Abstract
The blood-brain barrier (BBB) is important physiologically. Pathologically, BBB disruption has been implicated in a wide spectrum of neurological disorders including Parkinson's disease (PD). Recent studies indicate that caffeine is protective against PD, but by poorly understood mechanisms. Using a MPTP neurotoxin model of PD we tested the hypothesis that the protective actions of caffeine were due to, at least in part, preventing MPTP-induced BBB dysfunction. FVB mice were pretreated with caffeine (10 mg/kg, i.p.) or saline for 7 days prior to initiation of neurotoxin treatments; during the 7 days of neurotoxin treatment, caffeine or saline continued to be administered 10 min before each dose of MPTP (20 mg/kg, i.p.). Striatum (and for some studies hippocampus and cerebral cortex as well) were evaluated for BBB leakage, tight junction protein expression levels, integrity of dopaminergic neurons, and activation of astrocytes and microglia using immunostaining, immunoblotting and real-time PCR techniques. We found that caffeine blocked MPTP-induced decreases in numbers of TH-positive dopaminergic neurons, increases in leakage of Evan's blue dye and FITC-albumin in striatum but not in cerebral cortex or hippocampus, decreases in levels of the tight junction proteins occludin and ZO-1, and increases in reactive gliosis. Our results suggest that caffeine might protect against PD and PD-like features in animal models, in part, by stabilizing the BBB.
Keywords: Parkinson's disease, tight junction proteins, astrocytes, microglia, matrix metalloproteinase
INTRODUCTION
Parkinson's disease (PD) is characterized pathologically by progressive neuronal degeneration in the substantia nigra pars compacta and striatum. Clinically, PD patients present with tremor, rigidity, bradykinesia and postural instability (Obeso et al. 2000). Genetic factors have been linked to familial PD, however the majority of PD cases are sporadic in origin with unknown etiologies. The pathogenesis of PD has been linked increasingly to neuroinflammation, oxidative stress, and blood-brain barrier disruption (Hunot and Hirsch 2003; Rogers and Kovelowski 2003; Hawkins and Davis 2005; Abbott et al. 2006). Indeed, neuroinflammation and oxidative stress have been shown to have deliterious effects on blood-brain barrier (BBB) integrity and BBB leakage has been reported in PD patients (Kortekaas et al. 2005) and in animal models of PD including the MPTP mouse model (Zhao et al. 2007) and the 6-OHDA rat model (Carvey et al. 2005). Because BBB functions to protect the central nervous system and disruption of BBB induces loss of dopaminergic neurons (Rite et al. 2007) others and we have hypothesized that BBB dysfunction might underly, at least in part, the pathogenesis of PD (Desai et al. 2007; Monahan et al. 2008).
In addition to the mechanisms underling PD being unclear, pharmacological/medical interventions against PD are limited. Epidemiological studies, both prospective and retrospective, indicate that caffeine when administered chronically decreases the neuropathogenesis of PD (Benedetti et al. 2000; Ross et al. 2000b; Ross et al. 2000a; Ascherio et al. 2001). Experimental studies have also demonstrated that caffeine is neuroprotective against dopaminergic neurodegeneration in MPTP-induced mouse models of PD (Chen et al. 2001). However, although caffeine most potently blocks adenosine A2A receptors and other adenosine A2A receptor antagonists have protective actions against PD, the mechanisms whereby caffeine exerts its protecitve effects against PD are still not fully understood. We recently reported that chronic ingestion of caffeine prevented BBB dysfunction in a rabbit model of Alzheimer's disease (Chen et al. 2008). Here, given that BBB dysfunction has been demonstrated in the well-established MPTP mouse model of PD, we tested the hypothesis that caffeine would protect against the BBB dysfunction that occurs in this animal model of PD.
MATERIALS AND METHODS
Aminals and treatment
Male FVB mice (25–30 gm; 2–3 months old) were divided into 4 groups. Group 1 control mice were treated for two weeks with vehicle (PBS). Group 2 control mice received caffeine (10 mg/kg/day, i.p.) for two weeks. Group 3 mice received vehicle for the first week and then MPTP (20 mg/kg/day) for the second week. Group 4 mice received caffeine (10 mg/kg/day) for two weeks and during the second week received as well injections of MPTP (20 mg/kg/day, i.p.). For MPTP-treated mice, PBS or caffeine was injected 10 min prior to MPTP administration. One day after final treatments were administered, animals were anesthetized with isoflurane and perfused transcardially with ice-cold PBS. One half of the brain was taken intact and from the other hemisphere cerebral cortex, hippocampus and striatum were dissected; all brain tissue was frozen on a liquid nitrogen-cooled surface and stored at −80°C until taken for experimentation. All experimental procedures were approved by the Committee for Animal Care and Use at the University of North Dakota.
FITC-albumin leakage assay
The leakage of FITC-albumin (Sigma, St. Louis, MO) from vasculature into brain parenchyma as a measure of BBB integrity was assessed as described previously (Carvey et al. 2005). Briefly, isoflurane-anesthetized mice were injected intracardially with heparin (100 units/kg) and then infused with 5 ml of FITC-albumin (5 mg/ml, dissolved in 0.1 M PBS) at a rate of 1.5 ml/min. After perfusion, brains were removed immediately and immersed into 4% paraformaldehyde. Three days later, the fixative was replaced with three changes of 30% sucrose in 0.1 M PBS buffer. Each brain was sectioned with a sliding microtome and 30 μm sections were mounted onto gelatin-coated slides, dehydrated, cover-slipped and analyzed for FITC-albumin leakage using a confocal microscope (Olympus).
