication records, had only transient elevations in A1c, BP, and LDL, had suspected A1c or LDL measurement errors, had missing demographic information, or were not regularly seen by a primary care physician associated with BWH and MGH over the study period. The remaining 14,293 hyperglycemic, 26,128 hypertensive, and 15,739 hyperlipidemic patients were included in the study.
Figure 1.
Counts of patients excluded from analysis
Across the study period, only median DBP was below the treatment target (75 mm Hg); median A1c was 7.4%, SBP 130 mm Hg, and LDL 106.7 mg/dL (Table 1). The average number of uncontrolled periods per patient over the course of the study ranged from 1.4 for hyperlipidemia to 3.4 for hypertension. Hyperglycemic patients had A1c above target 46% of the time, hypertensive patients had uncontrolled BP 42.7% of the time, and hyperlipidemic patients had elevated LDL cholesterol 46.3% of the time. At least one of the study measurements was not under control 88.4% of the time.
TABLE 1.
Patient Characteristics
| Variable | Hyperglycemic Period Patients | Hyperlipidemic Period Patients | Hypertensive Period Patients | Combined Measures Period Patients |
|---|---|---|---|---|
| Study patients, n | 14,293 | 15,739 | 26,128 | 26,496 |
| Age2, mean (± SD), years | 59.9 (13.8) | 58.1 (13.4) | 60.2 (13.9) | 59.3 (14.2) |
| Women, n (%) | 7,509 (52.5) | 8,817 (56.0) | 14,078 (53.9) | 14,061 (53.1) |
| Race/Ethnicity n (%) | ||||
| White | 8,568 (59.9) | 9,481 (60.2) | 17,447 (66.8) | 17,705 (66.8) |
| Black | 2,112 (14.8) | 2,287 (14.5) | 3,192 (12.2) | 3,048 (11.5) |
| Hispanic | 2,244 (15.7) | 2,484 (15.8) | 3,279 (12.5) | 3,377 (12.7) |
| Other3 | 1,369 (9.6) | 1,487 (9.4) | 2,210 (8.5) | 2,366 (8.9) |
| English as the primary language, n (%) | 11,416 (79.9) | 12,671 (80.5) | 21,784 (83.4) | 21,963 (82.9) |
| Health insurance, n (%) | ||||
| Private | 5,402 (37.8) | 6,638 (42.2) | 10,371 (39.7) | 10,735 (40.5) |
| Medicare | 7,110 (49.7) | 7,216 (45.8) | 13,088 (50.1) | 13,029 (49.2) |
| Medicaid | 1,521 (10.6) | 1,634 (10.4) | 2,297 (8.8) | 2,339 (8.8) |
| None/ Unknown | 260 (1.8) | 251 (1.6) | 372 (1.4) | 393 (1.5) |
| Median income by zip code, mean (± SD), $1,000s | 51.1 (20.6) | 51.9 (21.2) | 52.4 (20.4) | 52.7 (20.7) |
| Number of uncontrolled periods, mean (± SD), median | 1.5 (0.8) 1.0 |
1.4 (0.7) 1.0 |
3.4 (2.7) 3.0 |
2.2 (1.8) 2.0 |
| Hemoglobin A1c, mean (± SD), % | 7.7 (1.2) | 7.3 (1.3) | ||
| Systolic blood pressure, mean (± SD), mm Hg | 130.8 (10.1) | 129.7 (10.7) | ||
| Diastolic blood pressure, mean (± SD), mm Hg | 75.0 (6.8) | 74.7 (6.9) | ||
| LDL cholesterol, mean (± SD), mg/dL | 109.7 (23.5) | 100.7 (27.7) | ||
| Follow-up time, mean (± SD), months | 75.2 (24.5) | 77.6 (24.1) | 69.8 (26.8) | 68.8 (26.7) |
| Total time above treatment target, mean (± SD), months | 34.6 (28.1) | 35.9 (25.9) | 29.8 (23.2) | 60.8 (59.2) |
Only values of relevance to the individual period analysis were included in this table (i.e. A1c values for hyperglycemic patients, LDL for hyperlipidemic patients, etc.).
Age calculated at the start date of the first uncontrolled period
Includes unknown
Median time between encounters ranged from 1.1 months for hypertensive periods to 1.8 months for hyperlipidemic periods (Table 2). The mean rate of anti-hyperglycemic medication intensification was approximately once per year, anti-hypertensive medications once every four months, and anti-hyperlipidemic medications once every 17 months. Overall, patients with at least one measurement above target had their treatment intensified on average once every 2.8 months.
Table 2.
