Abstract
Background
Bilateral breast reconstruction utilising autologous free tissue transfer is a complex procedure with multiple options for donor tissue available. Autogenous breast reconstruction techniques have evolved over the last three decades to meet this goal. The aim of this study was to determine the outcomes of patients undergoing bilateral breast reconstruction with DIEAP, TRAM or SIEA flaps.
Material/Methods
A prospective study was performed in our Interdisciplinary Breast Centre from July 2004 until December 2011 in 144 patients. Demographic information, diabetes mellitus type I status, tobacco use, tumor stage, primary/secondary reconstruction, operative technique, adjuvant therapy received, length of follow-up, and complications were evaluated. Complications were divided into donor site and recipient site. To investigate which risk factors were independently related to flap loss (complete or partial), multiple linear regression analysis was performed.
Results
The study identified 144 patients who had bilateral breast reconstruction with DIEAP, TRAM or SIEA flaps. For all flaps (n=248), outcome included 98.4% survival and 0.7% vein microanastomosis revision. Recipient site complications included 1.6% complete flap loss, 0.8% fat necrosis, 2.9% partial skin loss/dehiscence flap necrosis and 2.0% haematoma rate. Donor site complications included 3.7% partial skin loss/dehiscence. There was evidence of abdominal bulges in TRAM patients (1.1%) but no hernias in any patients. BMI is a major determinant of flap loss (complete or partial) in these patients.
Conclusions
The primary goal of bilateral breast reconstruction is to provide a treatment option that can create a natural, symmetric breast mounds with minimal donor-site morbidity following bilateral mastectomies. These results support weight loss therapy prior to bilateral breast reconstruction.
Keywords: breast cancer, bilateral autologous breast reconstruction, DIEAP, TRAM SIEA
Background
Choices for postablative reconstruction of the breast include implant reconstruction and/or autologous tissue flaps from various donor sites [1–5]. The role of breast reconstruction in the breast cancer patient is becoming more complex as the number of options for reconstruction available to the patient continues to increase [5]. This fact is compounded when one considers bilateral reconstruction in the breast cancer patient. Implantable prostheses with silicone gel or saline implants are a viable and popular option for reconstruction and are naturally associated with less operative and hospital time when compared with autologous tissue reconstruction, but the results deteriorate over time, especially in the setting of radiation therapy [5]. However, autologous tissue reconstruction allows for the creation of a soft, naturally textured and ptotic breast mound, which generally does not change with time [4–6]. Breasts reconstructed with the patient’s own tissue behave naturally, achieving the consistency of the native breast, becoming less oedematous and softer as the patient ages [4–6]. Breast reconstruction utilising free abdominal flaps, both free and pedicled, outperform implant-based breast reconstruction in aesthetic measures [7]. In bilateral cases, symmetry and aesthetics are consistently superior to unilateral reconstructions [6,9]. Use of autologous tissue is acceptable only if it can be performed with reasonable morbidity [5]. Given the abdominal donor-site morbidity associated with the free transverse rectus abdominis musculocutaneous (TRAM) procedure, free flaps utilising deep inferior epigastric artery perforator (DIEAP) flap have received considerable attention [5,6,9]. The DIEAP flap is harvested with preservation of the underlying musculofascial system [6,9]. Therefore, the principal advantages of and indications for the DIEAP flap are to preserve the rectus abdominis muscle and the anterior rectus sheath to reduce the incidence of abdominal morbidity such as bulge, hernia, and weakness [6,9]. Despite the advantages of the DIEAP flap, risks include flap failure, venous congestion, and fat necrosis, which are related to the blood supply of the DIEAP flap [6,9].
We have previously demonstrated in our series of over 700 flaps that DIEAP-flaps and fasciasparing (fs) TRAM-flaps are reliable flaps for unilateral breast reconstruction, resulting in highly aesthetic results and low donor site morbidity [6,9]. The aim of this study was to determine and compare the outcomes of patients undergoing simultaneous bilateral breast reconstruction with TRAM and DIEAP flaps.