Evan's blue dye leakage assay
An alternate method using Evan's blue dye was also used as an indicator of BBB integrity. Three hours after Evan's blue dye (25 mg/kg) was injected i.p. mice were anesthetized with isoflurane (Chen et al. 2008), blood was collected from the heart, and animals were perfused with oxygenated PBS. Following perfusion, brains were removed quickly from the skull and cerebral cortex, hippocampus and striatum were dissected, weighed, and incubated for 72 h with formamide in the dark at room temperature. After incubation, samples were centrifuged at 10,000 × g for 10 minutes, supernatants were collected, and absorbance was measured at 620 nm. Evan's blue concentrations were calculated from standard curves and values were expressed as Evan's blue concentration/specimen weight and were normalized to plasma Evan's blue concentration.
Immunohistochemical staining for TH-positive neurons, tight junction proteins, and glia
Frozen coronal sections (14 μm) were air-dried, fixed in acetone for 10 min, treated with 0.3% hydrogen peroxide in methonal, and incubated with a blocking solution of 1.5% normal serum in PBS. Subsequently, sections were incubated overnight at 4°C with antibodies direct against target proteins. A tyrosine hydroxylase polycolonal antibody (Abcam) was used to assess the integrity of dopaminergic neurons. After washing, sections were incubated with biotinylated secondary antibodies and liquid diaminobenzidine/hydrogen peroxide, dehydrated through 70%, 95% and 100% alcohol, cleared in xylene, mounted with resinous mounting medium, and examined under conventional (Leica) microscopy.
Expression levels of tight junctions were determined using fluorescently labeled anti-ZO-1 (Zymed, clone ZO1-1A12) and anti-occludin (Zymed, clone OC-3F10) antibodies. An anti-GFAP monoclonal antibody (Sigma, Clone G-A-5) was used for detection of astrocyte activation. Microglial activation was determined by staining with biotinconjugated Griffonia simplicifolia isolectin B4 (Molecular Probe). In order to examine whether MMP9 was expressed on reactive astrocytes, double immunostaining for MMP9 (1:500, Abcam) and GFAP (1:2000, Sigma) was performed; sections were first incubated with anti-MMP9 and monoclonal anti-GFAP antibodies and then were incubated with either Texas-red conjugated goat anti-rabbit or FITC-conjugated goat anti-mouse secondary antibodies. Sections were examined by confocal (Olympus) microscopy. Images were analyzed with Image J software.
MMP-9 zymography in situ
In situ gelatinolytic activity was detected on frozen brain sections (14 μm thick) using a commercial kit (EnzChek Gelatinase Assay Kit; Molecular Probes). Briefly, brain sections were dried at room temperature and incubated with 20 μg/ml quenched FITC-labelled gelatin at 37°C for 3 h. Cleavage of quenched FITC-labelled gelatin by MMPs resulted in the formation of green fluorescent product which was detected with confocal microscopopy (Zeiss 501). EDTA (15 mM) was used to inhibit metalloprotease activity and determine specific levels of MMP activity.
Immunoblot analysis of protein expression levels of tyrosine hydroxylase, occludin and ZO-1
Striatum was gently homogenized using a Teflon homogenizer in 1 ml ice-cold suspension buffer consisting of 20 mM HEPES-KOH (pH 7.5), 250 mM sucrose, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM PMSF, and protease inhibitor cocktail (1:100, Sigma). Protein concentrations were determined by a Bradford method (Bio-Rad). Equal amount of proteins (10 μg) were separated by SDS–PAGE (10% gel), transfered to a polyvinylidene difluoride membrane, and incubated overnight at 4°C with monoclonal antibodies against tyrosine hydroxylase (1:2,000), occludin (1:1000) and ZO-1 (1:1000); β-actin (1:5,000, Abcam) was used as a loading control. The blots were developed with enhanced chemiluminescence, and then visualized and analyzed by LabWorks 4.5 software on a UVP Bioimaging System (Upland, CA, USA).
Quantitative RT-PCR for tight junction proteins and MMP-9
Total RNA from striatum was extracted with TRIzol-Reagent (Invitrogen) according to the manufacturer's instructions and levels were determined spectrophotometrically. Reverse transcription reactions were carried out using a SuperScript® III First-Strand Synthesis supermix (Invitrogen). The primers for tight junction proteins and GAPDH were as follows: 5'-TGACAGACGGAAACCTTAGAG-3' and 5'-AGACATCGTGCAGGCAGATG-3' for occludin; 5'-TGGACAACCAGATGTGGATTT-3' and 5'-TCCCGTCTTCATGAGCTGAAT-3' for ZO-1; 5′-CCAAGGGTACAGCCTGTTCCT-3′ and 5′-GCACGCTGGAATGATCTAAGC-3′ for MMP9; 5'-GCCAAGGTCATCCATGACAAC-3' and 5'-AGTGTAGCCCAAGATGCCCTT-3' for GAPDH. Samples were run with our iCycler IQ™ Multicolor Real-Time PCR Detection System (Bio-Rad) that monitors fluorescence as a direct indication of PCR product. All samples were run in triplicate and the averaged values were used for the relative quantification of the gene expression. Individual mRNA expression levels were calculated as the ratio of their expression compared with that of GAPDH.
Statististical analysis
All data were expressed as means and SEM. Statistical significance for multiple comparisons was determined by one-way ANOVA and a Tukey post-hoc test. p < 0.05 was considered to be statistically significant.
RESULTS
Caffeine attenuated MPTP-induced loss of dopaminergic neuron in striatum
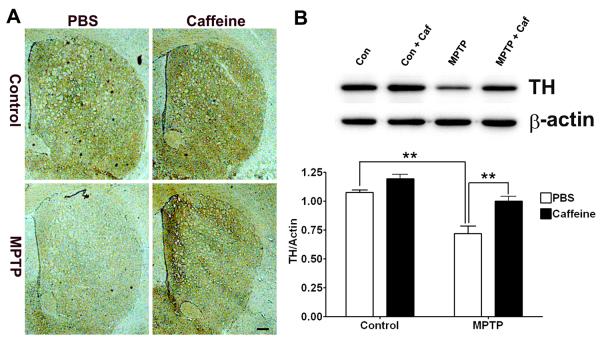
Caffeine protected against loss of dopaminergic neurons in a MPTP mouse model of PD (Chen et al. 2001). Accordingly, we determined first the extent to which caffeine protected against loss of striatal dopaminergic neurons in our MPTP mouse model of PD. Using immunostaining for tyrosine hydroxylase (TH) as a measure of dopaminergic neuron integrity, we noted a marked decrease in TH immunoreactivity in striatum that was attenuated by caffeine (Figure 1A). To confirm and extend these qualitative immunohistochemical results, we quantified TH protein levels by immunoblotting and found that MPTP decreased significantly (p < 0.01) TH protein levels and that caffeine blocked significantly (p < 0.01) these decreases (Figure 1B).
