Protein Z (PZ), a vitamin K-dependent plasma protein, dramatically enhances inhibition of coagulation factor Xa by protein Z-dependent protease inhibitor (ZPI), serpinA10 [1]. ZPI also directly inhibits factor XIa [2,3]. That PZ and ZPI knockout mice show enhanced responses in models of induced thrombosis supports a physiological relevant role for the PZ/ZPI system in the regulation of coagulation [4,5].
The broad range of plasma PZ levels has led to the suggestion that the inflammatory response might effect PZ expression [6–8]. Potentially consistent with this proposition, several studies in which plasma samples were obtained near the time of stroke reported high levels of PZ [9–12], whereas others using plasma samples obtained during convalescence found the opposite [13–15]. Two studies investigating the association between inflammation and PZ levels, however, have produced conflicting results [16,17]. Here, murine models of the acute phase response and the antiphospholipid syndrome (APS) are used to better define the relationship between PZ and ZPI levels and inflammation.
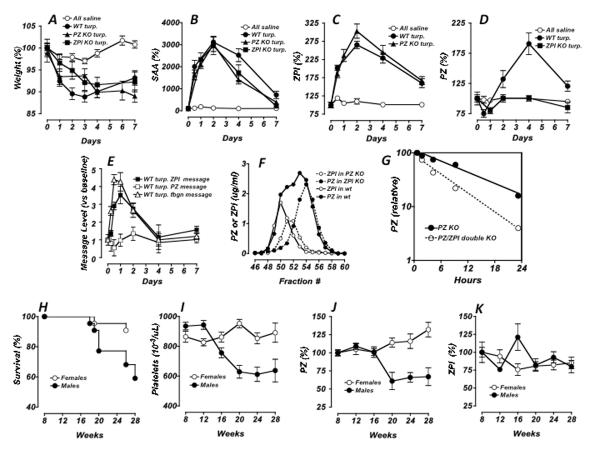
Subcutaneous injection of turpentine with the production of an aseptic abscess is a model of the acute phase response induced by local inflammation [18]. As previously reported for wild-type mice in this model, PZ knockout mice and ZPI knockout mice injected subcutaneously (SQ) with turpentine responded with significant weight loss (Fig. 1A), a dramatic increase in serum amyloid A (SAA) (Fig. 1B), a drop in albumin and an increase in fibrinogen (data not shown). Plasma ZPI levels significantly increased in response to turpentine in both wild-type mice and PZ knockout mice, with maximal levels occurring around day 2 (Fig. 1C). Plasma PZ levels significantly increased in response to turpentine in wild-type mice with maximal levels occurring on day 4, but there was no effect of turpentine on PZ levels in ZPI knockout mice (Fig. 1D).
Figure 1. PZ and ZPI responses.
PZ and ZPI proteins in the acute phase response (A–E). Wild-type (C57BL/6J; Jackson labs, Bar Harbor, ME) and PZ and ZPI knockout mice [4,5], 12–20 weeks of age, were administered 100 uL of turpentine [or phosphate buffered saline (PBS) as a control] SQ into the hindquarters; mouse weights were monitored (A). At various times, retro-orbital blood samples were collected into heparinized-capillary tubes, plasma was prepared and analyzed by immunoassays for SAA (B), using the E-90SAA kit (Immunology Consultants Lab, Inc., Newberg, OR); for ZPI (C), using human factor XIa (Enzyme Research Laboratories, South Bend, IN) as capture reagent, HRP-labeled polyclonal antibody raised to recombinant human ZPI that cross-reacts with mouse ZPI as the detection reagent and a standard curve generated with pooled C57BL/6J plasma [28]; and for PZ (D), with the Zymutest Protein Z ELISA kit RK031A (Aniara Corp. Mason, OH), which cross-reacts with mouse PZ, and a standard curve generated with pooled C57BL/6J plasma. PZ and ZPI Messages in Acute phase response (E). Livers, isolated from C57BL/6J mice injected SQ with 100 uL turpentine, were collected at the indicated times into RNAlater; RNA was extracted using RNeasy kits and treated with RNase-free DNase as suggested (Qiagen, Valencia, CA). First-strand DNA synthesis was performed using SuperScript III First Strand cDNA synthesis kit and random hexamer primers (Invitrogen). Samples were analyzed with TaqMan gene expression assays using Applied Biosystem StepOnePlus real-time PCR system and FAM-probe kits for murine GAPDH (Mm99999915.g1), PZ (Mm00482203.m1), ZPI (Mm00522856.m1) and fibrinogen (Mm00513575.m1) (Applied Biosystems, Inc, Foster City, CA). PZ, ZPI and fibrinogen messages were normalized to GAPDH message. (A–E) Results in control, PBS treated, mice were similar for all mouse groups; therefore the data were pooled and are represented as a single group (PBS). Shown for all data sets are means +/− standard