Material and Methods
Data regarding patients who underwent simultaneous bilateral breast reconstruction with free abdominal flaps from 2004 to 2011 was prospectively collected (Table 1). Demographic information, diabetes mellitus type I status, tobacco use, tumor stage, primary/secondary reconstruction, operative technique (Table 2), adjuvant therapy received, length of follow-up, and complications were evaluated. Complications were divided into donor site and recipient site (Table 3). Specific abdominal donor-site complications assessed included hematoma, seroma, skin necrosis/dehiscence, bulging, and hernia formation. Recipient-site complications included hematoma, seroma, infection, fat necrosis, and flap loss. Abdominal bulging was defined as abdominal wall bulging without a fascia defect as assessed by physical examination and/or radiographic study. Abdominal hernia was defined as a fascia defect identified by physical examination and radiographic study and/or confirmed at the time of operative repair. Fat necrosis was defined as a palpable discrete firmness greater than 2 cm identified by physical examination during postoperative evaluation or by intraoperative finding for any subsequent procedure.
Table 1.
Patient demographics and history.
| DIEAP | TRAM/DIEAP/SIEA | |
|---|---|---|
| No. of patients | 75 | 69 |
| Age, years (mean) | 47.7±0.9 | 48.1±1.0 |
| Body mass index | 27.2±0.4 | 26.1±0.3 |
| Active smoker (%) | 18.7 | 7.2 |
| Diabetes mellitus type 1 (%) | 1.3% | 0 |
| Chemotherapy history (%) | 58.7 | 26.1 |
| Radiation history (%) | 62.7 | 34.8 |
| Primary reconstruction (%) | 40 | 40.8 |
| Secondary reconstruction (%) | 60 | 59.4 |
| Previous implant reconstruction (%) | 18.7 | 26.1 |
| Follow-up (years) | 2.5±0.2 | 3.9±0.2 |
Results are presented as mean ±SEM.
Table 2.
Description of the procedures performed on patients in each group.
| DIEAP patients | TRAM/DIEAP/SIEA patients | |
|---|---|---|
| Bilateral free TRAM | 17 | |
| Bilateral SIEA | 1 | |
| Bilateral DIEAP | 75 | |
| TRAM/DIEAP | 49 | |
| DIEAP/SIEA | 2 | |
| Total | 75 | 69 |
Table 3.
Complications following bilateral breast reconstruction.
| DIEAP | TRAM/DIEAP/SIEA | Overall | |
|---|---|---|---|
| Recipent site | |||
| Venous congestion requiring revision (%) | 0.7 | 0.7 | 0.7 |
| Complete flap loss (%) | 0 | 4.3 (2 out of 4 in SIEA flaps) | 1.6 |
| Fat necrosis (%) | 1.3 | 0 | 0.8 |
| Partial skin loss/dehiscence (%) | 4 | 1.1 | 2.9 |
| Haematoma (%) | 2 | 2.1 | 2.0 |
| Donor site | |||
| Hematoma (%) | 0 | 0 | 0 |
| Seroma (%) | 0 | 0 | 0 |
| Partial skin loss/dehiscence (%) | 4 | 3.2 | 3.7 |
| Hernia (%) | 0 | 0 | 0 |
| Abdominal bulge (%) | 0 | 1.1 | 0.4 |
Data was also collected regarding patients who had a recurrence of malignancy in the previously resected site.
Results were tabulated in a Microsoft Excel spreadsheet, Version 2007 (Microsoft Corp., Redmond, WA). To investigate which risk factors were independently related to flap loss (complete or partial), multiple linear regression analysis was performed using data from the bilateral breast reconstruction patients, and estimated effects, 95% CIs, and P values were tabulated. Multiple correlation coefficients were calculated as an overall measure of the relationship between risk factors and flap loss (complete or partial). In addition, multiple correlation coefficients were calculated as an overall measure of the relationship between risk factors and donor site partial skin loss/wound dehiscence. All statistical analyses were performed using Instat software Version 3.05 (GraphPad Software Inc., San Diego, CA). Unless otherwise indicated, results are shown as mean ±SEM., including 95% CIs where appropriate, and P<0.05 was considered significant.
