Abstract
We investigated how the broadly neutralizing monoclonal antibody 2F5 affects the human immunodeficiency virus type 1 envelope glycoprotein as it undergoes receptor-induced conformational changes and show that 2F5 binds both native and fusion-intermediate conformations, suggesting inhibition of a late step in virus entry. We also demonstrate conformational changes in the C heptad of gp41.
The envelope glycoprotein (Env) of human immunodeficiency virus type 1 (HIV-1) consists of a surface (gp120) subunit that attaches virus to target cells and a noncovalently associated transmembrane protein (gp41) (Fig. 1A) that mediates fusion between virus and target cell membranes. These subunits are synthesized as a fusion-inactive precursor (gp160) that is cleaved in the synthetic pathway to generate mature Env. gp120 binding to CD4 and chemokine receptors on the host cell triggers fusion-inducing conformational changes in gp41, leading to entry of the viral nucleocapsid into the host cell cytoplasm.
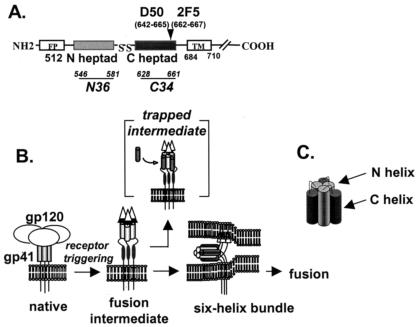
FIG. 1.
(A) Linear representation of gp41 domains and approximate localization of epitopes for 2F5 and D50 monoclonal antibodies (MAbs). Numbering is based on the HIV-1 HXB2 strain according to the Los Alamos National Laboratory database. N36 peptide contains the following residues (546 to 581): SGIVQQQNNLLRAIEAQQHLLQLTVWGIKQLQARIL. C34 peptide contains the following residues (628 to 661): WMEWDREINNYTSLIHSLIEESQNQQEKNEQELL. FP, fusion peptide; TM, transmembrane domain. (B) Model of HIV-1 entry involving conformational changes in gp41. Brackets show a putative fusion intermediate that is trapped with the C-heptad repeat peptide, preventing formation of the six-helix bundle. (C) Schematic representation of the six-helix bundle structure generated by self assembly of the N- and C-heptad repeat domains.
The mechanism of gp41-mediated membrane fusion is not fully understood. A widely accepted model of HIV entry postulates that gp41 undergoes major refolding steps as it mediates membrane fusion (Fig. 1B, reviewed in reference 5). In this model, gp41 transitions from its native, metastable conformation as it exists on the surface of virus or infected cells to a final fusion-active conformation consisting of a thermostable six-helix bundle structure (Fig. 1C). This structure forms when two heptad repeat regions in the gp41 ectodomain self assemble into a trimer of hairpins, where the N- and C-terminal heptad repeats align in an antiparallel manner in each hairpin monomer (4, 35, 37). The N heptads form a triple-stranded coiled coil in the internal layer of the six-helix bundle, and the C-heptad repeat helices form the external layer. It is believed that gp120 binding to receptors loosens the association of gp120 with gp41, which in turn releases the fusion peptide at the N terminus of gp41 to insert into the target membrane. Subsequent folding of this fusion-intermediate conformation into the six-helix bundle structure probably facilitates fusion by bringing membranes together as Env adopts a more thermodynamically stable conformation.
We investigated gp41 conformational changes by analyzing how two gp41 monoclonal antibodies (MAbs) with epitopes in the C heptad of gp41 bind Env under various conditions (Fig. 1A). The first antibody, 2F5, well known for being one of the few broadly neutralizing and protective HIV antibodies (20, 28), binds to a core epitope containing the residues ELDKWA at the C terminus of the C heptad (24, 27). The second antibody, D50, also binds a linear peptide from the C heptad but is not neutralizing (7). D50 was generated in mice immunized with a secreted, uncleaved, oligomeric form of Env (8). In enzyme-linked immunosorbent assay experiments (data not shown), we confirmed that both MAbs bind the same C-heptad gp41 peptide (DP-178/T20, residues 638 to 673 of the HXB2 Env) but not an overlapping C-heptad gp41 peptide (C34, residues 628 to 661 of the HXB2 Env).