Uncontrolled Period Characteristics.
| Variable | Hyperglycemic Periods | Hyperlipidemic Periods | Hypertensive Periods | Combined Measures Periods |
|---|---|---|---|---|
| Study periods, n | 21,696 | 22,092 | 89,419 | 58,559 |
| Period length, mean (± SD), months | 22.8 (24.0) | 25.6 (23.6) | 8.7 (11.7) | 22.3 (27.0) |
| Average initial hemoglobin A1c, mean (± SD), % | 8.1 (1.5) | |||
| Average initial LDL, mean (± SD), mg/dL | 127.7 (25.9) | |||
| Average initial systolic blood pressure, mean (± SD), mm Hg | 140.1 (12.9) | |||
| Average initial diastolic blood pressure, mean (± SD), mm Hg | 78.3 (10.8) | |||
| Average maximum hemoglobin A1c, mean (± SD), % | 8.8 (1.9) | 7.8 (2.1) | ||
| Average maximum systolic blood pressure, mean (± SD), mm Hg | 148.6 (17.5) | 149.4 (19.7) | ||
| Average maximum diastolic blood pressure, mean (± SD), mm Hg | 84.1 (10.6) | 85.1 (11.0) | ||
| Average maximum LDL, mean (± SD), mg/dL | 137.7 (30.9) | 113.4 (40.7) | ||
| Periods where treatment target was reached, n (%) | 14,835 (69.4) | 16,753 (75.8) | 81,531 (91.2) | 41,155 (70.3) |
| Rate of treatment intensification per month mean, (± SD) | 0.08 (0.14) | 0.06 (0.12) | 0.23 (1.1) | 0.36 (1.68) |
| Encounter interval, mean (± SD), months | 1.9 (1.7) | 2.4 (2.0) | 1.6 (1.6) | 1.9 (1.8) |
| Periods with patients on insulin, n (%) | 5,672 (26.1) | 10,624 (18.1) |