Surgical technique
Preoperative markings are performed with the patient in the standing and supine positions. The superior margin of the flap is shifted slightly above the umbilicus to include periumbilical perforators (Figure 1). Vertical dimensions of the flap rarely exceed 12 cm, allowing for closure under minimal tension. Perforators were identified preoperatively using a Doppler probe (Mini Dopplex D900, Huntleigh Healthcare Ltd., Cardiff, United Kingdom) until July 2008 and from August 2008 to January 2010 using Color flow Duplex ultrasonography (Phillips iU22 (Philips GmbH, Hamburg, Germany) apparatus with a 12.5 MHz linear array [10]. From January 2010 until now we are using a CT scan angiography protocol to determine the perforators preoperatively (Data in progress).
Figure 1.
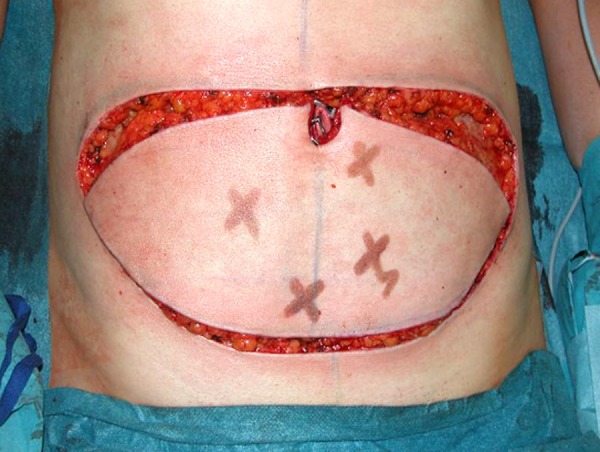
Perioperative Markings depending on the perioperative Doppler detection.
For immediate reconstruction, suggested markings are made for the skin-sparing mastectomy. The inframammary crease is marked for reference. Using a 2-team approach, the DIEAP flaps are elevated during the mastectomy. The internal mammary vessels at the level of the third or fourth rib are the recipient vessels of choice. The pectoralis major muscle is divided to expose the costal cartilage. The perichondrium is elevated to allow removal of 2 cm of cartilage. The posterior perichondrium is opened to expose the internal mammary vessels. In failed implant cases, a pericapsular dissection is preferred.
The DIEAP flap is elevated from lateral to medial in a suprafascia plane. The superficial inferior epigastric vein (SIEV) is preserved. Once the lateral perforators are identified, the panniculus is split down the midline into 2 flaps. Skin and fat are elevated from the midline laterally until the medial row perforators are seen. The largest perforators are selected (Figure 2). The anterior rectus sheath is opened around the perforating vascular bundle, allowing the perforators to be traced to the deep inferior epigastric vessels. Intercostal nerves are left intact to avoid denervating the muscles medially. The rectus sheath and muscle is separated to allow isolation of the pedicle (Figure 2). After dividing the pedicle, the flap is brought to the chest for anastomosis to the internal mammary vessels [6,9,11–14].
Figure 2.
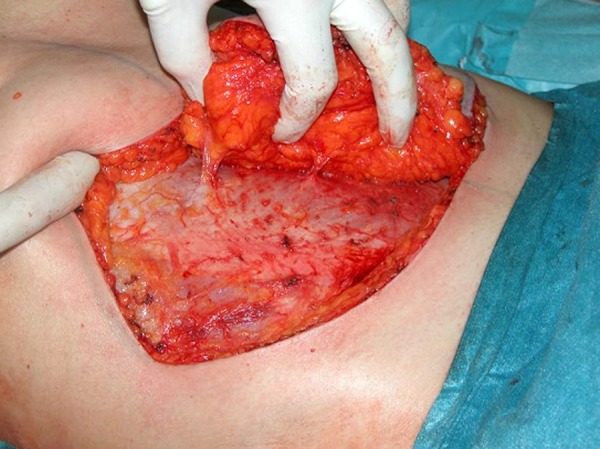
Selection of Perforators on the left side of the abdomen.