errors with 5–20 animals per group. Gel chromatography profiles of ZPI and PZ for various mouse plasmas (F). Heparinized mouse plasmas (0.5 ml), obtained from wild-type, PZ knockout and ZPI knockout mice as indicated, were passed through two 10 mm × 300 mm Superdex 200 GL columns (GE Healthcare, Piscataway, NJ, USA) that were connected in series, equilibrated and run with HS buffer (20 mM Hepes, 100 mM NaCl, pH 7.4). Half mL fractions were assayed for PZ and ZPI by immunoassay and quantified using purified recombinant murine PZ or ZPI (see below). PZ and ZPI circulating half-life determinations (G). PZ knockout and PZ/ZPI double knockout mice were injected sub-orbitally with 50 uL PBS with 0.1 mg/mL bovine serum albumin (BSA) containing 1 ug of 125I-PZ/PCtag recombinant mouse protein (2 × 104 cpm/ug) labeled using Na125I (M.P. Biomedical, Solon, OH) and Iodogen tubes and separated from free label with Zeba desalt spin columns (Pierce Biotechnology, Rockford, IL). Blood samples were collected from the non-injected eye at the indicated times post-injection and 10 uL of each blood sample was counted in an LKB 1275 mini gamma counter. For each animal, the 30-minute time point count was assigned a value of 100 and the counts for each successive time point were normalized relative to this value. Shown are the normalized averages and standard deviations for the five animals in each group (G). Recombinant mouse PZ with a protein C tag (PCtag) was prepared as follows: Mouse PZ cDNA was cloned into pDCNA4.0 with the 36 base sequence encoding for the PCtag epitope (EDQVDPRLIDGK) inserted between the PZ C-terminus codon and the stop codon. This plasmid was transfected into Trex293 cells; stable cells expressing PZ/PCtag were identified by ELISA and expanded in DMEM with 10% fetal calf serum, 50 units/mL penicillin, 50 ug/mL streptomycin, 300 ug/mL Zeocin, 5 ug/mL blasticitin, 1 ug/mL tetracycline and10 ug/mL vitamin K. Upon reaching ~80% confluence, media was replaced with serum-free Opti-MEM which other than serum contained the same additives as the DMEM media. Conditioned-media was harvested and replaced every other day. PZ/PCtag was purified from conditioned-media using an anti-PCtag antibody as described by the manufacturer (Invitrogen). Purified protein was >90% pure as judged by SDS-PAGE with Coomassie staining and quantified by Bio-Rad protein assay (Bio-Rad, Hercules, CA) using BSA as standard. Recombinant mouse ZPI was produced and isolated in the same fashion except the PCtag was placed at the mature N-terminus of the recombinant ZPI. PZ and ZPI responses in a model of APS (H–K). F1 offspring (48 males and 39 females) of BXSB/MpJ males crossed with NZW/LacJ females (Jackson labs) were monitored up to 28 weeks of age for survival (H); retro-orbital blood samples were collected into heparinized tubes for platelet counts (I); plasma was prepared and analyzed by immunoassays for PZ (J); and ZPI (K); with each data analysis including a minimum of 20 samples; shown are means +/− standard errors.
Both PZ and ZPI are expressed in the liver and RT-PCR performed on liver-derived mRNA showed that ZPI, but not PZ, mRNA was increased substantially in response to turpentine; fibrinogen mRNA, as a positive control for the acute phase response, was also increased in response to turpentine (Fig. 1E). The increase in ZPI message and protein in wild-type mice in response to turpentine defines it as an acute phase response protein. In contrast, the increase in the PZ protein level was ZPI dependent and not related to a change in PZ message implicating a mechanism other than PZ gene induction. Administration of lipopolysaccharide (LPS) to mice mimics the acute phase response to infection [19]. Relative to the turpentine model, the LPS (intraperitoneal 100 ug E. coli serotype 0111:B4, Sigma, St. Louis, MO) model showed similar, although more transient and less robust, responses in weight loss, SAA, ZPI, and PZ (data not shown).
