Summary
The relationship between protein Z levels and thrombosis is controversial. We performed a systematic review and meta-analysis of the available studies to assess the association between protein Z and vascular thrombotic diseases. We conducted an electronic literature search through MedLine, Embase, Google Scholar, Web of Science, The Cochrane Library, bibliographies of retrieved articles and abstracts of congresses up to October, 2009. Studies were included if they analysed protein Z levels in patients with vascular thrombotic diseases. After the review process, 28 case-control studies (33 patient cohorts), including 4,218 patients with thrombotic diseases and 4,778 controls, were selected for analysis. The overall analysis using a random-effects model showed that low protein Z levels were associated with an increased risk of thrombosis (odds ratio [OR] 2.90, 95% confidence interval [CI] 2.05–4.12; p<0.00001). On subgroup analysis, a significant association was found between low protein Z levels and arterial vascular diseases (OR 2.67, 95%CI 1.60–4.48; p=0.0002), pregnancy complications (OR 4.17, 95%CI 2.31–7.52; p<0.00001), and venous thromboembolic diseases (OR 2.18, 95%CI 1.19–4.00; p=0.01). The results of this meta-analysis are consistent with a role for protein Z deficiency in thrombotic diseases, including arterial thrombosis, pregnancy complications and venous thromboembolism.
Keywords: Protein Z, thrombosis, coagulation, meta-analysis
Introduction
Protein Z is a vitamin K-dependent plasma glycoprotein described in its human form in 1984 (1). The amino acid sequence of protein Z shows wide homology with many coagulation factors, such as VII, IX, X, and protein C (2). In contrast to many other vitamin K-dependent coagulation factors, however, protein Z is not a zymogen of a serine protease (2). The physiological function of protein Z was uncertain for many years until in vitro and in vivo studies demonstrated that protein Z plays an important role in inhibiting coagulation, serving as a cofactor for the inactivation of activated factor X by plasma protein Z-dependent protease inhibitor (3).
The potential role of alterations of protein Z levels in the pathogenesis of thrombotic diseases has been evaluated in several studies that have produced conflicting findings (4–32). On one side, many of the clinical studies reported low protein Z levels to be associated with the occurrence and progression of several types of ischaemic vascular diseases, such as ischaemic stroke, coronary heart disease, venous thromboembolic diseases and fetal loss (5, 7, 8, 10–12, 14, 17, 19, 21, 23, 25–29, 31), whereas, on the other side, some studies either did not observe an association or reported an association between high, instead of low, levels of protein Z and thrombotic disease (6, 9, 13, 15, 16, 18, 20, 22, 24, 30, 32).
In order to evaluate this complex issue, we systematically reviewed and analysed all the available studies addressing the potential association of protein Z levels with the risk of thrombosis.
Methods
Selection of studies
Studies that investigated the possible association between protein Z and an increased risk of thrombosis in adults were identified through a computerised search in all the electronic databases: MedLine (source: PubMed, 1966 to October 2009), Embase (1980 to October 2009), Web of Science, The Cochrane Library (source: The Cochrane Central Register of Controlled Trials, 2009, issue 1), Clinicaltrials.org, and Google Scholar. Relevant keywords relating to protein Z in combination as MeSH terms and text words (“protein Z”, “Z protein”, “vitamin K-dependent protein”, “vitamin K-dependent glycoprotein” or “blood protein”) were used in combination with words relating to thrombosis (“thrombosis”, “haemostasis”, “thrombotic diseases”, “venous thrombosis”, “venous thromboembolism”, “arterial thrombosis”, “atherothrombosis”, “vascular diseases”, “embolism”, “cardiovascular disease”, “cerebrovascular disease”, “stroke”, “peripheral arterial disease”, “pregnancy”, “fetal loss”, “blood coagulation”, or “coagulation disorders”). The search strategy had no language restrictions and was supplemented by manually reviewing the reference list of all retrieved articles as well as abstracts from the congresses of the International Society on Thrombosis and Haemostasis (ISTH), American Society of Haematology (ASH), American Heart Association (AHA), American College of Cardiology (ACC), and European Society of Cardiology (ESC) to October, 2009.