Vessel anastomosis is performed with 9–0 nylon for both the artery and vein. The flaps are tailored to desired breast shape and contour. Each opening in the rectus sheath is closed without tension (Figure 3). The abdominal apron is advanced and closed in routine fashion with superficial fascia system repair (Figure 4) [15].
Figure 3.
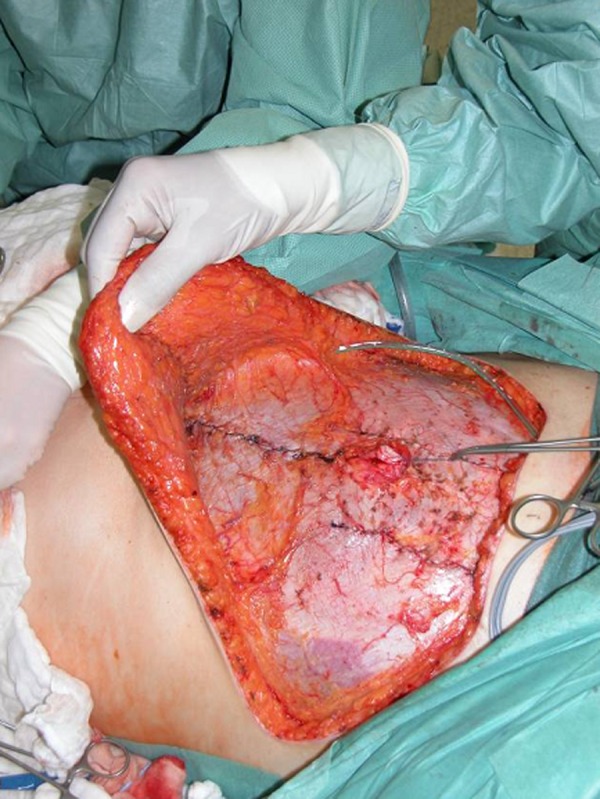
Morbidity of the donor site after bilateral DIEAP flaps. Wide abdominal rectus plication has been performed to achieve better cosmesis.
Figure 4.
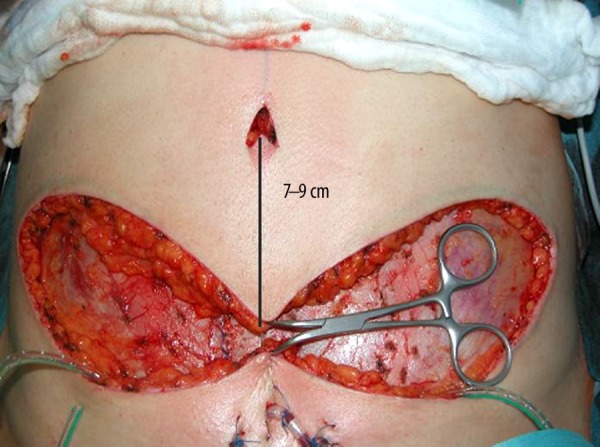
Closing the abdomen with inserting the belly button in the middle approximately 7–9 cm from the horizontal scar.