Plasma levels of PZ and ZPI appear to correlate in both man and mice and a PZ/ZPI complex has been identified in man [5,20]. On size-exclusion chromatography of plasma from wild-type mice, all the ZPI appeared to elute with PZ in a PZ/ZPI complex; ~35% of the PZ co-eluted with ZPI and ~65% eluted as free PZ (Fig. 1F). Using recombinant mouse PZ and ZPI as standards in immunoassays, mouse PZ circulates at 15 ± 8 ug/mL (mean ± SD, n=20) with a range from 8–22 ug/mL while mouse ZPI circulates at 5 ± 3 ug/mL (mean ± SD, n=20) with a range from 3–9 ug/mL. Thus, PZ and ZPI circulate as a complex in both human and mouse plasma. In man, there is excess free ZPI [20], whereas in the mouse there is excess free PZ. Still, a reduction in PZ levels in either species, as exemplified by warfarin treatment in man and by murine protein Z deficiency, is associated with reduced plasma levels of ZPI and murine ZPI deficiency is associated with reduced plasma levels of PZ [5,20].
Since the increase in PZ following turpentine administration was dependent on ZPI and PZ/ZPI complexes circulate in mice, we tested whether PZ/ZPI complex formation affected the circulating half-lives of PZ and ZPI. In preliminary studies, size-exclusion chromatography of plasma taken 30 min post-injection of 1 ug of labeled recombinant mouse PZ into a PZ/ZPI double knockout mouse demonstrated that >90% of label eluted at a size consistent with free PZ. In contrast, plasma taken 30 min post-injection of 1 ug of labeled PZ in a PZ knockout mouse demonstrated that >90% of label eluted at a size consistent with a PZ/ZPI complex. Similarly, 1 ug of labeled recombinant ZPI injected into a wild-type mouse (which naturally contain excess free PZ; see Fig. 1A) forms a complex with PZ as indicated by its size exclusion chromatography profile (data not shown). Subsequent studies showed a PZ half-life of ~210 minutes in PZ/ZPI double knockout mice and ~580 minutes in PZ knockout mice (Fig. 1G). In a similarly designed study evaluating ZPI, the ZPI half-life was ~320 minutes in PZ knockout mice versus ~660 minutes in wild-type mice (with excess circulating PZ) (Fig. 1G). Taken together, these results demonstrate formation of a PZ/ZPI complex extends the half-life of each protein relative to its free form.
APS is an autoimmune state that is associated with circulating, predominantly β2-glycoprotein I-dependent, antiphospholipid antibodies (anticardiolipin, lupus anticoagulant), thrombocytopenia, thrombosis, and fetal wastage. Reduced levels of PZ have been consistently reported in individuals with antiphospholipid antibodies and low levels of PZ are associated with the thrombotic complications of APS [21–23]. ZPI antigen levels, however, are not reduced in individuals with APS [23]. Therefore, PZ and ZPI levels were evaluated in a mouse model of APS. Crosses of NZW females with BXSB males produce F1 males who, much more frequently than females, develop thrombocytopenia, vascular thrombosis and increased mortality (Fig. 1H, 1I) [24,25]. A drop in plasma PZ protein levels occurred with disease progression (Fig. 1J), but plasma ZPI levels remained unchanged (Fig. 1K). Mean SAA levels did not change significantly over the 28-week course of the mouse experiment (data not shown), which is consistent with the low levels of SAA and limited inflammatory response reported in humans with primary APS [26,27].
In summary, the murine models show ZPI, but not PZ, to be a typical acute phase reactant. The increase in murine plasma PZ levels in the acute phase models was dependent on ZPI and potentially due in part to prolongation of the PZ half-life when it circulates in complex with ZPI. In regard to the formation of the PZ/ZPI complex in plasma, however, ZPI is limiting in the mouse, but in excess in man. Therefore, the degree to which an increase in plasma ZPI secondary to an acute phase response would affect PZ-ZPI complexation and the circulating half-life of PZ in humans is not known. ZPI, of course, could also influence PZ levels through alternative mechanisms, for example by affecting PZ synthesis, secretion, proteolysis or extra-plasma localization.
In contrast to the vigorous acute phase response induced by SQ turpentine, the NZW x BXSB F1 murine model of APS and human primary APS are associated with a muted inflammatory response and ZPI levels are not increased. The murine APS model demonstrates an acquired reduction in PZ levels that mirrors that seen in human APS, despite the differing relative proportions of PZ and ZPI in mouse and human plasma. Why the typical correlation between PZ and ZPI plasma levels is not maintained in mouse and human APS is not clear.