Two investigators (FS, FC) assessed potentially relevant articles for eligibility. The decision to include or exclude studies was hierarchical and initially made on the basis of the study title, then of the study abstract, and finally of the complete study manuscript. In the event of conflicting opinions, resolution of the disagreement was resolved through discussion. Eligible studies were included if they met all of the following criteria: (i) conducted on adults (i.e. older than 18 years); (ii) evaluated patients with thrombotic events; and (iii) presented data on protein Z levels and on cut-off levels of protein Z in relation to the disease. Accordingly, studies were excluded if they were not conducted in humans, if they considered outcomes different from those of interest for the meta-analysis, if they failed to report concentrations of protein Z for cases and control subjects, if they did not report proportions of patients with abnormal protein Z levels or if they failed to present estimates of association with the disease.
Outcomes of interest for the current meta-analysis were vascular thrombotic diseases, and included arterial vascular diseases such as cerebro- and cardio-vascular diseases, peripheral arterial disease, ischaemic colitis, and retinal thrombosis, pregnancy complications such as fetal loss, and preeclampsia as well as venous thromboembolic diseases such as deep-vein thrombosis, venous thromboembolism, pulmonary embolism, portal vein thrombosis, and retinal vein occlusion.
Data extraction
All data were reviewed and separately extracted by two independent investigators (FS, FC) using a standardised form. The following baseline characteristics were collected: author’s name and country of the study patients, number of patients, mean age of the study populations, gender, type of disease, cut-off levels of protein Z, number of patients and controls with altered protein Z values, odds ratios (OR) and confidence intervals (CI), as well as adjustment for confounding factors in multivariate analysis.
Statistical analysis
Review Manager (RevMan; version 5.0.18 for Macintosh; Copenhagen, Denmark; The Cochrane Collaboration, 2008) was used to pool results from the individual studies.
Pooled results are reported as OR and are presented with 95%CI with two-sided p-values using both a fixed-effects model (Mantel-Haenszel method) and a random-effects model (DerSimonian and Laird method). The two models yielded very similar estimates. Therefore, we decided to present the results based on the random-effects model because of the presence of significant heterogeneity in the analyses. A p-value less than 0.05 was considered statistically significant. The results of the original studies using multivariate models with the most complete adjustment for potential confounders were used when available; the confounding variables included in these analyses are shown in the Supplementary data (see Supplementary data available online at www.thrombosis-online.com). When not provided, estimates of an association were calculated using the number of patients and controls with abnormal protein Z levels. For studies reporting high, instead of low, protein Z levels to be associated with an increased risk of thrombosis, we recalculated the OR, by using conventional procedures.
Statistical heterogeneity was evaluated using the I2 statistic, which assesses the appropriateness of pooling the individual study results. The I2 value provides an estimate of the amount of variance across studies due to the heterogeneity rather than chance.
Where I2 was greater than 50%, heterogeneity was considered substantial. Moreover, to further investigate the heterogeneity across the studies we performed sensitivity analyses by dividing studies into groups according to their main characteristics. Subgroup analyses were then performed according to mean sample size of the patient population (<128; >128), to the country of origin of the patients (European countries; non-European countries), to the mean age of the patients’ populations (<45 years; >45 years), as well as to the presence or absence of adjustment for possible confounding factors (yes; no). Small study bias and/or publication bias was appraised by visual inspection of funnel plot of effect size against standard error and, analytically, by the Egger’s test.
Results
Study identification and selection
Our search strategy yielded 158 articles (▶Fig. 1). Of these 56 articles were excluded either because they were not conducted on humans or because they were reviews or reports of case studies. The selected articles were carefully reviewed and a further 38 articles were excluded because they did not include patients with outcomes of interest. Thirty-four additional studies were excluded because they reported no data on protein Z levels (n=23) or cut-off levels of protein Z for an increased risk of disease (n=11). Finally, one study was excluded because it lacked a control group and one because it was a study aimed at testing the efficacy of thromboprophylaxis in women with an unexplained previous pregnancy loss.
Figure 1.
Flow chart of search strategy.
As a result, 28 case-control studies were included and entered into the final analysis. Of these, five papers reported different cohorts of patients for separate clinical outcomes. Therefore, as a whole, 33 groups of patients were analysed. Included studies ranged in size from 16 to 443 cases and from 31 to 593 controls, for a total number of 4,218 patients and 4,778 controls.