We first assessed MAb binding to native or receptor-triggered Env from the cell or virion surface (Fig. 2). Approximately 107 293T cells transiently expressing HXB2 Env were suspended in 500 μl of Dulbecco's modified Eagle's medium (DMEM) and were preincubated for 1 h at 37°C in the presence or absence of 2 to 4 μg of a soluble form of CD4 (sCD4; kindly provided by Ray Sweet, SmithKline Beecham, King of Prussia, Pa.) to enrich for fully triggered Env. We and others have shown that CD4 is sufficient for triggering Env into the six-helix bundle (6, 15). The transfection was performed by using the pSM-HXB2 and pREV expression plasmids and FUGENE 6 (Roche, Indianapolis, Ind.) as previously described (13). 2F5 (1 μg) or D50 containing supernatant (50 μl) was then added to cells and incubated for an additional hour at 37°C before being washed twice with phosphate-buffered saline to remove unbound antibodies. Cells were then lysed and immunoprecipitated with protein A-agarose beads (Roche) as previously described (38). The same assay was performed on HIV-1 pseudovirions (HXB2 Env) with approximately 1 μg of p24-containing pseudovirion stocks, prepared as previously described (38), except that unbound antibodies were eliminated by centrifugation (2 h at 20,000 × g) instead of washing.
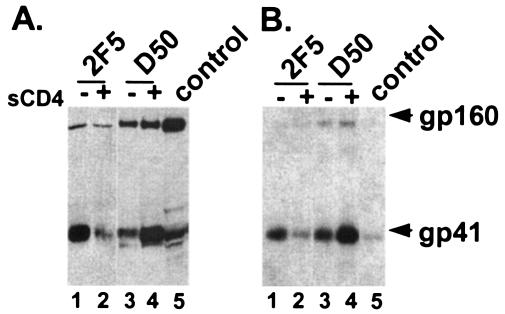
FIG. 2.
CD4 dependence of antibody binding to gp41. 2F5 and D50 MAbs were used to immunoprecipitate gp41 on the surface of Env-expressing cells (A) or virions (B) in the absence (−) or presence (+) of sCD4. Blots were probed with an antibody to gp41. Control is an immunoblot of Env-expressing cell lysate. Results shown are representative of at least three independent experiments.
These experiments showed that 2F5 preferentially binds native gp41 (prior to receptor activation) (Fig. 2A, lane 1) but that D50 prefers the triggered form after receptor activation (Fig. 2A, lane 4), consistent with some previous reports using flow cytometry assays (25, 31). Similar results were seen with intact virions (Fig. 2B). The close proximity of the two MAb epitopes on the linear sequence of gp41 combined with the opposite responses to pretreatment with sCD4 strongly argues that this region of gp41 undergoes major conformational changes after gp120 binds CD4. While the increased binding of the D50 MAb after receptor activation could be solely due to increased exposure of this region after gp120 triggering by CD4, the decreased binding of the 2F5 MAb after receptor activation makes this explanation less likely. Additionally, a recent report by Barbato et al. involving structural studies of gp41 peptides provides further support for conformational changes in this region (1). It is possible that conformational changes could involve interaction of the pretransmembrane domain with the membrane (1, 19, 34), which could disrupt the 2F5 epitope, but our experiments do not directly address this issue.