Figure 1. Caffeine attenuated MPTP-induced loss of tyrosine hydroxylase (TH) positive dopaminergic neurons in striatum.
(A) MPTP decreased markedly TH immunoreactivity in striatum, and this effect was attenuated by caffeine. Bar = 100 μm. (B) MPTP decreased significantly (p < 0.01) TH protein levels in striatum, and this effect was blocked significantly (p < 0.01) by caffeine. (n = 4, **p < 0.01)
Caffeine attenuated MPTP-induced gliosis in striatum
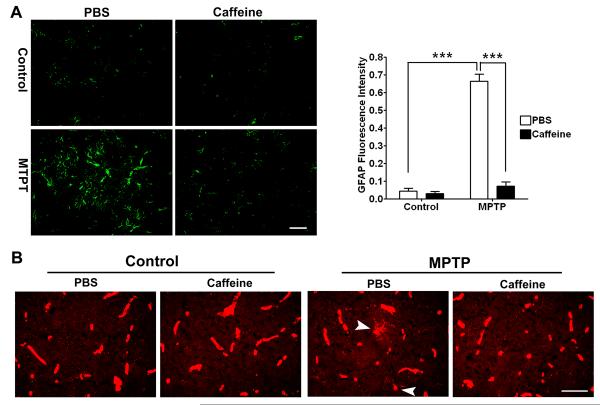
Activation of astrocytes and microgila occurs in PD patients (Forno et al. 1992; Ouchi et al. 2005; Gerhard et al. 2006) and in animal models of PD including MPTP (Francis et al. 1995; Kohutnicka et al. 1998; Liberatore et al. 1999). Indeed, gliosis is considered a prominent feature of PD (McGeer and McGeer 2008). Accordingly, we examined next the effects of caffeine on gliosis in striatum of MPTP-treated animals. Activation of astrocytes was evident immunohistochemically; marked increases in GFAP staining were observed throughout the striatum. When GFAP protein expression levels were quantified from immunoblots we found that MPTP significantly (p < 0.001) and markedly increased GFAP levels and that this effect was blocked significantly (p < 0.001) by caffeine (Figure 2A). MPTP also markedly increased activation of microglia as assessed by immunostaining for lectin and this effect was attenuated by caffeine (Figure 2B). Morphologically, and consistent with the findings of others (Soltys et al. 2001), astocytes and microglia in MPTP-treated animals were found to be more compact, rounded, and with obvious cell thickening than were cells from control and caffeine-treated mice; this is indicative of an activated state.
Figure 2. Caffeine attenuated MPTP-induced gliosis.
(A) MPTP significantly (p < 0.001) increased GFAP immunoreactivity in striatum and this effect was blocked significantly (p < 0.001) by caffeine. The upper left panel of four photomicrographs was representative of findings observed in 3 animals with 6 sections taken from each animal. ***p < 0.001, Bar = 50 μm. (B) In addition to microglia, lectin staining also revealed blood vessels. Amoeboid-shaped microglia (arrow heads) were observed only in striatum from MPTP treated animals; not in control or caffeine-treated animals. Representative images were taken from 3 animals; 6 sections were taken from each animal. Bar = 50 μm.
Caffeine blocked MPTP-induced leakge of the BBB in striatum
To examine the effects of MPTP in the absence and presence of caffeine on BBB integrity, we first assessed the integrity of the BBB using confocal microscopy to determine if FITC-albumin, a large molecular weight protein (MW range = 69–70 kD), entered brain parenchyma. In control animals, indicative of an intact BBB FITC-albumin was confined exclusively to blood vessels and the staining showed sharp outlines of the vasculature in both striatum and hippocampus. In MPTP-treated animals, indicative of BBB disruption the outline of the vasculature was obscured and patchy areas of FITC-albumin leakage was observed in striatum (Figure 3A). Although others have reported increased vascularization in substantia nigra of MPTP-treated monkeys (Barcia et al. 2005), the increased staining for FITC-albumin was not due to increased angiogenesis because when we stained blood vessels with collagen IV no increases in blood vessels (numbers or volume) were observed (data not included). In contrast to striatum, FITC-albumin was still confined to blood vessels in hippocampus of MPTP-treated animals and this is indicative of an intact BBB (Figure 3B). In caffeine-treated control and PBS-treated animals, FITC-albumin was confined to blood vessels in striatum and hippocampus. This indicates that caffeine treatment protected against MPTP-induced disruption of the BBB in striatum.
Figure 3. Caffeine blocked MPTP-induced increases in striatum FITC-albumin leakage.