Characteristics of the studies included in the meta-analysis are summarised as supplementary data (see Supplementary data available online at www.thrombosis-online.com). Most were conducted in European countries. The vast majority of the studies investigated low protein Z levels in association with an increased risk of thrombosis (5, 7, 8, 10–12, 14–23, 25–32), whereas four studies evaluated the opposite hypothesis, i.e. whether high protein Z levels were associated with an increased risk of thrombotic events (6, 9, 13, 24). Therefore, the cut-off levels for the proposed abnormal protein Z levels differed substantially among the included studies, ranging from < 520 ng/ml to > 2,600 ng/ml. In three out of 28 studies protein Z levels were measured in terms of percentage values and not as absolute levels (6, 18, 31). Moreover, a multivariate logistic regression model was tested only in some (n=10) of the included studies, with significant differences in the variables used to adjust the analyses (6, 12, 13, 19, 22, 23, 26–28, 31).
Meta-analysis
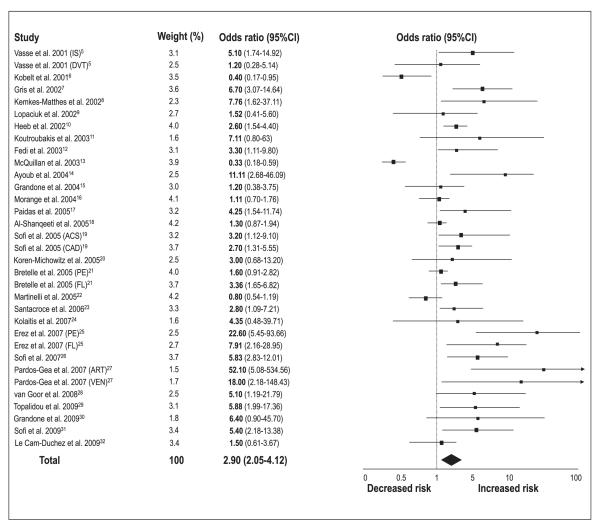
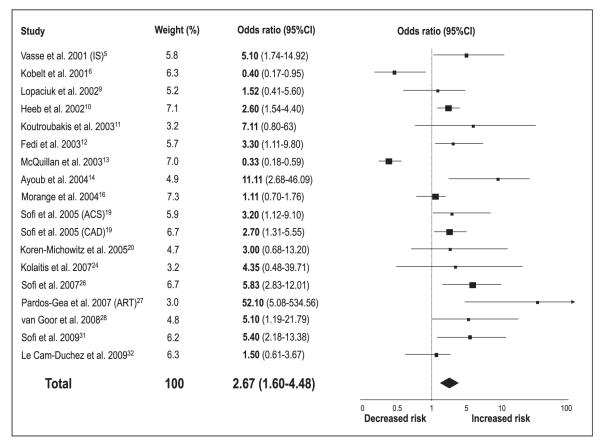
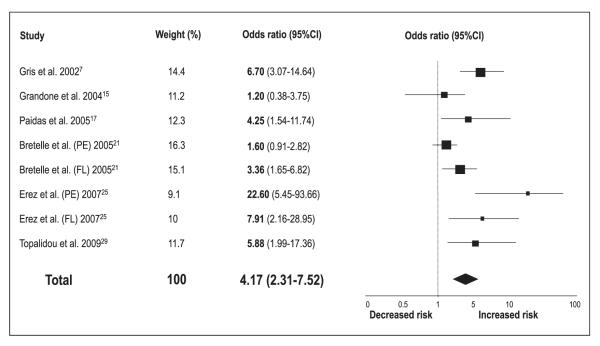
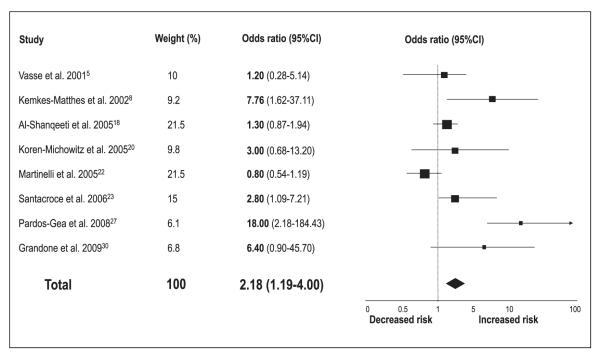
Meta-analytic pooling under a random-effects model showed that patients with low protein Z levels are at increased risk of thrombotic events (OR 2.90, 95%CI 2.05–4.12; p<0.00001) (▶Fig. 2). However, significant statistical heterogeneity across the studies (I2=80%) was detected. When studies evaluating different clinical outcomes were grouped, we found that low protein Z levels were a significant risk factor for arterial vascular diseases (n=18 reports, with a total of 2,054 patients and 3,033 controls) (OR 2.67, 95%CI 1.60–4.48; p=0.0002) (▶Fig. 3), pregnancy complications (n=8 reports, with a total of 714 patients and 515 controls) (OR 4.17, 95%CI 2.31–7.52; p<0.00001) (▶Fig. 4), and venous thromboembolic diseases (n=8 reports, with a total of 1,297 patients and 1,399 controls) (OR 2.18, 95%CI 1.19–4.00; p=0.01) (▶Fig. 5). ▶Table 1 reports all the results according to both fixed and random models. Significant statistical heterogeneity across the studies was also noted in the subgroup analyses: I2=82% for the outcome of arterial vascular diseases; I2=69% for the outcome of pregnancy complications, and I2=70% for the outcome of venous thromboembolic diseases.
Figure 2. Risk of vascular thrombotic diseases for low protein Z levels.