We then investigated binding of the MAbs to the gp41 fusion intermediate. Receptor-activated gp41 was trapped with peptides corresponding to the C-heptad repeat (C34, which lacks the 2F5 core epitope) or the N-heptad repeat (N36) (Fig. 1A) before immunoprecipitation with the MAbs. These peptides are potent inhibitors of HIV entry (16, 21, 39, 40) and have been shown to preferentially bind gp41 after receptor activation (11, 13, 18), mimicking interactions in the six-helix bundle. By a dominant-negative mechanism, the C peptide binds the N-heptad repeat of gp41 (Fig. 1B) and the N peptide binds the C-heptad repeat of gp41, preventing formation of the six-helix bundle using the endogenous heptad repeats from gp41 (reviewed in reference 5). One hour prior to immunoprecipitation with D50 (50 μl) or 2F5 (1.5 μg), 500 μl of 107 CHO Env-expressing cells (36) was preincubated with 2 to 4 μg sCD4 and 10 μg of either N36 or C34 inhibitory gp41 to trap the fusion intermediate. Significantly, the loss of the 2F5 epitope is prevented when the fusion intermediate is trapped with peptides (Fig. 3). This effect is more pronounced when the fusion intermediate is trapped with the C peptide (Fig. 3A, lane 4) than it is with the N peptide (Fig. 3A, lane 6), despite apparently comparable abilities of the N and C peptides to trap gp41 when excess peptides are used (13).
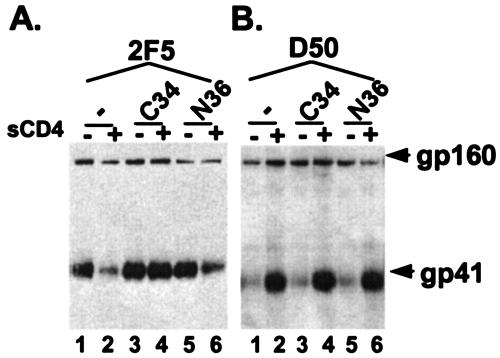
FIG. 3.
Antibody binding to the fusion intermediate. 2F5 (A) and D50 (B) MAbs were used to immunoprecipitate peptide-trapped Env from gp41 expressed on cell surface with (+) or without (−) sCD4 in the absence (−) or presence (+) of the C34 or N36 peptide. The C34 peptide binds the N heptad of gp41, and the N36 peptide binds the C heptad of gp41, preventing formation of the six-helix bundle using endogenous heptad repeats from gp41. Blots were probed with an antibody to gp41. Results shown are representative of at least three independent experiments.
The results with the trapped fusion intermediates show that the 2F5 epitope remains present shortly after gp120 has been triggered by CD4 but may be lost by the time the six-helix bundle has fully formed. This finding further suggests that the C-heptad repeat region of gp41 may need to interact with the endogenous N heptad of gp41 in order to change to a conformation that abolishes the 2F5 epitope. The hypothesis is consistent with the observation that the C peptide, which binds the N heptad of gp41 (13, 29), is better at preserving the 2F5 epitope in the triggered conformation than is the N peptide, which binds the C heptad of gp41 (13, 30) (Fig. 3). Because the N peptide probably binds the C-heptad repeat near the 2F5 epitope to make a six-helix bundle-like structure, the N peptide may facilitate helix formation in the C heptad, and in doing so may perturb the conformation of the 2F5 epitope. This possibility was suggested in a previous study involving 2F5 binding to gp41 peptides (12). Our demonstration that the 2F5 epitope is present on the fusion intermediate agrees with two publications that used different assays (9, 10), but there are inconsistencies in the literature on this point (1).
In contrast to 2F5, no significant changes were seen with immunoprecipitations using D50 when Env was first triggered and trapped with either peptide (Fig. 3B, lanes 1 to 6). Thus, it seems that the D50 epitope is equally present in the fusion-intermediate and six-helix bundle conformations, suggesting a linear epitope. The finding that the C peptide does not increase immunoprecipitation by D50 is also significant, because it rules out the possibility that the increased immunoprecipitation of gp41 by 2F5 is due to stabilization of gp41 oligomers as a result of interactions with the C heptad.