Results
The mean age was 47±1.0 years, 13.2% of patients had a recent history of actively smoking tobacco and the mean BMI was 26.9±0.4. Additional demographic details are given in Table 1. We devided our patients in two groups: The DIEAP group and the TRAM/DIEAP/SIEA group. The DIEAP group included a bilateral reconstruction with DIEAP flaps in one patient only. The TRAM/DIEAP/SIEA group included either TRAM lfaps, DIEAP flaps or SIEA flaps in one patient (Table 2). The internal mammary vessels were used in all flaps. The most common reason for performing a secondary reconstruction was capsular fibrosis or failed implants (22.2%), while 40.3% of flaps were performed in unreconstructed mastectomy patients. Most patients were staged at T1/T2 level, with only 5 T3 tumors. The average follow-up period was 2.5±0.2 years in the DIEAP group and 3.9±0.2 years in the TRAM/DIEAP/SIEA group. Twenty four patients (16.7%) experienced a total of 26 complications. Two patients experienced 2 complications, and 22 patients experienced a single complication. Twenty three patients experienced early complications (Figure 5) (Table 3). Eleven patients (0.8%) experienced postoperative complications. All of them returned to the operating theatre: 6 venous thromboses and 4 haematomas.
Figure 5.
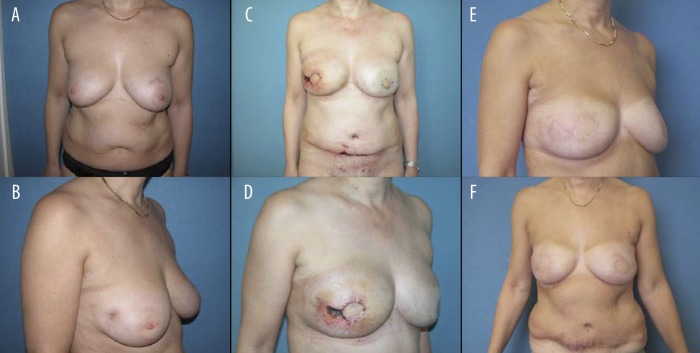
(A, B) Bilateral breast cancer, bilateral breast conserving therapy, Radiotherapy both sides. (C, D) 11 days after bilateral Skin sparing mastectomy and reconstruction with DIEAP flaps. Wound healing problems with skin necrosis on the original breast skin and at the abdominal scar. (E, F) One year after surgery with complete healing of the wounds.
Four (1.4%) complete flap losses occurred; all of which were in the TRAM/DIEAP/SIEA group. However, 2 of these complete flap losses were found in one patient reconstructed with the SIEA flap alone. This was in our hands a complete flap loss of 50% in SIEA flaps.
A small dehiscence occurred in 7 flaps (2.4%). Fat necrosis was found in 2 DIEAP flaps (0.8%) in 2 patients. Patients with partial flap loss were considered to have fat necrosis. One patient with a TRAM flap developed abdominal bulging (0.3%) (Table 3). One patient (0.7%) experienced local cancer recurrence, 26 months post reconstruction.
Multiple regression analysis using risk factors that could be related to flap loss (complete or partial) in patients undergoing bilateral breast reconstruction with TRAM, DIEAP or SIEA flaps was conducted on the total population studied (144 patients). The analysis identified body mass index as a highly significant predictor of flap loss (complete or partial) in patients undergoing bilateral breast reconstruction (P=0.002).
There was no evidence that age, active smoking, diabetes status, and previous radiotherapy contributed to flap loss (complete or partial) (Table 4). In addition, there was no evidence that age, active smoking, BMI or diabetes status contributed to donor site partial skin loss/wound dehiscence (data not included).
Table 4.
Analysis of the relationship between risk factors and flap loss (complete or partial) in patients undergoing bilateral breast reconstruction with TRAM, DIEAP or SIEA flaps.
| Variable | Complete or partial flap loss | |
|---|---|---|
| P | β coefficient (CI) | |
| Age (years) | 0.751 | 0.001 (−0.002, 0.003) |
| BMI (kg/m2) | 0.002 | 0.014 (0.005, 0.024) |
| Smoking (0=no, 1=yes) | 0.844 | 0.051 (−0.069, 0.171) |
| Diabetic (0=no, 1=yes) | 0.662 | −0.088 (−0.489, 0.312) |
| Radiotherapy (0=no, 1=yes) | 0.189 | −0.047 (−0.119, 0.0237) |
Analysis of variance p-values for the null hypothesis of no relationship between risk factors and complete or partial flap loss are displayed. Regression coefficients (β coefficient) and their 95% Confidence Intervals (CI) for the risk factors are also given. The coefficient of determination (R2) for this analysis is 10.0%. BMI, Body mass index. Smoking refers to current active smoking.