Squares represent the effect size; extended lines, 95%CI; diamond, total effect size. IS, ischaemic stroke; DVT, deep-vein thrombosis; ACS, acute coronary syndromes; CAD, coronary artery disease; PE, preeclampsia; FL, fetal loss; ART, arterial vascular diseases; VEN, venous thromboembolic diseases.
Figure 3. Risk of arterial vascular diseases for low protein Z levels.
Squares represent the effect size; extended lines, 95%CI; diamond, total effect size. IS, ischaemic stroke; ACS, acute coronary syndromes; CAD, coronary artery disease; ART, arterial vascular diseases.
Figure 4. Risk of pregnancy complications for low protein Z levels.
Squares represent the effect size; extended lines, 95%CI; diamond, total effect size. PE, preeclampsia; FL, fetal loss.
Figure 5. Risk of venous thromboembolic diseases for low protein Z levels.
Squares represent the effect size; extended lines, 95%CI; diamond, total effect size.
Table 1.
Results of fixed and random models.
| Outcome (n, reports) | Patients, n | Controls, n | Fixed model | Random model | ||
|---|---|---|---|---|---|---|
|
|
||||||
| OR (95%CI) | I2; p (het.) | OR (95% CI) | I2; p (het.) | |||
| All outcomes (33) | 4,218 | 4,778 | 1.87 (1.62–2.15) | 80%; p<0.0001 | 2.90 (2.05–4.12) | 80%; p<0.0001 |
| Arterial vascular events (18) | 2,054 | 3,033 | 1.86 (1.51–217) | 82%; p<0.0001 | 2.67 (1.60–4.48) | 82%; p<0.0001 |
| Pregnancy complications (8) | 714 | 515 | 3.42 (2.51–4.66) | 69%; p=0.002 | 4.17 (2.31–7.52) | 69%; p=0.002 |
| Venous thromboembolic diseases (8) |
1,297 | 1,399 | 1.28 (1.01–1.65) | 70%; p=0.002 | 2.18 (1.19–4.00) | 70%; p=0.002 |
Sensitivity analyses
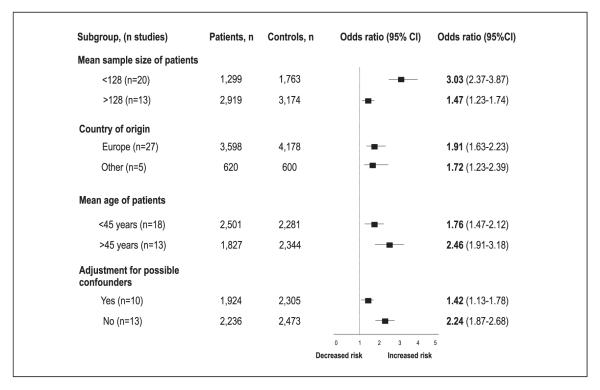
In order to analyse the nature of this heterogeneity across the studies we performed sensitivity analyses by grouping studies according to characteristics such as size of the studies (mean size of the patients sample: 128), country of origin of the patients (European countries versus other countries), age of the patients (mean age: 45) and adjustment for confounding factors at the multivariate analysis (yes; no) (▶Fig. 6). Studies conducted on patients with an age above the mean age of all the population of patients (>45 years) and smaller studies showed a tendency towards a more elevated estimate of association with the increased risk of thrombosis with respect to those conducted in younger patients and to larger studies.