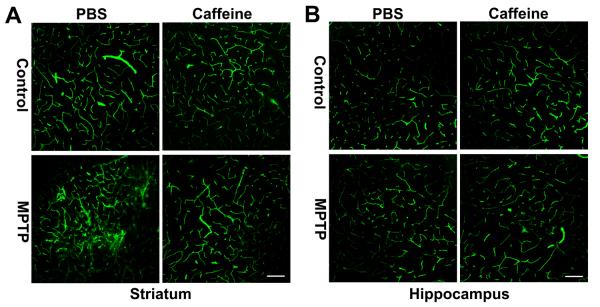
(A) MPTP markedly increased FITC-albumin leakage in striatum, and this effect was blocked by caffeine. (B) Neither caffeine nor MPTP increased FITC-albumin leakage in hippocampus. Bar = 100 μm (n = 4)
To confirm and extend the qualitative histological results on BBB integrity, we further evaluated BBB integrity in discrete brain regions using a quantitative Evan's blue dye leakage assay. Consistent with the FITC-albumin leakage results, we found that MPTP injection significantly (p < 0.01) increased leakage of Evan's blue dye in striatum (Figure 4A), but MPTP did not change significantly Evan's blue dye leakage in hippocampus (Figure 4B) or cerebral cortex (Figure 4C). Caffeine treatment alone did not affect significantly leakage of Evan's blue dye in any of the three brain regions examined. However, caffeine blocked significantly (p < 0.01) MPTP-induced increases in leakage of Evan's blue dye in striatum (Figure 4A).
Figure 4. Caffeine blocked MPTP-induced increases in striatum Evan's blue dye leakage.
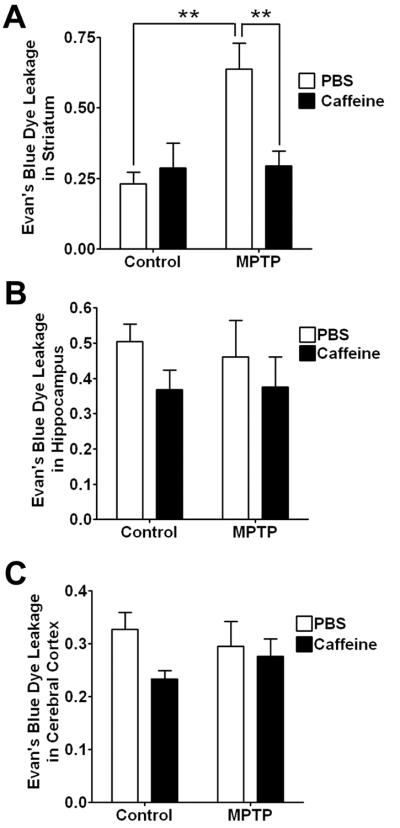
(A) MPTP-induced increases (p < 0.01) in leakage of Evan's blue dye in striatum were decreased significantly (p < 0.01) by caffeine. Neither caffeine nor MPTP changed significantly Evan's blue dye leakage in cerebral cortex (B) or in hippocampus (C). (n = 6, **p < 0.01)
Caffeine blocked MPTP-induced decreases in levels of striatal tight junction proteins
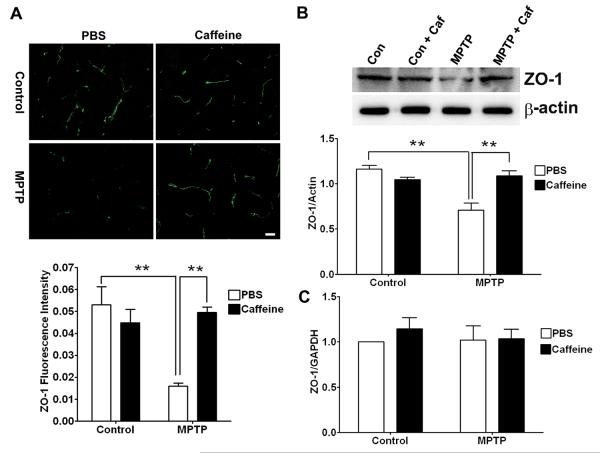
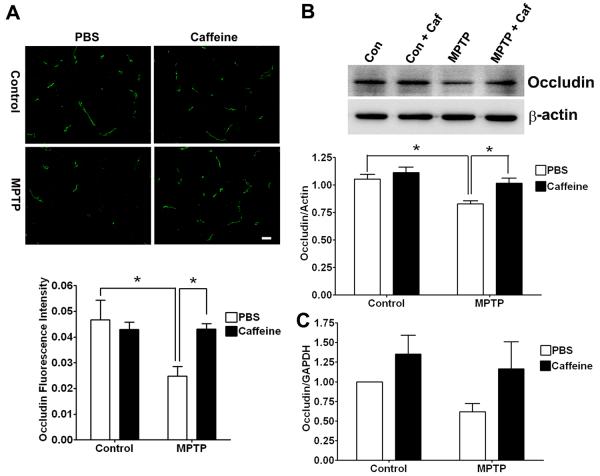
The restrictive nature of the BBB that exists between adjacent endothelial cells is due to a complex network of tight junction proteins such as occludin, claudins and zonula occludens (ZO) that link transmembrane proteins to the actin cytoskeleton (Begley and Brightman 2003). Accordingly, we next determined the effects of MPTP in the absence or presence of caffeine on expression levels of these tight junction proteins. In striatum of MPTP-treated animals, levels of ZO-1 (Figure 5A, p < 0.01) and occludin (Figure 6A, p < 0.05) as determined immunohistochemically were decreased significantly and this effect on ZO-1 (p < 0.01) and occludin (p < 0.05) was blocked significantly by pretreatment with caffeine. Using immunoblotting techniques to quantify levels of the tight junction proteins, we found that MPTP treatment decreased significantly the protein levels of both ZO-1 (Fig 5B, p < 0.01) and occludin (Fig 6B, p < 0.05) in striatum, and these effects on ZO-1 (p < 0.01) and occludin (p < 0.05) were blocked significantly by caffeine. Caffeine treatment alone had no effect on the protein levels of these two tight junction proteins. However, real-time RT-PCR measurements of tight junction protein mRNA revealed that neither ZO-1 (Figure 5C) nor occludin (Figure 6C) mRNA levels were affected significantly by the MPTP and/or caffeine treatments. Thus, changes in tight junction proteins appeared to occur at the protein level and not transcriptionally.
Figure 5. Caffeine blocked MPTP-induced decreases in the expression of ZO-1.