Only values of relevance to the individual period analysis were included in the table (i.e. A1c values for hyperglycemic periods, LDL for hyperlipidemic periods, etc.). Initial measures were not reported for the combined measure periods because not all measures were always available on the start date of a period. Periods with missing data were excluded from the calculations of the average maximum measures per period.
Encounter Interval and Time to Treatment Target Achievement
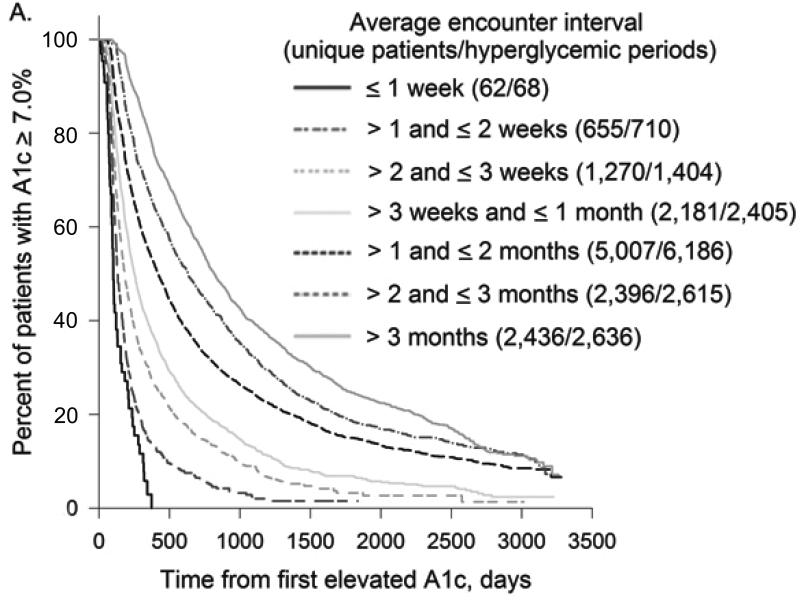
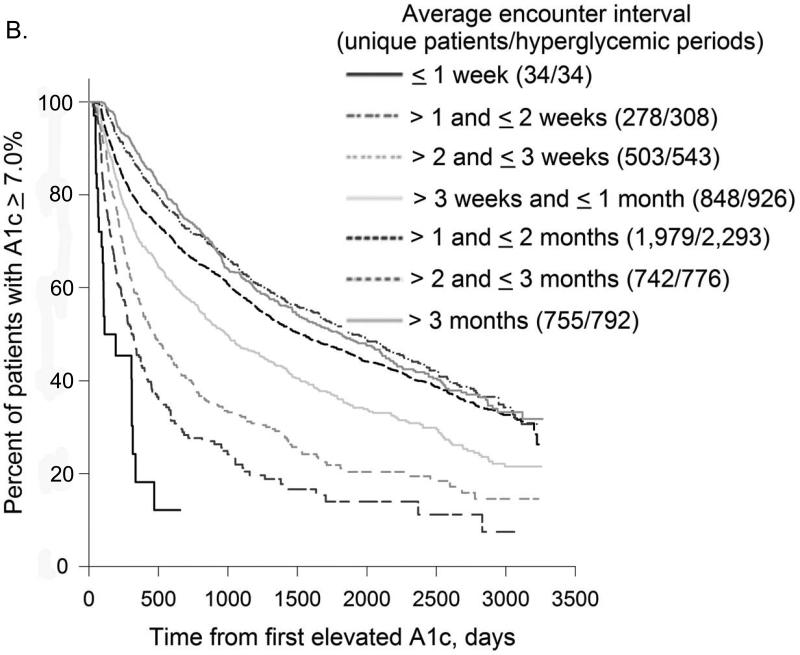
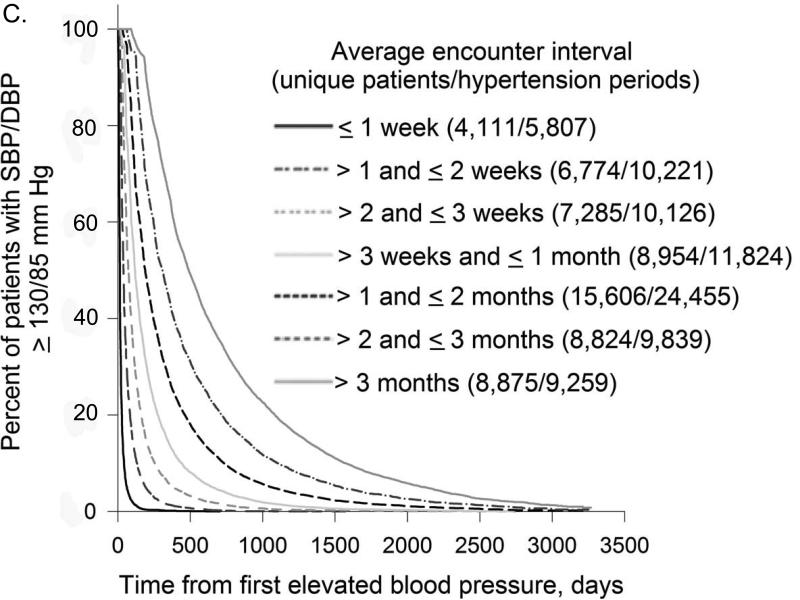
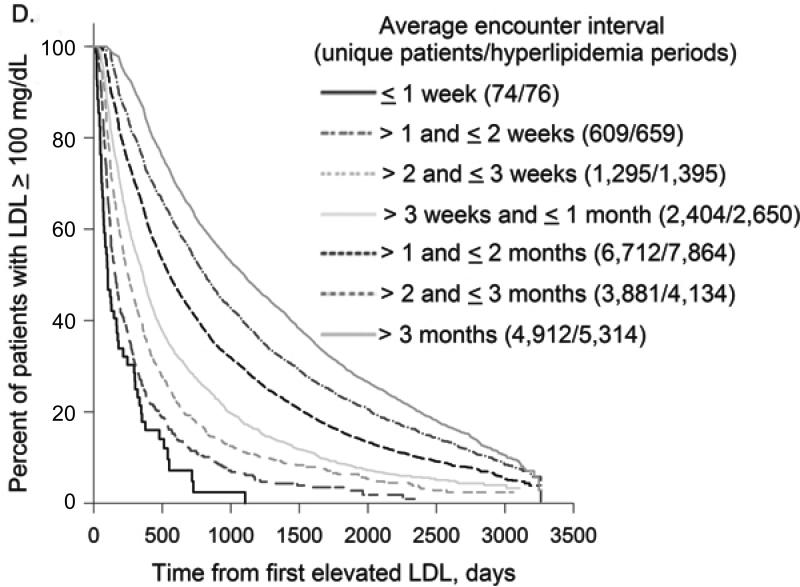
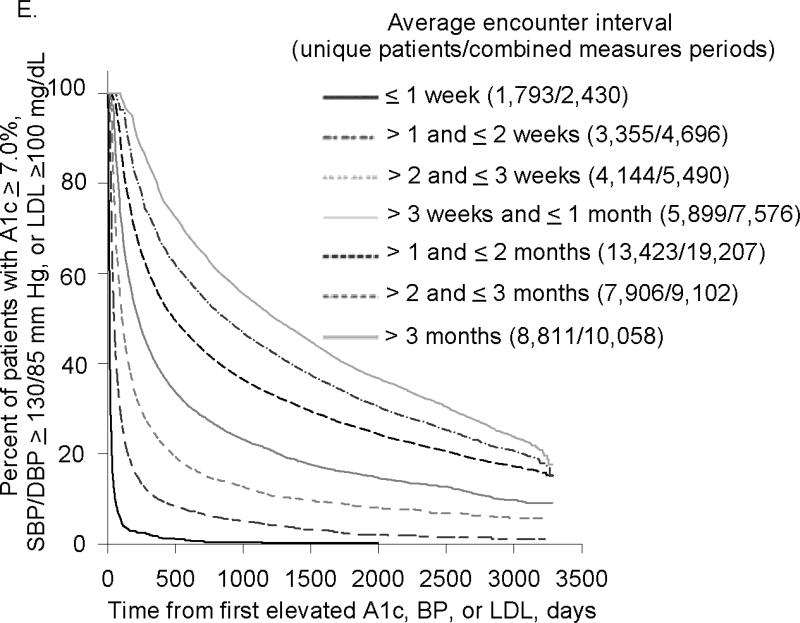
In all treatment categories, time to treatment target rose progressively as the interval between encounters increased (Figure 2). Compared to patients with mean encounter interval between 1-2 weeks, median time to A1c target for patients whose mean encounter interval was 3-6 months was 4.4 vs. 24.9 months (not on insulin) and 10.1 vs. 52.8 months (on insulin); time to BP target was 1.3 vs. 13.9 months and LDL target 5.1 vs. 32.8 months, respectively. For all treatment targets combined, median time to target was 1.5vs. 36.9 months with mean encounter interval between 1-2 weeks vs. 3-6 months.
Figure 2. Encounter Frequency and Time to Treatment Target.
Kaplan-Meier curves for time to treatment target from first elevated A1c, BP, or LDL were plotted for different average encounter intervals. Distinct uncontrolled periods (from the first elevated to the first normal measurement) for the same patient were analyzed separately.