Discussion
Muscle-sparing abdominal flaps have evolved in an effort to minimize abdominal morbidity while continuing to provide the benefits of autologous breast restoration. In particular, the DIEAP flap is harvested with preservation of the underlying musculofascia system of the abdominal wall. Reports of the DIEAP flap are encouraging, demonstrating lower abdominal wall morbidity together with highly aesthetic breast reconstructions [4–9]. However, it has been questioned whether perforator dissection is of benefit when compared with other muscle-sparing flaps [4,5,9]. Whether DIEAP flap reconstruction can be accomplished safely in the setting of bilateral reconstruction must be critically analyzed. We have carried out bilateral breast reconstruction with abdominal free flaps with an acceptable overall morbidity in 144 patients.
No correlation was found between age, smoking, diabetes, radiation, and the incidence of flap loss (complete or partial) (Table 4). BMI is a major determinant of flap loss (complete or partial) in these patients. Focus on the rate of fat necrosis with the DIEAP flap has increased over the last few years. Previously reported fat necrosis rates for series of bilateral breast reconstruction with abdominal flaps range from 11–27% [16–18]. These numbers are at odds with our series (1.3% DIEAP flaps experienced fat necrosis but no TRAM/DIEAP/SIEA patients) and this may be due to the comparatively earlier experience with deep inferior epigastric artery perforator at these departments. We must be aware of that complication rates are much higher in the beginning with a steep learning curve in time and dependability, regardless which operation being performed. In this series, using stringent inclusion criteria, we did not experience such high rates of fat necrosis, this may be due to our attention to preventing excessive thinning of skin flaps, improper use of electrocautery, and excessive tension on skin flaps in an attempt to increase breast projection. The elevated rate of fat necrosis in these studies may be due to the inability to turn to the other side of the abdomen and choose the most ideal perforator(s) to carry the flap. There is no “safety net” with the use of bilateral flaps. Each flap must be transferred on ipsilateral perforating vessels, which are occasionally less than ideal.
The morbidity to the abdominal wall is largely eliminated with preservation of muscle and fascia. Complete closure of the abdominal wall can be accomplished without tension and without using a mesh. Only 0.4% patients in this series experienced abdominal hernias or bulges; all were bilateral TRAM patients. A study of bilateral free TRAM flaps reported an incidence of hernia and bulge formation of 11.6%, almost 30 times our rates [19]. A study of bilateral free DIEAP flaps reported an incidence of hernia and bulge formation of 6%, which was an improvement on the aforementioned bilateral free TRAM flap series [18]. In our personal experience, true hernias have never occurred in unilateral or bilateral DIEAP flaps. Bulges are certainly seen following DIEAP flap procedures, particularly during the learning curve before the importance of preserving the segmental motor supply of the rectus is understood. Hernias can have an important impact on abdominal wall functioning, chronic pain, and the risk for repetitive corrective surgery. We believe that the risk of hernia formation is independent of the type of TRAM flap used, as long as secure fascia repair is performed. The main advantages in preserving the musculofascia components with the DIEAP flap are preservation of the rectus muscle which has been shown to reduce postoperative pain and shorten hospital stay [20,21]. We have previously demonstrated that refinements in technique lowered the time involved with bilateral DIEAP flap reconstruction [9].
Use of the internal mammary vessels allows medial placement of the flap with greater comfort for the assistant during anastomosis and leads to a more aesthetic breast restoration.