(A) MPTP decreased significantly (p < 0.01) ZO-1 immunostaining in striatum, and this effect was blocked significantly (p < 0.01) by caffeine. Bar = 20 μm. (B) MPTP decreased significantly (p < 0.01) ZO-1 protein levels in striatum, and this effect was blocked significantly (p < 0.01) by caffeine. (C) Neither MPTP nor caffeine changed significantly ZO-1 mRNA levels in striatum. (n = 4, **p < 0.01)
Figure 6. Caffeine blocked MPTP-induced decreases in occludin expression levels.
(A) MPTP decreased significantly (p < 0.05) occludin immunostaining in striatum, and this effect was blocked significantly (p < 0.05) by caffeine. Bar = 20 μm. (B) MPTP decreased significantly (p < 0.05) occludin protein levels in striatum, and this effect was blocked significantly (p < 0.05) by caffeine. (C) Neither MPTP nor caffeine changed significantly occludin mRNA levels in striatum. (n = 4, *p < 0.05).
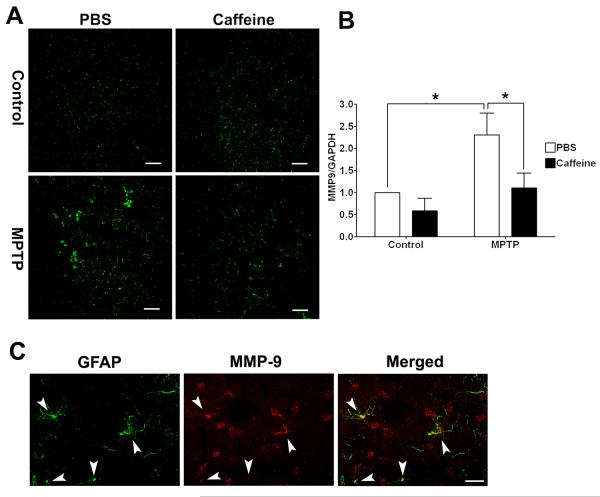
Caffeine blocked MPTP-induced increases in MMP9 activity
Metallomatrixprotease 9 (MMP9) is capable of degrading tight junction proteins including ZO-1 and occludin (Asahi et al. 2001; Behzadian et al. 2001; Petty and Lo 2002; Pflugfelder et al. 2005) and MMP9 activity is increased in striatum of MPTP-treated mice (Lorenzl et al. 2004). We therefore determined next the levels of striatal MMP9 activity and protein in MPTP-treated mice in the abscence or presence of caffeine. Using an in situ gelatinolytic activity zymography method we observed that MPTP markedly increased in situ gelatinolytic activity in stiatum and that this effect was blocked by caffeine (Figure 7A). MPTP also significantly (p < 0.05) increased mRNA levels of MMP9 in striatum, and this effect was blocked signficantly (p < 0.05) by caffeine (Figure 7B). MMP9 is expressed mainly on CNS neurons and astrocytes (Zhao et al. 2006). Immunohistochemically, we observed marked increases in the numbers of reactive astrocytes in striatum from MPTP-treated animals. Using double immunofluorescent staining, we found in striatum of MPTP-treated mice that MMP9 co-distributed with GFAP-positive astrocytes (Figure 7C).
Figure 7. Caffeine attenuated MPTP-induced increases in in situ gelatinolytic activity and increases in the expression of MMP9.
(A) MPTP increased in situ gelatinolytic activity in striatum and this effect was attenuated by caffeine. Bar = 100 μm. (B) MPTP increased significantly (p < 0.05) mRNA levels of MMP-9 in striatum and this effect was blocked significantly (p < 0.05) by caffeine. (n = 4, *p < 0.05) (C) MMP-9 co-localized (see arrow heads) with GFAP in striatum of MPTP-treated mice.
DISCUSSION
The BBB is an important physical and metabolic barrier that helps keep separate the central nervous system and the systemic circulation. This barrier helps regulate and protect the microenvironment of the brain and once disrupted synaptic and neuronal functions can be compromised (Zlokovic 2008). Increasingly, BBB disruption has been implicated in the pathogenesis of a number of acute and chronic neurodegenerative disorders including brain trauma (Hoane et al. 2006), stroke (McGill et al. 2005), multiple sclerosis (Minagar and Alexander 2003), HIV-1 dementia (Petito and Cash 1992), Alzheimer's disease (Zipser et al. 2007) and Parkinson's disease (Kortekaas et al. 2005). Indeed, BBB disruption is reportedly one of the earliest pathological events underlying Alzheimer's disease (Ujiie et al. 2003).
Parkinson's disease is a devastating chronic neurodegenerative disease characterized clinically by muscle rigidity, tremor and bradykinesia and pathologically by loss of dopaminergic neurons mainly in the substantia nigra pars compacta, neuroinflammation and dysruption of the BBB (Kortekaas et al. 2005). Useful animal models for PD now exist and dysruption of the BBB has been noted in MPTP-treated mice (Zhao et al. 2007) and 6-OHDA-treated rats (Carvey et al. 2005). Our results confirm and extend findings of earlier studies by showing using two separate but complementary methods that the BBB becomes increasingly leaky in MPTP-treated mice and that this is likely due to increased neuroinflammation, increased levels of MMP9, and ultimately decreased expression levels of tight junction proteins. Of further significance is our finding that caffeine attenuated MPTP-induced increases in BBB leakage, as indicated by FITC-albumin leakage and Evan's blue dye leakage, decreases in the protein levels of tight junction proteins, ZO-1 and occludin, increases in activation of astrocyte and microglia, and increases in MMP9.