2A. Encounter Frequency and Time to A1c Target for Patients off Insulin
2B. Encounter Frequency and Time to A1c Target for Patients on Insulin
2C. Encounter Frequency and Time to BP Target
2D. Encounter Frequency and Time to LDL Target
2E. Encounter Frequency and Time to Combined Target
As encounter intervals increased, the proportion of patients who never reached treatment targets also rose steadily. Comparing patients with mean encounter interval between 1-2 weeks to > 6 months, the fraction of uncontrolled periods that never reached treatment target was 35.4% vs. 55.6% for hyperglycemic patients on insulin and 5.4% vs. 15.9% for hypertensive patients. For hyperglycemic patients not on insulin and hyperlipidemic patients, the lowest proportion of uncontrolled periods that did not achieve treatment target was for encounter intervals between 1 and 2 weeks: 14.8% and 16.8%, compared to 36.8% and 31.9% for encounter intervals > 6 months. For all treatment targets combined, the proportion of uncontrolled periods that never achieved all targets was 11.0% for encounter intervals ≤ 1 week vs. 43.4% for encounter intervals > 6 months.
In the multivariable Weibull model adjusted for demographic characteristics, CCI, insulin administration (in hyperglycemic and combined uncontrolled periods), maximum A1c, SBP, DBP, and LDL (where relevant), A1c and LDL testing rates (where relevant), and treatment intensification, doubling the time between physician encounters resulted in a 35% (not on insulin) and 17% (on insulin) increase in median time to A1c normalization, an 87% increase in time to BP normalization, and a 27% increase in median time to LDL normalization (p < 0.0001 for all). Higher rates of treatment intensification, lower A1c, BP and LDL levels, and not being treated with insulin (for hyperglycemic patients) were also associated with shorter periods (p < 0.0001 for all). In a Weibull model of combined uncontrolled periods, doubling the time between physician encounters led to an 84% increase in the time to achievement of all treatment targets (p < 0.0001). When treatment intensification was not included in the model, doubling the time between physician encounters translated into 38%, 20%, 90%, 32%, and 88% increases in time to A1c when not and on insulin, BP, LDL, and combined control, respectively (p < 0.0001 for all). In a post-hoc multivariable sensitivity analysis including periods for patients treated by endocrinologists, encounter frequency had similar effects on time to A1c, BP, and LDL normalization (results not shown).
Multivariable sequential comparison of time to treatment target for encounter interval categories adjusted for patients’ demographics, highest A1c, BP and LDL (where relevant) during the uncontrolled period, rate of treatment intensification, and insulin treatment (for hyperglycemic patients) showed that differences between most consequent encounter interval categories were highly significant (p < 0.0001). Exceptions included encounter intervals ≤ 1 week vs. 1-2 weeks for hyperglycemic patients not treated with insulin (p = 0.0057) and hyperlipidemic patients (p = 0.90), and encounter intervals 1-2 vs. 2-3 weeks (p = 0.13) and 2-3 weeks vs. > 3 months (p = 0.68) for hyperglycemic patients on insulin.
Encounter Interval and Rates of Outcome Measure Change
In multivariable analysis adjusted for demographic characteristics, CCI, insulin treatment (in hyperglycemic patients), highest A1c, BP and LDL (where relevant) during the uncontrolled period, rate of treatment intensification and A1c and LDL measurement (where relevant), and clustering within individual physicians and repeated measurements within patients, for every additional month between encounters, rate of A1c decrease declined an additional 0.014% per month, rate of SBP decreased by 2.5 mm Hg per month, rate of DBP decreased by 1.0 mm Hg per month, and rate of LDL decreased by 0.28 mm/dL per month (p < 0.0001 for all). More frequent treatment intensification led to faster rates of decrease for all diabetes measures (p < 0.0001 for all).
COMMENT
In this large retrospective study we found a strong association between encounter frequency and A1c, BP, and LDL control in patients with diabetes. This relationship was confirmed in individual and combined analyses of time to normalization, rate of measure decrease, and rate of target achievement. A strong dose-response relationship between encounter frequency and the outcomes was evident in all associations we analyzed.
Current guidelines provide little guidance for how frequently patients with diabetes should be seen by their physicians, apart from the recommendation for A1c measurement every three months14. Our findings provide evidence that for many patients with elevated A1c, BP or LDL, more frequent patient-physician encounters were associated with a shorter time to treatment target, and control was fastest at two-week intervals. Bi-weekly encounters may therefore be appropriate for the most severely uncontrolled patients or under a different treatment care model.
More frequent opportunities for medication intensification are likely an important mediator of the encounter frequency effect. This explanation is corroborated by a decrease in the encounter frequency effect when treatment intensification rate is included in the model. Many textbooks recommend a lower limit of 4-6 weeks on the medication intensification frequency out of concern for a stacking effect and overdose27,28. However, time to maximum effect for most medications is shorter than commonly believed. Majority of antihyperglycemics achieve most of their effect within 2 weeks29-32 and others in under 4 weeks33-36; antihypertensives (except thiazides), in under 2 weeks37-42; and statins, within 2 weeks43. These results are consistent with our findings that bi-weekly encounters are associated with fastest achievement of glucose, BP, and LDL control.