REFERENCES
- 1.Han X, Fiehler R, Broze GJ., Jr. Isolation of a protein Z-dependent plasma protease inhibitor. Proc Natl Acad Sci USA. 1998;95:9250–5. doi: 10.1073/pnas.95.16.9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han X, Huang Z-F, Fiehler R, Broze GJ., Jr. The protein Z-dependent protease inhibitor is a serpin. Biochemistry. 1999;38:11073–8. doi: 10.1021/bi990641a. [DOI] [PubMed] [Google Scholar]
- 3.Rezaie AR, Sun MF, Gailani D. Contributions of basic amino acids in the autolysis loop of factor XIa to serpin specificity. Biochemistry. 2006;45:9427–33. doi: 10.1021/bi060820+. [DOI] [PubMed] [Google Scholar]
- 4.Yin Z-F, Huang Z-F, Cui J, Fiehler R, Lasky N, Ginsburg D, Broze GJ., Jr. Prothrombotic phenotype of protein Z deficiency. Proc Natl Acad Sci USA. 2000;97:6734–8. doi: 10.1073/pnas.120081897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Tu Y, Lu L, Lasky N, Broze GJ. Protein Z-dependent protease inhibitor deficiency produces a more severe murine phenotype than protein Z deficiency. Blood. 2008;111:4973–8. doi: 10.1182/blood-2007-12-126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fedi S, Sofi F, Brogi D, Tellini I, Cesari F, Sestini I, Gazzini A, Comeglio M, Abbate R, Gensini GF. Low protein Z plasma levels are independently associated with acute coronary syndromes. Thromb Haemost. 2003;90:1173–8. doi: 10.1160/TH03-04-0237. [DOI] [PubMed] [Google Scholar]
- 7.Al-Shanqeeti A, van Hylckama Vlieg A, Berntorp E, Rosendaal FR, Broze GJJr. Protein Z and protein Z-dependent protease inhibitor. Determinants of levels and risk of venous thrombosis Thromb Haemost. 2005;93:411–13. doi: 10.1160/TH04-11-0715. [DOI] [PubMed] [Google Scholar]
- 8.Miletich JP, Broze GJ., Jr. Human plasma protein Z antigen: range in normal subjects and effect of warfarin therapy. Blood. 1987;69:1580–6. [PubMed] [Google Scholar]
- 9.Kobelt K, Biasiutti F, Mattle H, Lammle B, Wuillemin W. Protein Z in ischaemic stroke. Br J Haematol. 2001;114:169–173. doi: 10.1046/j.1365-2141.2001.02913.x. [DOI] [PubMed] [Google Scholar]
- 10.Lichy C, Kropp S, Dong-Si T, Genius J, Dolan T, Hampe T, Stoll F, Reuner K, Grond-Ginsbach C, Grau A. A common polymorphism of the protein Z gene is associated with protein Z plasma levels and with risk of cerebral ischemia in the young. Stroke. 2003;35:40–45. doi: 10.1161/01.STR.0000106909.75418.E4. [DOI] [PubMed] [Google Scholar]
- 11.Staton J, Sayer M, Hankey GJ, Cole V, Thom J, Eikelboom JW. Protein Z gene polymorphisms, protein Z concentrations, and ischemic stroke. Stroke. 2005;36:1123–1127. doi: 10.1161/01.STR.0000166058.49577.ca. [DOI] [PubMed] [Google Scholar]
- 12.McQuillan AM, Eikelboom JW, Hankey GJ, Baker R, Thom J, Staton J, Yi Q, Cole V. Protein Z in ischemic stroke and its etiologic subtypes. Stroke. 2003;34:2415–2419. doi: 10.1161/01.STR.0000092124.52084.4B. [DOI] [PubMed] [Google Scholar]
- 13.Vasse M, Guegan-Massardier E, Borg J-Y, Woimant F, Soria C. High frequency of protein Z deficiency in patients with ischemic stroke. Lancet. 2001;357:933–934. doi: 10.1016/S0140-6736(00)04218-5. [DOI] [PubMed] [Google Scholar]
- 14.Heeb M, Paganini-Hill A, Griffin J, Fisher M. Low protein Z levels and risk of ischemic stroke; differences by diabetic status and gender. Blood Cells Mol Dis. 2002;29:139–144. doi: 10.1006/bcmd.2002.0549. [DOI] [PubMed] [Google Scholar]