Figure 6. Subgroup analyses.
Squares represent the effect size; extended lines, 95%CI; diamond, total effect size.
In addition, we conducted multiple leave-one-out analyses for the secondary endpoints. Specifically, we found that the studies by Kobelt et al. (6), McQuillan et al. 13), and Morange et al. (16), were the major contributors to statistical heterogeneity in the analysis for arterial vascular diseases (p for heterogeneity from 0.00001 overall to 0.19 after their exclusions, with I2 going from 82% to 24%) whereas the studies by Bretelle et al. (21), and Erez et al. (25), were the main contributors to statistical heterogeneity for pregnancy complications (p for heterogeneity from 0.002 overall to 0.17 after their exclusions, with I2 going from 69% to 35%). The studies by Kemkes-Matthes et al. (8), and Pardos-Gea et al. (27) were the main contributors to statistical heterogeneity for venous thromboembolic disease (p for heterogeneity from 0.002 overall to 0.28 after their exclusion, with I2 going from 70% to 22%).
Interestingly, however, none of these exclusions led to loss of statistical significance in favour of low protein Z levels as a predisposing factor in the occurrence of thrombosis.
Publication bias
The funnel plots of effect size versus standard error, performed in order to investigate for a possible publication bias, were broadly asymmetric (see Supplementary data available online at www.thrombosis-online.de), suggesting the presence of some statistical outliers among the included studies (Egger’s test: p<0.0001). After the exclusion of the studies that contributed substantially to the heterogeneity of the results, however, publication biases were not detected based on the p-value of the Egger’s test (p=0.08 for the outcome of vascular arterial diseases; p=0.2 for the outcome of pregnancy complications; and p=0.3 for the outcome of venous thromboembolic diseases).
Discussion
This is the first systematic review with meta-analysis conducted to evaluate the role of protein Z on the occurrence of thrombosis-related clinical outcomes. All the available studies on protein Z and clinical thrombotic events were systematically reviewed and separated into three different clinical outcomes, arterial vascular diseases such as ischaemic stroke, coronary heart disease, peripheral arterial disease or other ischaemic events involving the arteries, pregnancy complications such as fetal loss and preeclampsia, and venous thromboembolic diseases. This search strategy allowed us to analyse the role of protein Z in an overall population of more than 4,000 patients with thrombotic events as compared to control subjects.
In this meta-analytical study, low levels of protein Z were found to be a significant risk factor for all the thrombotic events, for arterial thrombosis, pregnancy complications and venous thromboembolic diseases. Overall, patients with low levels of this coagulation regulatory protein show nearly a three-fold increased risk of thrombotic events with respect to control subjects.
An association between protein Z deficiency and thrombosis has been demonstrated in animal models (33, 34), but human studies have produced conflicting results (5–32). Indeed, in four of these studies high circulating levels of protein Z were found to be associated with an increased risk of disease (6, 9, 13, 24). Although the aim of the present study was to determine whether low protein Z levels were a significant risk factor for the occurrence of thrombosis, these four studies were included in our analysis by computing estimates of association in a reverse way. By analysing subgroups of studies according to the clinical outcome, we were able to identify some studies as statistical outliers for the present meta-analysis. For arterial thrombotic events, these were the studies by Kobelt et al. (6), and McQuillan et al. (13), that represent two out of the three studies that investigated the role of high levels of protein Z in relation to the clinical events, and the study by Morange et al. (16) that is a cohort prospective study, but used as a nested case-control study for the aim of this meta-analysis. Acute, rather than convalescent protein Z levels were measured in one of these studies (13). Similarly, the main contributors for heterogeneity among pregnancy complication studies were those conducted by Bretelle et al. (21) and Erez et al. (25) that investigated preeclampsia as main clinical outcome and, for venous thromboembolic diseases, the studies by Kemkes-Matthes et al. (8) and Pardos-Gea et al. (27) that included either patients with positivity for factor V Leiden mutation or patients with venous thromboses in different localisations.