Our data lead us to propose that the C-heptad/pretransmembrane region may not be in a complete helical structure in the native conformation of Env. Instead, helicity may increase as gp41 folds into the six-helix bundle, perhaps as the gp41 C heptad interacts with its N heptad. Such a scenario is reminiscent of the spring-loaded mechanism of fusion activation for influenza hemagglutinin (3). This hypothesis is also supported by peptide (26) and recombinant protein (14) studies, suggesting that the 2F5 epitope is in a β-turn-like conformation. On the other hand, other studies indicate that the 2F5 epitope is present in helical peptides (2, 17, 32). Based on these peptide models, peptides were chemically constrained to be either helical or β-turn (17, 23), but broadly potent neutralizing antibodies have not been produced from these immunogens. In this regard, our data support an alternative strategy for eliciting 2F5-like antibodies based on exposure of the 2F5 epitope in the fusion intermediate.
Finally, the peptide-trapping studies suggested to us that the mechanism of 2F5 neutralization does not involve fixing Env in the native conformation but instead involves interfering with Env-mediated entry at some later step, after initial receptor-induced conformational changes. To investigate whether the 2F5 MAb neutralizes HIV-1 by preventing formation of the six-helix bundle, we pretreated 500 μl of CHO-Env-expressing cells with 10.5 μg of the 2F5 MAb before receptor activation (5 μl of sCD4) and immunoprecipitated gp41 with two sets of anti-six-helix bundle antibodies (Fig. 4). The first antibody is the NC-1 MAb (15), which was raised against a recombinant gp41 (35); it binds a conformational epitope in the six-helix bundle and is nonneutralizing (15). The second antibody set is a pair of polyclonal anti-peptide sera (α-b1 and α-b2) that preferentially bind the six-helix bundle (6). It was previously shown that sCD4 triggers formation of the fusion intermediate and six-helix bundle with the HXB2 Env (11, 15). Presumably, displacement of gp120 from gp41 by sCD4 is sufficient to allow gp41 to fold into its most thermostable conformation, though the HXB2 gp120 may be more easily released from gp41 than some other HIV-1 strains. Surprisingly, the presence of high concentrations of 2F5 had no effect on the ability of increasing concentrations of the NC-1 (Fig. 4A, lanes 1 to 7) or the purified sera against the six-helix bundle (Fig. 4A, lanes 9 to 12 and 15 to 18) to bind gp41. Similarly, when increasing amounts of the 2F5 MAb were added to Env-expressing cells that were subsequently incubated with NC-1 in the presence or absence of sCD4, we detected no changes in the amount of gp41 immunoprecipitated by NC-1 (Fig. 4B, lanes 4 to 6). As shown in negative control lines, we were able to selectively pull down gp41 with the mouse NC-1 MAb (Fig. 4A, lane 7) or the rabbit sera (Fig. 4A, lanes 13 and 19) rather than the human 2F5 MAb by using species-specific magnetic beads.
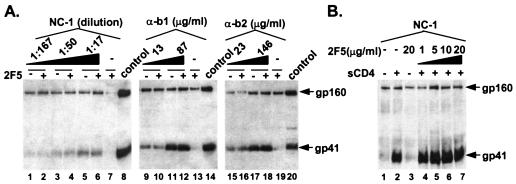
FIG. 4.
Antibodies binding to the six-helix bundle after pretreatment with 2F5. (A) Increasing concentrations of the NC-1 MAb or anti-six-helix bundle antibodies (α-b1, α-b2) are able to immunoprecipitate gp41 equally well in the presence or absence of pretreatment with 2F5. (B) Increasing concentrations of 2F5 do not impair the ability of the NC-1 to precipitate gp41. Blots were probed with an antibody to gp41. Control is an immunoblot of Env-expressing cell lysate. Results shown are representative of at least three independent experiments.