Although median time between patient-physician encounters was only 1.4 months for hyperglycemic patients, treatment intensification occurred just once per year. Target A1c is commonly reached much more slowly than recommended by guidelines; the incongruity between encounter frequency and rates of treatment intensification suggests there are many opportunities for physicians to alter medications that may lead to faster A1c control during encounters.
Treatment intensification may not be the sole factor responsible for the association between encounter frequency and patient outcomes, as illustrated by the strong residual association between encounter frequency and time to normalization when controlling for treatment intensification. Other studies have shown that more frequent encounters are also associated with better medication adherence44,45. During encounters, physicians may also be providing lifestyle coaching or other education that lead to better diabetes control.
There is evidence that faster control of intermediate end points (A1c, BP, LDL) that could be achieved by more frequent provider encounters translates into improvement in clinical outcomes. Early intensive insulin therapy in patients with newly diagnosed diabetes leads to more durable control and improvement in beta-cell function46. The VALUE trial found that lower BP in the first three months decreased rates of stroke and myocardial infarction47. Several studies have shown that statins lowered rates of cardiovascular events in high-risk patients within 3-6 months of initiation48-52.
As more frequent encounters could increase demand on healthcare resources, straining an already taxed53 and dwindling primary care environment54,55, increased encounter implementation may require innovative approaches to patient care delivery. Medical homes may help coordinate care of patients, while some interactions could be accomplished through group visits, phone, fax, email, or internet communications56. Studies have shown that mid-level providers can alleviate physician workload without any negative impact on patient outcomes56-60.
Once a patient achieves diabetes control, the frequency of the encounters may be decreased to alleviate the strain on healthcare resources and possibly to also reward the patient61. Studies have shown that among patients with controlled hypertension, provider-patient encounters can be 6 months apart without adverse effects62.
Our study has a number of strengths. With access to records from two large hospital systems, we were able to analyze over 26,000 patients with uncontrolled diabetes from diverse backgrounds and health insurance coverage plans. We have focused on the primary care setting, where most patients with diabetes are treated. Importantly, our results were consistent with pharmacodynamic data, providing a physiologic basis for our findings.
Our study also had several limitations. It was conducted in clinics affiliated with two academic medical centers in Eastern Massachusetts, and thus may not be generalizable to all settings. These clinics do not include many mid-level providers, primarily limiting these conclusions to primary care physicians. Uncontrolled periods were censored at the beginning of the study; however, unless encounter frequency was systematically uneven over the duration of the study, this should not have biased our results. We were unable to distinguish between routine scheduled encounters and last-minute appointments with physicians; the focus of care (routine vs. urgent) probably differs between these two visit types. A1c, BP, and LDL were only measured during the course of routine care, possibly leading to an ascertainment bias as patients with shorter encounter intervals had more frequent opportunities to have measurements below target. However, a separate analysis showed that higher encounter frequency was linked to higher probability of A1c, BP, and LDL target achievement at 2 years after the first abnormal level was measured (data not shown). This finding supports our interpretation in a manner not subject to bias by the missing measurement data. The retrospective nature of this data does not allow us to assess the availability or motivation of patients to see their physicians, which may be another indicator of adherence. We were also unable to consider how individual patient and provider goals may have differed from published guidelines or which physicians may practice in clinics that institute diabetes management protocols; however, we did correct for clustering within providers and repeated measures within patients, which helps to mitigate this confounder. There were several potential confounders we could not measure, including the type of diabetes, face-to-face vs. remote encounters, focus of treatment at an encounter, patient motivation and medication adherence. We were also unable to measure potential costs and risks associated with higher encounter frequency, making a full risk-benefit analysis impossible. Although some clinical trials found evidence that faster attainment of intermediate measures can result in improved clinical outcomes, this study is limited to intermediate outcomes and we do not have evidence that the association between higher encounter frequency and faster A1c, BP, and LDL control reported here leads to improved clinical outcomes in this study population. The retrospective nature of our study prevents us from establishing a causal relationship between encounter frequency and patient outcomes. A randomized interventional study is therefore needed to definitively establish optimal encounter frequency for patients with diabetes.
Table 3.
Effects of Patient and Treatment Characteristics on Time to Treatment Target.