Inflammation appears to play an important role in the pathogenesis of PD (McGeer et al. 1988; Hunot and Hirsch 2003; McGeer and McGeer 2008) and inflammation resulting from MPTP treatment might be a major contributor to BBB disruption (Carvey et al. 2006; Monahan et al. 2008). Brain inflammation is integrally associated with activation of astrocytes and microglia and activation of astrocytes and microglia have been observed in substantia nigra pars compacta and striatum of MPTP-treated mice (Francis et al. 1995; Kohutnicka et al. 1998; Kurkowska-Jastrzebska et al. 1999). Consistent with these reports, we found that MPTP treatment induced a marked increase in activation of astrocytes and microglia in striatum. Reactive gliosis can release a cascade of proinflammatory and neurotoxic factors including TNF-α and IL-1, and free radicals (Mogi et al. 1998; Przedborski and Jackson-Lewis 1998; Koutsilieri et al. 2002; Tichauer et al. 2007; McGeer and McGeer 2008); all of which can disrupt the BBB (Abbott 2000). The BBB leakage could potentiate further neuroinflammatory responses by allowing peripheral inflammatory cells to infiltrate into brain parenchyma, thus creating a vicious cycle. Moreover, it has been shown that disruption of the BBB can induce the loss of dopaminergic neurons (Rite et al. 2007). Thus disruption of the BBB may underlie, at least in part, the pathogenesis of PD (Desai et al. 2007). Disruption of BBB may also help explain why even short-term exposures to MPTP result in progressive PD-like disorders.
Epidemiological and experimental studies indicate that caffeine, when administered chronically, has beneficial effects against a number of neurological disorders including stroke, Alzheimer's disease, and PD (Rudolphi et al. 1989; Sutherland et al. 1991; Bona et al. 1995; Benedetti et al. 2000; Ross et al. 2000b; Ross et al. 2000a; Ascherio et al. 2001; Lindsay et al. 2002; Maia and de Mendonca 2002; Dall'Igna et al. 2003; Arendash et al. 2006; Higdon and Frei 2006; Ritchie et al. 2007). One of the features common to these diseases is the presence of a disrupted BBB. Thus caffeine might exert its neuroprotective effect by virtue of its action on the BBB. We reported recently that chronic ingestion of caffeine protected against BBB leakage in a rabbit model of Alzheimer's disease (Chen et al. 2008). This finding promoted us to examine the effects of caffeine on BBB integrity in MPTP mouse model of PD.
Our observation that caffeine blocks MPTP-induced loss of TH-positive neurons in striatum confirms the findings of others that caffeine is neuroprotective against MPTP-induced lesions of dopaminergic neurons in striatum (Chen et al. 2001). Caffeine also blocked MPTP-induced activation of astrocytes and microglia and this finding appears consistent with clinical findings of an inverse relationship between coffee consumption and inflammation (Lopez-Garcia et al. 2006). More importantly, we found that caffeine blocked MPTP-induced BBB leakage in striatum, as evidence by the qualitative leakage of FITC-albumin and quantitative leakage of Evan's blue dye. Caffeine also blocked MPTP-induced decreased expression of the tight junction proteins ZO1 and occludin. The observation that neither caffeine nor MPTP significantly changed the mRNA levels of these tight junction proteins indicates that caffeine and MPTP might affect the expression of these tight junction proteins at a post-transcriptional, translational or post-translational level. It has been shown that phosphorylation status affects dynamic assembly of these tight junction proteins (Sakakibara et al. 1997; Antonetti et al. 1999; Persidsky et al. 2006), and that activation of matrix metallomatrixprotease (MMPs), including MMP9, could degrade these tight junction proteins (Asahi et al. 2001; Behzadian et al. 2001; Petty and Lo 2002; Pflugfelder et al. 2005). Thus, caffeine and MPTP might affect the expression these tight junction proteins post-translationally. Although we did not test whether caffeine or MPTP affects the phosphorylation status of these tight junction proteins, we did observe that caffeine blocked MPTP-induced increases in in situ activity and expression of MMP9. Thus, changes in the expression of these tight junction proteins may be associated with changes in the activity of MMP9.
The sequence of events and mechanisms by which MPTP and caffeine cause these effects was not investigated and is not evident. It could be that MPTP first induces loss of dopaminergic neuron and subsequently inflammatory responses are initiated thus causing disruption of the BBB or that BBB disruption precedes neuronal loss (Rite et al. 2007). Similarly, the protective effects of caffeine might be initiated as a result of direct neuroprotective actions or actions at the BBB per se. On the other hand, several lines of evidence indicate that caffeine and the adenosine receptors it is known to block can regulate neuroinflammation in in vitro models devoid of BBB (Schwaninger et al. 1997; Hasko et al. 2005; Horrigan et al. 2006). Therefore, the protective effects of caffeine against MPTP-induced BBB disruption might result from its ability to inhibit inflammation. Indeed, we did observe that caffeine attenuated MPTP-induced activation of astrocytes and microglia, markers of neuroinflammation, and increased activity of MMPs. The current study could not exclude the possibility that caffeine's protective effect against BBB leakage is secondary to its neuroprotective effects, but we do favor the notion that caffeine is acting at the BBB and that neuroprotection is secondary to the BBB effects because of our findings in a rabbit model of Alzheimer's disease that caffeine protects against increased BBB leakage in the absence of neuronal damage by the cholesterol-enriched diet (Chen et al. 2008).
Caffeine has diverse pharmacological effects, but its mechanism(s) of actions remain ill-understood (Nehlig et al. 1992). Caffeine at low concentrations can block all four subtypes of adenosine receptors, at intermediate concentrations can elevate intracellular cAMP levels by inhibiting PDE4 activity, and at higher concentrations can mobilize calcium from endoplasmic reticulum stores through actions on IP3 and ryanodine receptors (Fredholm et al. 1999). Although detailed molecular mechanisms whereby caffeine protects against BBB disruption were not explored in the present study, two of the most likely pharmacological actions whereby caffeine might be exerting its effects are blocking adenosine receptors and/or inhibiting cAMP phosphodiesterase especially because both actions are known to affect mechanisms that control BBB permeability (Rubin et al. 1991; Folcik et al. 1999; Ishizaki et al. 2003). Clearly, additional detailed studies are warranted to determine the detailed mechanisms by which caffeine exerts its protective actions against disruptive effects on BBB integrity.