Subgroup analysis demonstrated a significant association between low protein Z and arterial vascular diseases (mainly ischaemic stroke and atherosclerotic vascular diseases), pregnancy complications (fetal loss and preeclampsia), and venous thromboembolic diseases (mainly deep-vein thrombosis). The link between protein Z and arterial disease is supported by the observation of protein Z in the atherosclerotic vascular lesions and the interaction between protein Z and certain arterial risk factors (35, 36). With regard to venous thromboses, on the other hand, significant associations were reported particularly when accompanied with other acquired or genetic risk factors for thrombosis such as factor V Leiden mutation (8).
Actually, the reasons why protein Z levels are decreased in patients with clinical manifestations of thrombotic diseases are different and not yet determined. One possibility could be that the persistent activation of the coagulatory process is able to determine an increased consumption of such protein. Another possibility could be that protein Z is reduced in its synthesis by liver and/or by other sources such as endothelial cells. A further option can be that related to the presence of anti-protein Z antibodies and another possibility could be the association between protein Z and some traditional risk factors. In addition, another explanation could reside on the association with the inflammatory state present in such kind of patients. All these hypotheses, together with a possible influence of medical therapies, might explain the variability observed in the present meta-analysis.
Some limitations can be identified in the present study. First, the meta-analysis was performed on case-control studies and most of the included studies were of small size, thus the overall results have to be interpreted carefully. Second, the overall and the subgroup results showed a moderate heterogeneity across the studies. Consistent results were obtained, however, following sensitivity and multiple leave-out-one analyses. Third, the possibility of publication bias cannot be fully eliminated despite the lack of evidence for such on funnel plot and Egger’s test performed after the exclusion of outlier studies.
In conclusion, the present meta-analysis conducted on 28 case-control studies and 33 cohorts of patients, with an overall population of more than 8,000 subjects, demonstrated that low protein Z levels are significantly associated with all thrombotic events, arterial thromboses, pregnancy complications, and venous thromboembolic diseases. Additional issues concerning protein Z, however, still need to be addressed and clarified: the need of prospective and larger studies, whether protein Z is influenced by the acute phase response, the best method of protein Z measurement, and the optimal cut-off value for protein Z in the estimation of thrombotic risk.
Supplementary Material
What is known about this topic?
Protein Z is a vitamin K-dependent glycoprotein whose sequence has wide homology with many coagulation factors.
Many studies have investigated the possible role of protein Z on the occurrence of thrombotic diseases.
The relationship between protein Z and thrombosis remains, however, controversial.
What does this study add?
Low levels of protein Z are significantly associated with thrombotic diseases including arterial, venous thromboses as well as pregnancy complications.
Footnotes
Authorship F.S. handled conception and design, analysed and interpreted the data, drafted the article, gave final approval of the article, provided administrative, technical, or logistic support, and collected and assembled the data. F.C. handled conception and design, analysed and interpreted the data, and gave final approval of the article. R.A. and G.F.G. critically revised the article for important intellectual content, gave final approval of the article. G.B. drafted the article, critically revised the article for important intellectual content, and gave final approval of the article. S.F. handled conception and design, drafted the article, and gave final approval of the article.