| Hyperglycemic Periods | ||||
|---|---|---|---|---|
| Variable | Estimate | 95% Confidence Limits | P-value (chi-square) | |
| Normalized Maximum A1c | 0.4881 | 0.4768 | 0.4994 | < 0.0001 |
| Normalized Age, years | 0.0039 | 0.0027 | 0.0051 | < 0.0001 |
| Female | 0.1255 | 0.1000 | 0.1509 | < 0.0001 |
| Not-English language | 0.0116 | -0.0239 | 0.0470 | 0.5223 |
| Minority race | -0.0498 | -0.0800 | -0.0196 | 0.0012 |
| Income by $1,000 | -0.0004 | -0.0011 | 0.0003 | 0.2377 |
| Non-private insurance | 0.0427 | 0.0127 | 0.0727 | 0.0053 |
| On Insulin | 0.3855 | 0.3527 | 0.4182 | < 0.0001 |
| PCP encounter interval, log(months) | 0.4050 | 0.3852 | 0.4248 | < 0.0001 |
| Charlson comorbidity index | -0.0038 | -0.0071 | -0.0005 | 0.0248 |
| Rate of A1c testing, per month | -0.6588 | -0.6805 | -0.6371 | < 0.0001 |
| Rate of antihyperglycemic medication intensification, per month | -0.9349 | -1.0278 | -0.8420 | < 0.0001 |
| Hypertensive Periods | ||||
|---|---|---|---|---|
| Variable | Estimate | 95% Confidence Limits | P-value (chi-square) | |
| Normalized Maximum SBP | 0.0236 | 0.0232 | 0.0239 | <0.0001 |
| Normalized Maximum DBP | 0.0186 | 0.0180 | 0.0193 | <0.0001 |
| Normalized Age, years | 0.0034 | 0.0029 | 0.0039 | <0.0001 |
| Female | 0.0554 | 0.0453 | 0.0655 | <0.0001 |
| Not-English language | -0.0325 | -0.0469 | -0.0180 | <0.0001 |
| Minority race | -0.1127 | -0.1248 | -0.1006 | <0.0001 |
| Income by $1,000 | 0.0003 | 0.0001 | 0.0006 | 0.0159 |
| Non-private insurance | 0.0146 | 0.0027 | 0.0265 | 0.0161 |
| PCP encounter interval, log(months) | 0.9047 | 0.8985 | 0.9110 | <0.0001 |
| Charlson comorbidity index | -0.0098 | -0.0111 | -0.0086 | <0.0001 |
| Rate of antihypertensive medication intensification, per month | -0.0972 | -0.0999 | -0.0945 | <0.0001 |
| Hyperlipidemic Periods | ||||
|---|---|---|---|---|
| Variable | Estimate | 95% Confidence Limits | P-value (chi-square) | |
| Normalized Maximum LDL | 0.0173 | 0.0168 | 0.0178 | <0.0001 |
| Normalized Age, years | -0.0083 | -0.0094 | -0.0073 | <0.0001 |
| Female | 0.1517 | 0.1291 | 0.1744 | <0.0001 |
| Not-English language | -0.0359 | -0.0673 | -0.0044 | 0.0255 |
| Minority race | -0.0611 | -0.0879 | -0.0344 | <0.0001 |
| Income by $1,000 | -0.0003 | -0.0008 | 0.0003 | 0.3882 |
| Non-private insurance | 0.0199 | -0.0065 | 0.0463 | 0.1402 |
| PCP encounter interval, log(months) | 0.3489 | 0.3320 | 0.3657 | <0.0001 |
| Charlson comorbidity index | -0.0019 | -0.0049 | 0.0011 | 0.2081 |
| Rate of LDL testing, per month | -0.9678 | -0.9886 | -0.9470 | <0.0001 |
| Rate of antihyperlipidemic medication intensification, per month | -1.4805 | -1.5319 | -1.4291 | <0.0001 |
| Combined Uncontrolled Periods | ||||
|---|---|---|---|---|
| Variable | Estimate | 95% Confidence Limits | P-value (chi-square) | |
| Normalized Maximum A1c | 0.3571 | 0.3494 | 0.3649 | <0.0001 |
| Normalized Maximum SBP | 0.0184 | 0.0178 | 0.0190 | <0.0001 |
| Normalized Maximum DBP | 0.0203 | 0.0192 | 0.0215 | <0.0001 |
| Normalized Maximum LDL | 0.0136 | 0.0133 | 0.0139 | <0.0001 |
| Normalized Age, years | -0.0017 | -0.0025 | -0.0008 | 0.0001 |
| Female | 0.0973 | 0.0797 | 0.1149 | <0.0001 |
| Not-English language | 0.0219 | -0.0047 | 0.0485 | 0.1068 |
| Minority race | -0.0833 | -0.1054 | -0.0613 | <0.0001 |
| Income by $1,000 | 0.0014 | 0.0009 | 0.0018 | <0.0001 |
| Non-private insurance | 0.0055 | -0.0155 | 0.0265 | 0.6084 |
| On Insulin | 0.1200 | 0.0949 | 0.1450 | <0.0001 |
| PCP encounter interval, log(months) | 0.8843 | 0.8727 | 0.8960 | <0.0001 |
| Charlson comorbidity index | 0.0000 | -0.0022 | 0.0022 | 0.9920 |
| Rate of A1c testing, per month | 1.4278 | 1.2365 | 1.6191 | <0.0001 |
| Rate of LDL testing, per month | -0.0235 | -0.0606 | 0.0136 | 0.2150 |
| Rate of medication intensification, per month | -0.0517 | -0.0540 | -0.0493 | <0.0001 |
ACKNOWLEDGMENTS
This study was supported in part by grants from Agency for Healthcare Research and Quality (5R18HS017030), National Library of Medicine (5RC1LM010460) and Diabetes Action Research and Education Foundation.
Footnotes
The authors have no significant primary financial arrangements with commercial companies that produce or sell products that are the subject of studies reported in the manuscript, or with competitors of such companies.
No potential conflicts of interest relevant to the article were reported.