Caffeine protects against MPTP-induced loss of dopaminergic neuron, activation of astrocytes and microglia, and disruption of the BBB. Our findings that caffeine, a safe and readily available drug, can stabilize BBB have important implications for therapeutic interventions against PD and other neurological disorders.
ACKNOWLEDGEMENTS
This work was supported by P20RR17699 from the National Center for Research Resources, a component of the NIH.
REFERENCE
- Abbott NJ. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell Mol Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, Shippy D, Tan J. Caffeine protects Alzheimer's mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- Barcia C, Bautista V, Sanchez-Bahillo A, Fernandez-Villalba E, Faucheux B, Poza y Poza M, Fernandez Barreiro A, Hirsch EC, Herrero MT. Changes in vascularization in substantia nigra pars compacta of monkeys rendered parkinsonian. J Neural Transm. 2005;112:1237–1248. doi: 10.1007/s00702-004-0256-2. [DOI] [PubMed] [Google Scholar]
- Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB. TGF-beta increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci. 2001;42:853–859. [PubMed] [Google Scholar]
- Benedetti MD, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Smoking, alcohol, and coffee consumption preceding Parkinson's disease: a case-control study. Neurology. 2000;55:1350–1358. doi: 10.1212/wnl.55.9.1350. [DOI] [PubMed] [Google Scholar]
- Bona E, Aden U, Fredholm BB, Hagberg H. The effect of long term caffeine treatment on hypoxic-ischemic brain damage in the neonate. Pediatr Res. 1995;38:312–318. doi: 10.1203/00006450-199509000-00007. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Punati A, Newman MB. Progressive dopamine neuron loss in Parkinson's disease: the multiple hit hypothesis. Cell Transplant. 2006;15:239–250. doi: 10.3727/000000006783981990. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, Snyder J, Zhu YG, Ling ZD. 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur J Neurosci. 2005;22:1158–1168. doi: 10.1111/j.1460-9568.2005.04281.x. [DOI] [PubMed] [Google Scholar]
- Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr., Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson's disease. J Neurosci. 2001;21(RC143):141–146. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gawryluk JW, Wagener JF, Ghribi O, Geiger JD. Caffeine blocks disruption of blood brain barrier in a rabbit model of Alzheimer's disease. J Neuroinflammation. 2008;5:12. doi: 10.1186/1742-2094-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Igna OP, Porciuncula LO, Souza DO, Cunha RA, Lara DR. Neuroprotection by caffeine and adenosine A2A receptor blockade of beta-amyloid neurotoxicity. Br J Pharmacol. 2003;138:1207–1209. doi: 10.1038/sj.bjp.0705185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood-brain barrier pathology in Alzheimer's and Parkinson's disease: implications for drug therapy. Cell Transplant. 2007;16:285–299. doi: 10.3727/000000007783464731. [DOI] [PubMed] [Google Scholar]
- Folcik VA, Smith T, O'Bryant S, Kawczak JA, Zhu B, Sakurai H, Kajiwara A, Staddon JM, Glabinski A, Chernosky AL, Tani M, Johnson JM, Tuohy VK, Rubin LL, Ransohoff RM. Treatment with BBB022A or rolipram stabilizes the blood-brain barrier in experimental autoimmune encephalomyelitis: an additional mechanism for the therapeutic effect of type IV phosphodiesterase inhibitors. J Neuroimmunol. 1999;97:119–128. doi: 10.1016/s0165-5728(99)00063-6. [DOI] [PubMed] [Google Scholar]
- Forno LS, DeLanney LE, Irwin I, Di Monte D, Langston JW. Astrocytes and Parkinson's disease. Prog Brain Res. 1992;94:429–436. doi: 10.1016/s0079-6123(08)61770-7. [DOI] [PubMed] [Google Scholar]
- Francis JW, Von Visger J, Markelonis GJ, Oh TH. Neuroglial responses to the dopaminergic neurotoxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mouse striatum. Neurotoxicol Teratol. 1995;17:7–12. doi: 10.1016/0892-0362(94)00048-i. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Hasko G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci. 2005;26:511–516. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr. 2006;46:101–123. doi: 10.1080/10408390500400009. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Kaplan SA, Ellis AL. The effects of nicotinamide on apoptosis and blood-brain barrier breakdown following traumatic brain injury. Brain Res. 2006;1125:185–193. doi: 10.1016/j.brainres.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: friend or foe? Pharmacol Ther. 2006;111:877–892. doi: 10.1016/j.pharmthera.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S49–58. doi: 10.1002/ana.10481. discussion S58–60. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Chiba H, Kojima T, Fujibe M, Soma T, Miyajima H, Nagasawa K, Wada I, Sawada N. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp Cell Res. 2003;290:275–288. doi: 10.1016/s0014-4827(03)00354-9. [DOI] [PubMed] [Google Scholar]
- Kohutnicka M, Lewandowska E, Kurkowska-Jastrzebska I, Czlonkowski A, Czlonkowska A. Microglial and astrocytic involvement in a murine model of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Immunopharmacology. 1998;39:167–180. doi: 10.1016/s0162-3109(98)00022-8. [DOI] [PubMed] [Google Scholar]
- Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–179. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Scheller C, Grunblatt E, Nara K, Li J, Riederer P. Free radicals in Parkinson's disease. J Neurol. 2002;249(Suppl 2):II1–5. doi: 10.1007/s00415-002-1201-7. [DOI] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia E, van Dam RM, Qi L, Hu FB. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84:888–893. doi: 10.1093/ajcn/84.4.888. [DOI] [PubMed] [Google Scholar]