Together these results suggest that 2F5 does not strictly prevent folding of gp41 into the six-helix bundle, nor does it enhance six-helix bundle formation by prematurely triggering the fusion-active state. These observations were unexpected but can be explained in several ways. Although unlikely, we cannot rule out the possibility that the NC-1 monoclonal and our two sets of polyclonal antibodies recognize epitopes present in an incomplete six-helix bundle that is not fully formed. It is also possible that despite using high concentrations of 2F5, only a small fraction of Env are bound by 2F5, which would be enough to inhibit fusion but not enough to reduce triggering of a majority of Envs. This scenario would suggest that 2F5 neutralization is extremely efficient; 2F5 may only need to bind a few Envs to neutralize virus. Nonetheless, our data indicate that neutralization by 2F5 involves a novel mechanism that apparently impairs a post-fusion-intermediate step in the fusion process. Perhaps when membrane-bound receptors activate Env, as happens during natural infection, attachment of 2F5 to gp41 impedes close apposition of the target and viral membranes and/or interaction of the ectodomain of gp41 with membranes. In this case, interference of membrane merger by the antibody could impair complete formation of the six-helix bundle (22) or interfere with post-six-helix fusion events that involve bending and ordering of multiple fusion-active Envs to form a fusion pore. This hypothesis is supported by recent reports of two other potent neutralizing antibodies, 4E10 and Z13, which bind near the 2F5 epitope in the pretransmembrane region (33, 41) and could conceivably neutralize by a similar mechanism. 4E10, in contrast to 2F5, binds a linear epitope in both native and triggered conformations (41; data not shown) and would therefore not be expected to directly prevent formation of the six-helix bundle.
In summary, we have used membrane-anchored Env and antibodies in physiological conditions to show that the C-heptad repeat region of gp41 undergoes conformational changes after gp120 binds the receptor. We also demonstrated that 2F5 appears to bind both native and fusion-intermediate conformations of gp41 and neutralizes virus at a relatively late step in virus entry. These findings provide insights into the mechanism of Env-mediated membrane fusion and suggest ways to interfere with this process.
Acknowledgments
We thank Ira Berkower, Keith Peden, and Hana Golding (Center for Biologics Evaluation and Research [CBER], Bethesda, Md.) for critical reading of the manuscript, Nga Nguyen for peptide production (CBER Facility for Biotechnology Resources, Bethesda, Md.), and Pat Earl (NIH, Bethesda, Md.) for providing the D50 MAb.
We also acknowledge the support of the NIH Intramural AIDS Targeted Antiviral Program.
REFERENCES
- 1.Barbato, G., E. Bianchi, P. Ingallinella, W. H. Hurni, M. D. Miller, G. Ciliberto, R. Cortese, R. Bazzo, J. W. Shiver, and A. Pessi. 2003. Structural analysis of the epitope of the anti-HIV antibody 2F5 sheds light into its mechanism of neutralization and HIV fusion. J. Mol. Biol. 330:1101-1115. [DOI] [PubMed] [Google Scholar]
- 2.Biron, Z., S. Khare, A. O. Samson, Y. Hayek, F. Naider, and J. Anglister. 2002. A monomeric 3(10)-helix is formed in water by a 13-residue peptide representing the neutralizing determinant of HIV-1 on gp41. Biochemistry 41:12687-12696. [DOI] [PubMed] [Google Scholar]
- 3.Carr, C. M., and P. S. Kim. 1993. A spring-loaded mechanism for the conformational changes of influenza hemagglutinin. Cell 73:823-832. [DOI] [PubMed] [Google Scholar]