REFERENCES
- 1.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010 Mar;33(3):562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004 May;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Sep 30;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005 Dec 22;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 6.Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation. 1998 Dec 8;98(23):2513–2519. doi: 10.1161/01.cir.98.23.2513. [DOI] [PubMed] [Google Scholar]
- 7.Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care. 1997 Apr;20(4):614–620. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- 8.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj. 1998 Sep 12;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 9.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999-2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006 Mar;29(3):531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 10.Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999-2004. Ann Epidemiol. 2008 Mar;18(3):222–229. doi: 10.1016/j.annepidem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Hong CS, Atlas SJ, Chang Y, et al. Relationship between patient panel characteristics and primary care physician clinical performance rankings. Jama. 2010 Sep 8;304(10):1107–1113. doi: 10.1001/jama.2010.1287. [DOI] [PubMed] [Google Scholar]
- 12.Turchin A, Goldberg SI, Shubina M, Einbinder JS, Conlin PR. Encounter frequency and blood pressure in hypertensive patients with diabetes mellitus. Hypertension. 2010 Jul;56(1):68–74. doi: 10.1161/HYPERTENSIONAHA.109.148791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guthmann R, Davis N, Brown M, Elizondo J. Visit frequency and hypertension. J Clin Hypertens (Greenwich) 2005 Jun;7(6):327–332. doi: 10.1111/j.1524-6175.2005.04371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ADA Standards of medical care in diabetes--2010. Diabetes Care. 2010 Jan;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009 Jan;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009 Sep-Oct;15(6):540–559. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2000 Jan;23(Suppl 1):S32–42. [PubMed] [Google Scholar]
- 18.Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T. Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research. Jama. 1997 Dec 3;278(21):1759–1766. [PubMed] [Google Scholar]
- 19.Turchin A, Shubina M, Chodos AH, Einbinder JS, Pendergrass ML. Effect of board certification on antihypertensive treatment intensification in patients with diabetes mellitus. Circulation. 2008 Feb 5;117(5):623–628. doi: 10.1161/CIRCULATIONAHA.107.733949. [DOI] [PubMed] [Google Scholar]
- 20.Berlowitz DR, Ash AS, Hickey EC, et al. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998 Dec 31;339(27):1957–1963. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 21.Turchin A, Kolatkar NS, Grant RW, Makhni EC, Pendergrass ML, Einbinder JS. Using regular expressions to abstract blood pressure and treatment intensification information from the text of physician notes. J Am Med Inform Assoc. 2006 Nov-Dec;13(6):691–695. doi: 10.1197/jamia.M2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turchin A, Shubina M, Breydo E, Pendergrass ML, Einbinder JS. Comparison of information content of structured and narrative text data sources on the example of medication intensification. J Am Med Inform Assoc. 2009 May-Jun;16(3):362–370. doi: 10.1197/jamia.M2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994 Nov 15;13(21):2233–2247. doi: 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]
- 24.Greenland S. Principles of multilevel modelling. Int J Epidemiol. 2000 Feb;29(1):158–167. doi: 10.1093/ije/29.1.158. [DOI] [PubMed] [Google Scholar]
- 25.Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73(3):751–754. [Google Scholar]
- 26.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. [Google Scholar]
- 27.Goodman LS, Brunton LL, Chabner B, Knollmann BC. Goodman & Gilman's pharmacological basis of therapeutics. 12th ed. McGraw-Hill; New York: 2011. [Google Scholar]
- 28.Current medical diagnosis & treatment. McGraw-Hill Companies; New York etc.: p. v. [Google Scholar]
- 29.Simonson DC, Kourides IA, Feinglos M, Shamoon H, Fischette CT. Efficacy, safety, and dose-response characteristics of glipizide gastrointestinal therapeutic system on glycemic control and insulin secretion in NIDDM. Results of two multicenter, randomized, placebo-controlled clinical trials. The Glipizide Gastrointestinal Therapeutic System Study Group. Diabetes Care. 1997 Apr;20(4):597–606. doi: 10.2337/diacare.20.4.597. [DOI] [PubMed] [Google Scholar]
- 30.Rosenstock J, Hassman DR, Madder RD, et al. Repaglinide versus nateglinide monotherapy: a randomized, multicenter study. Diabetes Care. 2004 Jun;27(6):1265–1270. doi: 10.2337/diacare.27.6.1265. [DOI] [PubMed] [Google Scholar]
- 31.Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med. 2009 Mar;26(3):268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009 Oct;52(10):2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006 Dec;29(12):2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 34.Chacra AR, Tan GH, Apanovitch A, Ravichandran S, List J, Chen R. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009 Sep;63(9):1395–1406. doi: 10.1111/j.1742-1241.2009.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kipnes MS, Krosnick A, Rendell MS, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med. 2001 Jul;111(1):10–17. doi: 10.1016/s0002-9343(01)00713-6. [DOI] [PubMed] [Google Scholar]