References
- 1.Broze GJ, Jr, Miletich JP. Human protein Z. J Clin Invest. 1984;73:933–938. doi: 10.1172/JCI111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ichinose A, Takeya H, Espling E, et al. Amino acid sequence of human protein Z, a vitamin K-dependent plasma glycoprotein. Biochem Biophys Res Comm. 1990;172:1139–1144. doi: 10.1016/0006-291x(90)91566-b. [DOI] [PubMed] [Google Scholar]
- 3.Han X, Fiehler R, Broze GJ., Jr Isolation of a protein Z-dependent plasma protease inhibitor. Proc Natl Acad Sci USA. 1998;95:9250–9255. doi: 10.1073/pnas.95.16.9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vasse M. Protein Z, a protein seeking a pathology. Thromb Haemost. 2008;100:548–556. [PubMed] [Google Scholar]
- 5.Vasse M, Guegan-Massardier E, Borg JY, et al. Frequency of protein Z deficiency in patients with ischaemic stroke. Lancet. 2001;357:933–934. doi: 10.1016/S0140-6736(00)04218-5. [DOI] [PubMed] [Google Scholar]
- 6.Kobelt K, Demarmels Biasiutti F, Mattle HP, et al. Protein Z in ischemic stroke. Br J Haematol. 2001;114:169–173. doi: 10.1046/j.1365-2141.2001.02913.x. [DOI] [PubMed] [Google Scholar]
- 7.Gris JC, Quere I, Dechaud E, et al. High frequency of protein Z deficiency in patients with unexplained early fetal loss. Blood. 2002;99:2606–2608. doi: 10.1182/blood.v99.7.2606. [DOI] [PubMed] [Google Scholar]
- 8.Kemkes-Matthes B, Nees M, Kunhel G, et al. Protein Z influences the prothrombotic phenotype in factor V Leiden patients. Thromb Res. 2002;106:183–185. doi: 10.1016/s0049-3848(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 9.Lopaciuk S, Bykowska K, Kwiecinski H, et al. Protein Z in young survivors of ischemic stroke. Thromb Haemost. 2002;88:536. [PubMed] [Google Scholar]
- 10.Heeb MJ, Paganini-Hill A, Griffin JH, et al. Low protein Z levels and risk of ischemic stroke: differences by diabetic status and gender. Blood Cells Mol Dis. 2002;29:139–144. doi: 10.1006/bcmd.2002.0549. [DOI] [PubMed] [Google Scholar]
- 11.Koutroubakis I, Theodoropoulou A, Sfiridaki A, et al. Low plasma protein Z levels in patients with ischemic colitis. Dig Dis Sci. 2003;48:1673–1676. doi: 10.1023/a:1025441405688. [DOI] [PubMed] [Google Scholar]
- 12.Fedi S, Sofi F, Brogi D, et al. Low protein Z plasma levels are independently associated with acute coronary syndromes. Thromb Haemost. 2003;90:1173–1178. doi: 10.1160/TH03-04-0237. [DOI] [PubMed] [Google Scholar]
- 13.McQuillan A, Eikelboom J, Hankey G, et al. Protein Z in ischemic stroke and its etiologic subtypes. Stroke. 2003;34:2415–2419. doi: 10.1161/01.STR.0000092124.52084.4B. [DOI] [PubMed] [Google Scholar]
- 14.Ayoub N, Esposito G, Barete S, et al. Protein Z deficiency in antiphospholipid-negative Sneddon’s syndrome. Stroke. 2004;35:1329–1332. doi: 10.1161/01.STR.0000127534.54538.15. [DOI] [PubMed] [Google Scholar]
- 15.Grandone E, Colaizzo D, Cappucci F, et al. Protein Z levels and unexplained fetal losses. Fertil Steril. 2004;82:982–983. doi: 10.1016/j.fertnstert.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 16.Morange PE, Juhan-Vague I, on behalf of the PRIME study group Protein Z plasma levels are not associated with the risk of coronary heart disease: the PRIME study. J Thromb Haemost. 2004;2:2050–2051. doi: 10.1111/j.1538-7836.2004.00981.x. [DOI] [PubMed] [Google Scholar]
- 17.Paidas MJ, Ku DHW, Lee MJ, et al. Protein Z, protein S levels are lower in patients with thrombophilia and subsequent pregnancy complications. J Thromb Haemost. 2005;3:497–501. doi: 10.1111/j.1538-7836.2005.01158.x. [DOI] [PubMed] [Google Scholar]
- 18.Al-Shanqeeti A, van Hylckama Vlieg A, Berntorp E, et al. Protein Z and protein Z-dependent protease inhibitor. Thromb Haemost. 2005;93:411–413. doi: 10.1160/TH04-11-0715. [DOI] [PubMed] [Google Scholar]
- 19.Sofi F, Cesari F, Vigiani S, et al. Protein Z levels in different phases of activity of coronary atherosclerosis. J Thromb Haemost. 2005;3:2254–2258. doi: 10.1111/j.1538-7836.2005.01536.x. [DOI] [PubMed] [Google Scholar]