- 4.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 5.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 6.de Rosny, E., V. Vassell, P. T. Wingfield, C. T. Wild, and C. D. Weiss. 2001. Peptides corresponding to the heptad repeat motifs in the transmembrane protein (gp41) of human immunodeficiency virus type 1 elicit antibodies to receptor-activated conformations of the envelope glycoprotein. J. Virol. 75:8859-8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Earl, P. L., C. C. Broder, R. W. Doms, and B. Moss. 1997. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J. Virol. 71:2674-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earl, P. L., C. C. Broder, D. Long, S. A. Lee, J. Peterson, S. Chakrabarti, R. W. Doms, and B. Moss. 1994. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J. Virol. 68:3015-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finnegan, C. M., W. Berg, G. K. Lewis, and A. L. DeVico. 2002. Antigenic properties of the human immunodeficiency virus transmembrane glycoprotein during cell-cell fusion. J. Virol. 76:12123-12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Follis, K. E., S. J. Larson, M. Lu, and J. H. Nunberg. 2002. Genetic evidence that interhelical packing interactions in the gp41 core are critical for transition of the human immunodeficiency virus type 1 envelope glycoprotein to the fusion-active state. J. Virol. 76:7356-7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuta, R. A., C. T. Wild, Y. Weng, and C. D. Weiss. 1998. Capture of an early fusion-active conformation of HIV-1 gp41. Nat. Struct. Biol. 5:276-279. [DOI] [PubMed] [Google Scholar]
- 12.Gorny, M. K., and S. Zolla-Pazner. 2000. Recognition by human monoclonal antibodies of free and complexed peptides representing the prefusogenic and fusogenic forms of human immunodeficiency virus type 1 gp41. J. Virol. 74:6186-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, Y., R. Vassell, M. Zaitseva, N. Nguyen, Z. Yang, Y. Weng, and C. D. Weiss. 2003. Peptides trap the human immunodeficiency virus type 1 envelope glycoprotein fusion intermediate at two sites. J. Virol. 77:1666-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho, J., K. S. MacDonald, and B. H. Barber. 2002. Construction of recombinant targeting immunogens incorporating an HIV-1 neutralizing epitope into sites of differing conformational constraint. Vaccine 20:1169-1180. [DOI] [PubMed] [Google Scholar]
- 15.Jiang, S., K. Lin, and M. Lu. 1998. A conformation-specific monoclonal antibody reacting with fusion-active gp41 from the human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 72:10213-10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang, S., K. Lin, N. Strick, and A. R. Neurath. 1993. HIV-1 inhibition by a peptide. Nature 365:113. [DOI] [PubMed] [Google Scholar]
- 17.Joyce, J. G., W. M. Hurni, M. J. Bogusky, V. M. Garsky, X. Liang, M. P. Citron, R. C. Danzeisen, M. D. Miller, J. W. Shiver, and P. M. Keller. 2002. Enhancement of alpha-helicity in the HIV-1 inhibitory peptide DP178 leads to an increased affinity for human monoclonal antibody 2F5 but does not elicit neutralizing responses in vitro. Implications for vaccine design. J. Biol. Chem. 277:45811-45820. [DOI] [PubMed] [Google Scholar]
- 18.Kilgore, N. R., K. Salzwedel, M. Reddick, G. P. Allaway, and C. T. Wild. 2003. Direct evidence that C-peptide inhibitors of human immunodeficiency virus type 1 entry bind to the gp41 N-helical domain in receptor-activated viral envelope. J. Virol. 77:7669-7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kliger, Y., S. A. Gallo, S. G. Peisajovich, I. Munoz-Barroso, S. Avkin, R. Blumenthal, and Y. Shai. 2001. Mode of action of an antiviral peptide from HIV-1. Inhibition at a post-lipid mixing stage. J. Biol. Chem. 276:1391-1397. [DOI] [PubMed] [Google Scholar]
- 20.Kunert, R., W. Steinfellner, M. Purtscher, A. Assadian, and H. Katinger. 2000. Stable recombinant expression of the anti HIV-1 monoclonal antibody 2F5 after IgG3/IgG1 subclass switch in CHO cells. Biotechnol. Bioeng. 67:97-103. [DOI] [PubMed] [Google Scholar]
- 21.Lu, M., and P. S. Kim. 1997. A trimeric structural subdomain of the HIV-1 transmembrane glycoprotein. J. Biomol. Struct. Dyn. 15:465-471. [DOI] [PubMed] [Google Scholar]