- 36.Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA. 2000 Apr 5;283(13):1695–1702. doi: 10.1001/jama.283.13.1695. [DOI] [PubMed] [Google Scholar]
- 37.Donnelly R, Elliott HL, Meredith PA, Kelman AW, Reid JL. Nifedipine: individual responses and concentration-effect relationships. Hypertension. 1988 Oct;12(4):443–449. doi: 10.1161/01.hyp.12.4.443. [DOI] [PubMed] [Google Scholar]
- 38.Donnelly R, Elliott HL, Meredith PA, Reid JL. Concentration-effect relationships and individual responses to doxazosin in essential hypertension. Br J Clin Pharmacol. 1989 Nov;28(5):517–526. doi: 10.1111/j.1365-2125.1989.tb03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donnelly R, Meredith PA, Elliott HL, Reid JL. Kinetic-dynamic relations and individual responses to enalapril. Hypertension. 1990 Mar;15(3):301–309. doi: 10.1161/01.hyp.15.3.301. [DOI] [PubMed] [Google Scholar]
- 40.Michelson EL, Frishman WH, Lewis JE, et al. Multicenter clinical evaluation of long-term efficacy and safety of labetalol in treatment of hypertension. Am J Med. 1983 Oct 17;75(4A):68–80. doi: 10.1016/0002-9343(83)90138-9. [DOI] [PubMed] [Google Scholar]
- 41.Pool JL, Guthrie RM, Littlejohn TW, 3rd, et al. Dose-related antihypertensive effects of irbesartan in patients with mild-to-moderate hypertension. Am J Hypertens. 1998 Apr;11(4 Pt 1):462–470. doi: 10.1016/s0895-7061(97)00501-3. [DOI] [PubMed] [Google Scholar]
- 42.van Brummelen P, Man in 't Veld AJ, Schalekamp MA. Hemodynamic changes during long-term thiazide treatment of essential hypertension in responders and nonresponders. Clin Pharmacol Ther. 1980 Mar;27(3):328–336. doi: 10.1038/clpt.1980.44. [DOI] [PubMed] [Google Scholar]
- 43.Bakker-Arkema RG, Davidson MH, Goldstein RJ, et al. Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. JAMA. 1996 Jan 10;275(2):128–133. [PubMed] [Google Scholar]
- 44.Patel NC, Crismon ML, Miller AL, Johnsrud MT. Drug adherence: effects of decreased visit frequency on adherence to clozapine therapy. Pharmacotherapy. 2005 Sep;25(9):1242–1247. doi: 10.1592/phco.2005.25.9.1242. [DOI] [PubMed] [Google Scholar]
- 45.Wannamaker BB, Morton WA, Jr., Gross AJ, Saunders S. Improvement in antiepileptic drug levels following reduction of intervals between clinic visits. Epilepsia. 1980 Apr;21(2):155–162. doi: 10.1111/j.1528-1157.1980.tb04057.x. [DOI] [PubMed] [Google Scholar]
- 46.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008 May 24;371(9626):1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 47.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004 Jun 19;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 48.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004 Apr 8;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 49.Colhoun HM, Betteridge DJ, Durrington PN, et al. Rapid emergence of effect of atorvastatin on cardiovascular outcomes in the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetologia. 2005 Dec;48(12):2482–2485. doi: 10.1007/s00125-005-0029-y. [DOI] [PubMed] [Google Scholar]
- 50.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004 Aug 21-27;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 51.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998 May 27;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 52.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995 Nov 16;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 53.Ostbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005 May-Jun;3(3):209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bodenheimer T. Primary care--will it survive? N Engl J Med. 2006 Aug 31;355(9):861–864. doi: 10.1056/NEJMp068155. [DOI] [PubMed] [Google Scholar]
- 55.Hauer KE, Durning SJ, Kernan WN, et al. Factors associated with medical students’ career choices regarding internal medicine. Jama. 2008 Sep 10;300(10):1154–1164. doi: 10.1001/jama.300.10.1154. [DOI] [PubMed] [Google Scholar]
- 56.Lee TH, Bodenheimer T, Goroll AH, Starfield B, Treadway K. Perspective roundtable: redesigning primary care. N Engl J Med. 2008 Nov 13;359(20):e24. doi: 10.1056/NEJMp0809050. [DOI] [PubMed] [Google Scholar]
- 57.Denver EA, Barnard M, Woolfson RG, Earle KA. Management of uncontrolled hypertension in a nurse-led clinic compared with conventional care for patients with type 2 diabetes. Diabetes Care. 2003;26(8):2256–2260. doi: 10.2337/diacare.26.8.2256. [DOI] [PubMed] [Google Scholar]
- 58.New JP, Mason JM, Freemantle N, et al. Specialist nurse-led intervention to treat and control hypertension and hyperlipidemia in diabetes (SPLINT): a randomized controlled trial. Diabetes Care. 2003 Aug;26(8):2250–2255. doi: 10.2337/diacare.26.8.2250. [DOI] [PubMed] [Google Scholar]
- 59.Taylor CB, Miller NH, Reilly KR, et al. Evaluation of a nurse-care management system to improve outcomes in patients with complicated diabetes. Diabetes Care. 2003;26(4):1058–1063. doi: 10.2337/diacare.26.4.1058. [DOI] [PubMed] [Google Scholar]
- 60.Vivian EM. Improving blood pressure control in a pharmacist-managed hypertension clinic. Pharmacotherapy. 2002 Dec;22(12):1533–1540. doi: 10.1592/phco.22.17.1533.34127. [DOI] [PubMed] [Google Scholar]
- 61.Wick A, Koller MT. Views of patients and physicians on follow-up visits: results from a cross-sectional study in Swiss primary care. Swiss Med Wkly. 2005 Mar 5;135(9-10):139–144. doi: 10.4414/smw.2005.10871. [DOI] [PubMed] [Google Scholar]
- 62.Birtwhistle RV, Godwin MS, Delva MD, et al. Randomised equivalence trial comparing three month and six month follow up of patients with hypertension by family practitioners. Bmj. 2004 Jan 24;328(7433):204. doi: 10.1136/bmj.37967.374063.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]