- 20.Koren-Michowitz M, Eting E, Rahimi-Levene N, et al. Protein Z levels and central retinal vein or artery occlusion. Eur J Haematol. 2005;75:401–405. doi: 10.1111/j.1600-0609.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 21.Bretelle F, Arnoux D, Shojai R, et al. Protein Z in patients with pregnancy complications. Am J Obstet Gynecol. 2005;193:1698–1702. doi: 10.1016/j.ajog.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Martinelli I, Razzari C, Biguzzi E, et al. Low levels of protein Z and the risk of venous thromboembolism. J Thromb Haemost. 2005;3:2817–2819. doi: 10.1111/j.1538-7836.2005.01664.x. [DOI] [PubMed] [Google Scholar]
- 23.Santacroce R, Sarno M, Cappucci F, et al. Low protein Z levels and risk of occurrence of deep vein thrombosis. J Thromb Haemost. 2006;4:2417–2422. doi: 10.1111/j.1538-7836.2006.02186.x. [DOI] [PubMed] [Google Scholar]
- 24.Kolaitis NJ, Felekis T, Dova L, et al. A high normal value of plasma protein Z is an uncommon finding in non-arteritic ischemic optic neuropathy (N-AION) patients. J Thromb Haemost. 2007;5(Suppl 2):P-S–101. [Google Scholar]
- 25.Erez O, Hoppensteadt D, Romero R, et al. Preeclampsia is associated with low concentrations of protein Z. J Matern Fetal Neonatal Med. 2007;20:661–667. doi: 10.1080/14767050701495011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sofi F, Cesari F, Pratesi G, et al. Low protein Z levels in patients with peripheral arterial disease. Thromb Haemost. 2007;89:1114–1117. [PubMed] [Google Scholar]
- 27.Pardos-Gea J, Ordi-Ros J, Serrano S, et al. Protein Z levels and anti-protein Z antibodies in patients with arterial and venous thrombosis. Thromb Res. 2008;121:727–734. doi: 10.1016/j.thromres.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 28.van Goor MPJ, Dippel DWJ, Jie KSG, et al. Low protein Z levels but not the protein Z gene G79A polymorphism are a risk factor for ischemic stroke. Thromb Res. 2008;123:213–218. doi: 10.1016/j.thromres.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Topalidou M, Effraimidou S, Farmakiotis D, et al. Low protein Z levels, but nor the intron F G79A polymorphism, are associated with unexplained pregnancy loss. Thromb Res. 2009;124:24–27. doi: 10.1016/j.thromres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Grandone E, Favuzzi G, De Stefano V, et al. Protein Z g-42a variant and the risk of pregnancy-related venous thromboembolism in a cohort of Italian patients. Thromb Res. 2009;123:848–850. doi: 10.1016/j.thromres.2008.12.045. [DOI] [PubMed] [Google Scholar]
- 31.Sofi F, Cesari F, Tu Y, et al. Protein Z-dependent protease inhibitor and protein Z in peripheral arterial disease patients. J Thromb Haemost. 2009;7:731–735. doi: 10.1111/j.1538-7836.2009.03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Cam-Duchez V, Soria C, Sollier CBD, et al. Rare genotypes of protein Z gene are a risk factor for premature myocardial infarction but not protein Z plasma level. Thromb Haemost. 2009;102:131–136. doi: 10.1160/TH09-01-0007. [DOI] [PubMed] [Google Scholar]
- 33.Yin ZF, Huang ZF, Cui J, et al. Prothrombotic phenotype of protein Z deficiency. Proc Natl Acad Sci. 2000;97:6734–6738. doi: 10.1073/pnas.120081897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Tu Y, Lu L, et al. Protein Z-dependent protease inhibitor deficiency produces a more severe murine phenotype than protein Z deficiency. Blood. 2008;111:4973–4978. doi: 10.1182/blood-2007-12-126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greten J, Kreis L, Liliensiek B, et al. Localisation of protein Z in vascular lesions of patients with atherosclerosis. VASA. 1998;27:144–148. [PubMed] [Google Scholar]
- 36.Heeb MJ, Fisher M, Paganini-Hill A. Association of low protein Z levels with ischemic stroke in young women. Thromb Haemost. 2007;97:495–496. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.