- 22.Markosyan, R. M., F. S. Cohen, and G. B. Melikyan. 2003. HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol. Biol. Cell 14:926-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGaughey, G. B., M. Citron, R. C. Danzeisen, R. M. Freidinger, V. M. Garsky, W. M. Hurni, J. G. Joyce, X. Liang, M. Miller, J. Shiver, and M. J. Bogusky. 2003. HIV-1 vaccine development: constrained peptide immunogens show improved binding to the anti-HIV-1 gp41 MAb. Biochemistry 42:3214-3223. [DOI] [PubMed] [Google Scholar]
- 24.Muster, T., F. Steindl, M. Purtscher, A. Trkola, A. Klima, G. Himmler, F. Ruker, and H. Katinger. 1993. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 67:6642-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neurath, A. R., N. Strick, K. Lin, and S. Jiang. 1995. Multifaceted consequences of anti-gp41 monoclonal antibody 2F5 binding to HIV type 1 virions. AIDS Res. Hum. Retrovir. 11:687-696. [DOI] [PubMed] [Google Scholar]
- 26.Pai, E. F., M. H. Klein, P. Chong, and A. Pedyczak. October 2000. World Intellectual Property Organization patent WO-00/61618.
- 27.Parker, C. E., L. J. Deterding, C. Hager-Braun, J. M. Binley, N. Schulke, H. Katinger, J. P. Moore, and K. B. Tomer. 2001. Fine definition of the epitope on the gp41 glycoprotein of human immunodeficiency virus type 1 for the neutralizing monoclonal antibody 2F5. J. Virol. 75:10906-10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, A. Jungbauer, et al. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 10:1651-1658. [DOI] [PubMed] [Google Scholar]
- 29.Rimsky, L. T., D. C. Shugars, and T. J. Matthews. 1998. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J. Virol. 72:986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Root, M. J., M. S. Kay, and P. S. Kim. 2001. Protein design of an HIV-1 entry inhibitor. Science 291:884-888. [DOI] [PubMed] [Google Scholar]
- 31.Sattentau, Q. J., S. Zolla-Pazner, and P. Poignard. 1995. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology 206:713-717. [DOI] [PubMed] [Google Scholar]
- 32.Schibli, D. J., R. C. Montelaro, and H. J. Vogel. 2001. The membrane-proximal tryptophan-rich region of the HIV glycoprotein, gp41, forms a well-defined helix in dodecylphosphocholine micelles. Biochemistry 40:9570-9578. [DOI] [PubMed] [Google Scholar]
- 33.Stiegler, G., R. Kunert, M. Purtscher, S. Wolbank, R. Voglauer, F. Steindl, and H. Katinger. 2001. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 17:1757-1765. [DOI] [PubMed] [Google Scholar]
- 34.Suarez, T., W. R. Gallaher, A. Agirre, F. M. Goni, and J. L. Nieva. 2000. Membrane interface-interacting sequences within the ectodomain of the human immunodeficiency virus type 1 envelope glycoprotein: putative role during viral fusion. J. Virol. 74:8038-8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan, K., J. Liu, J. Wang, S. Shen, and M. Lu. 1997. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc. Natl. Acad. Sci. USA 94:12303-12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss, C. D., and J. M. White. 1993. Characterization of stable Chinese hamster ovary cells expressing wild-type, secreted, and glycosylphosphatidylinositol-anchored human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 67:7060-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 38.Weng, Y., and C. D. Weiss. 1998. Mutational analysis of residues in the coiled coil domain of the human immunodeficiency virus type-1 (HIV-1) gp41. J. Virol. 72:9676-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wild, C., T. Greenwell, and T. Matthews. 1993. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res. Hum. Retrovir. 9:1051-1053. [DOI] [PubMed] [Google Scholar]
- 40.Wild, C., T. Oas, C. McDanal, D. Bolognesi, and T. Matthews. 1992. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc. Natl. Acad. Sci. USA 89:10537-10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]