- Lorenzl S, Calingasan N, Yang L, Albers DS, Shugama S, Gregorio J, Krell HW, Chirichigno J, Joh T, Beal MF. Matrix metalloproteinase-9 is elevated in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Neuromolecular Med. 2004;5:119–132. doi: 10.1385/NMM:5:2:119. [DOI] [PubMed] [Google Scholar]
- Maia L, de Mendonca A. Does caffeine intake protect from Alzheimer's disease? Eur J Neurol. 2002;9:377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGill JK, Gallagher L, Carswell HV, Irving EA, Dominiczak AF, Macrae IM. Impaired functional recovery after stroke in the stroke-prone spontaneously hypertensive rat. Stroke. 2005;36:135–141. doi: 10.1161/01.STR.0000149629.32525.b7. [DOI] [PubMed] [Google Scholar]
- Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Ogawa M, Ikeguchi K, Shizuma N, Fan D, Nakano I, Nagatsu T. Effects of repeated systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to mice on interleukin-1beta and nerve growth factor in the striatum. Neurosci Lett. 1998;250:25–28. doi: 10.1016/s0304-3940(98)00427-3. [DOI] [PubMed] [Google Scholar]
- Monahan AJ, Warren M, Carvey PM. Neuroinflammation and peripheral immune infiltration in Parkinson's disease: an autoimmune hypothesis. Cell Transplant. 2008;17:363–372. [PubMed] [Google Scholar]
- Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain ResRev. 1992;17:139–170. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW. Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci. 2000;23:S8–19. doi: 10.1016/s1471-1931(00)00028-8. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Heilman D, Haorah J, Zelivyanskaya M, Persidsky R, Weber GA, Shimokawa H, Kaibuchi K, Ikezu T. Rho-mediated regulation of tight junctions during monocyte migration across the blood-brain barrier in HIV-1 encephalitis (HIVE) Blood. 2006;107:4770–4780. doi: 10.1182/blood-2005-11-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Cash KS. Blood-brain barrier abnormalities in the acquired immunodeficiency syndrome: immunohistochemical localization of serum proteins in postmortem brain. Ann Neurol. 1992;32:658–666. doi: 10.1002/ana.410320509. [DOI] [PubMed] [Google Scholar]
- Petty MA, Lo EH. Junctional complexes of the blood-brain barrier: permeability changes in neuroinflammation. Prog Neurobiol. 2002;68:311–323. doi: 10.1016/s0301-0082(02)00128-4. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Farley W, Luo L, Chen LZ, de Paiva CS, Olmos LC, Li DQ, Fini ME. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V. Experimental developments in movement disorders: update on proposed free radical mechanisms. Curr Opin Neurol. 1998;11:335–339. doi: 10.1097/00019052-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Carriere I, de Mendonca A, Portet F, Dartigues JF, Rouaud O, Barberger-Gateau P, Ancelin ML. The neuroprotective effects of caffeine: a prospective population study (the Three City Study) Neurology. 2007;69:536–545. doi: 10.1212/01.wnl.0000266670.35219.0c. [DOI] [PubMed] [Google Scholar]
- Rite I, Machado A, Cano J, Venero JL. Blood-brain barrier disruption induces in vivo degeneration of nigral dopaminergic neurons. J Neurochem. 2007;101:1567–1582. doi: 10.1111/j.1471-4159.2007.04567.x. [DOI] [PubMed] [Google Scholar]
- Rogers J, Kovelowski CJ. Inflammatory Mechanisms in Parkinson's Disease. In: Wood PL, editor. Neuroinflammation, 2nd Edition: Mechanisms and Management. Humana Press Inc; Totowa, NJ: 2003. pp. 391–403. [Google Scholar]
- Ross GW, Abbott RD, Petrovitch H, White LR, Tanner CM. Relationship between caffeine intake and parkinson disease. Jama. 2000a;284:1378–1379. [PubMed] [Google Scholar]
- Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR. Association of coffee and caffeine intake with the risk of Parkinson disease. Jama. 2000b;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J, et al. A cell culture model of the blood-brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolphi KA, Keil M, Fastbom J, Fredholm BB. Ischaemic damage in gerbil hippocampus is reduced following upregulation of adenosine (A1) receptors by caffeine treatment. Neurosci Lett. 1989;103:275–280. doi: 10.1016/0304-3940(89)90112-2. [DOI] [PubMed] [Google Scholar]
- Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaninger M, Neher M, Viegas E, Schneider A, Spranger M. Stimulation of interleukin-6 secretion and gene transcription in primary astrocytes by adenosine. J Neurochem. 1997;69:1145–1150. doi: 10.1046/j.1471-4159.1997.69031145.x. [DOI] [PubMed] [Google Scholar]
- Soltys Z, Ziaja M, Pawlinski R, Setkowicz Z, Janeczko K. Morphology of reactive microglia in the injured cerebral cortex. Fractal analysis and complementary quantitative methods. J Neurosci Res. 2001;63:90–97. doi: 10.1002/1097-4547(20010101)63:1<90::AID-JNR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Peeling J, Lesiuk HJ, Brownstone RM, Rydzy M, Saunders JK, Geiger JD. The effects of caffeine on ischemic neuronal injury as determined by magnetic resonance imaging and histopathology. Neuroscience. 1991;42:171–182. doi: 10.1016/0306-4522(91)90157-j. [DOI] [PubMed] [Google Scholar]
- Tichauer J, Saud K, von Bernhardi R. Modulation by astrocytes of microglial cell-mediated neuroinflammation: effect on the activation of microglial signaling pathways. Neuroimmunomodulation. 2007;14:168–174. doi: 10.1159/000110642. [DOI] [PubMed] [Google Scholar]
- Ujiie M, Dickstein DL, Carlow DA, Jefferies WA. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol Dis. 2007;26:36–46. doi: 10.1016/j.nbd.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, Vitek MP, Hovanesian V, Stopa EG. Microvascular injury and blood-brain barrier leakage in Alzheimer's disease. Neurobiol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]