- 15.Lopaciuk S, Bykowska K, Kwiecinski H, Czlonkowska A, Kuczynska-Zardzewialy A. Protein Z in young survivors of ischemic stroke. Thromb Haemost. 2002;88:436. [PubMed] [Google Scholar]
- 16.Undar L, Karadogan I, Ozturk F. Plasma protein Z levels inversely correlate with plasma interleukin-6 levels in patients with acute leukemia and non- Hodgkin's lymphoma. Thromb Res. 1999;94:131–4. doi: 10.1016/s0049-3848(98)00210-2. [DOI] [PubMed] [Google Scholar]
- 17.Cesari F, Gori AM, Fedi S, Abbate R, Gensini GF, Sofi F. Modifications of protein Z and interleukin-6 during the acute phase of coronary artery disease. Blood Coagul Fibrinolysis. 2007;18:85–6. doi: 10.1097/MBC.0b013e3280124f2c. [DOI] [PubMed] [Google Scholar]
- 18.Gershenwald JE, Fong YM, Fahey TJ, 3rd, Calvano SE, Chizzonite R, Kilian PL, Lowry SF, Moldawer LL. Interleukin 1 receptor blockade attenuates the host inflammatory response. Proc Natl Acad Sci USA. 1990;87:4966–70. doi: 10.1073/pnas.87.13.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai L, Ji A, de Beer FC, Tannock LR, Van der Westhuyzen DR. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J Clin Invest. 2008;118:364–75. doi: 10.1172/JCI31539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabatabai A, Fiehler R, Broze GJ., Jr. Protein Z circulates in plasma in a complex with protein Z-dependent protease inhibitor. Thromb Haemost. 2001;85:655–60. [PubMed] [Google Scholar]
- 21.Steffano B, Forastiero R, Martinuzzo M, Kordich L. Low plasma protein Z levels in patients with antiphospholipid antibodies. Blood Coagul Fibrinolysis. 2001;12:411–2. doi: 10.1097/00001721-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 22.McColl MD, Deans A, Maclean P, Tait RC, Greer IA, Walker ID. Plasma protein Z deficiency is common in women with antiphospholipid antibodies. Br J Haematol. 2003;120:913–4. doi: 10.1046/j.1365-2141.2003.04151_5.x. [DOI] [PubMed] [Google Scholar]
- 23.Forastiero RR, Martinuzzo ME, Lu L, Broze GJ., Jr. Autoimmune antiphospholipid antibodies impair the inhibition of activated factor X by protein Z/protein Z-dependent protease inhibitor. J Thromb Haemost. 2003;8:1764–70. doi: 10.1046/j.1538-7836.2003.00303.x. [DOI] [PubMed] [Google Scholar]
- 24.Hang LM, Izui S, Dixon FJ. (NZW × BXSB) F1 hybrid. A model of acute lupus and coronary vascular disease with myocardial infarction. J Exp Med. 1981;154:216–21. doi: 10.1084/jem.154.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimoto Y, Kawamura M, Ichikawa K, Suzuki T, Sumida T, Yoshida S, Matsuura E, Ikeara S, Koike T. Anticardiolipin antibodies in NZW × BXSB F1 mice. A model of antiphspholipid syndrome. J. Immunol. 1992;149:1063–1068. [PubMed] [Google Scholar]
- 26.Sodin-Semrl S, Zigon P, Cucnik S, Kveder T, Blinc A, Tomsic M, Rozman B. Serum amyloid A in autoimmune thrombosis. Autoimmun. Rev. 2006;6:21–27. doi: 10.1016/j.autrev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Ames PRJ, Antinolfi I, Ciampa A, Batuca J, Scenna G, Lopez LR, Delgado Alves J, Iannaccone L, Matsurra E. Primary antiphospholipid syndrome: a low-grade autoinflammatory disease? Rheumatol. 2008;47:1832–1837. doi: 10.1093/rheumatology/ken382. [DOI] [PubMed] [Google Scholar]
- 28.Sofi F, Cesari F, Tu Y, Pratesi G, Pulli R, Pratesi C, Genesini R, Abbate R, Broze GJ., Jr. Protein Z-dependent protease inhibitor and protein Z in peripheral arterial disease patients. J Thromb Haemost. 2009;7:731–5. doi: 10.1111/j.1538-7836.2009.03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]