Dissection of the SIEV as an outlet for venous congestion facilitates postoperative flap management.
With regard to coagulation, we always administer heparin 2500 IU intraoperatively, after completion of the first anastomosis. At the start of our experience, we noted that administration of heparin 2500 IU twice intraoperatively, after completion of the first and second flap caused excessive bleeding.
In our microsurgically-orientated institution, abdominal free tissue transfer is the favoured method of breast reconstruction. Our preference towards flaps is based on our opinion that, in long term, living tissue might has less complication over long time period compared to implants. We have been using the DIEAP flap as our workhorse for autogenous breast reconstruction since 2004 [6,9]. This flap receives its blood supply via indirect perforating vessels originating from the deep inferior epigastric artery. These vessels can be dissected while sparing the musculofascial system of the abdominal wall to nourish the flap. The experience presented demonstrates that the DIEAP flap fares well in bilateral breast reconstruction when measured against other reconstructive options. However, in our hands the SIEA flaps are abandoned in our reconstructive practice, regardless of the SIEV vessels found. As we described we had a complete flap loss in SIEA flaps of 50% (2 out of 4), unacceptable for elective reconstructions with a high patient satisfaction demand (Figures 6 and 7).
Figure 6.
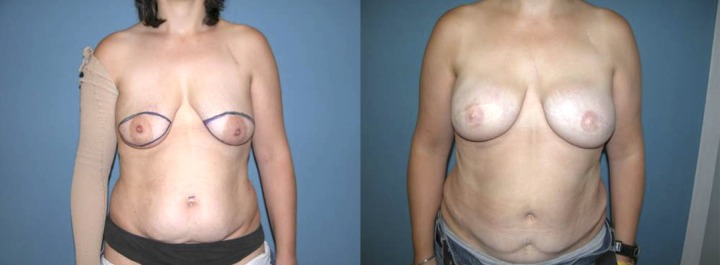
Bilateral reconstruction with DIEAP flaps after skin sparing mastectomy.
Figure 7.
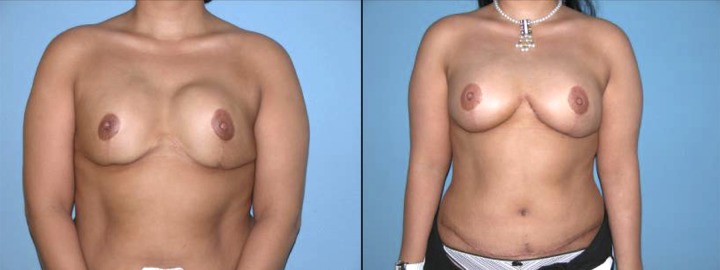
Bilateral reconstruction with DIEAP flaps after removing the capsular contracture and the implants.
BMI increases the incidence of flap loss (complete or partial). With a high rate of success, low fat necrosis and low abdominal wall morbidity, bilateral breast reconstruction with abdominal flaps seems well suited for patients in the high-risk category for bilateral breast carcinoma, and these patients will receive maximal benefit from having preservation of their abdominal wall. The DIEAP flap represents a natural progression in the evolution of breast reconstruction techniques. The time required for perforator dissection is worthwhile because it eliminates virtually all of the major abdominal morbidity, reduces postoperative pain, and can shorten hospital stay. Patients with an elevated body mass index should be cautioned regarding a potential increased risk of complications [22].
With many patients requiring bilateral reconstruction for a myriad of reasons and the high satisfaction rate associated with mastectomy procedures, the need for reconstruction with autologous tissue will likely continue to be in high demand [4]. Considering the multitude of reconstructive options available to the plastic surgeon, one must weigh the potential risks and benefits associated with these flap options in the patient requesting bilateral breast reconstruction [4].
Conclusions
Our prospective study demonstrates that bilateral autologous breast reconstruction can be performed safely and effectively. This is true of both free TRAM procedures, as well as procedures which aim to reduce donor-site morbidity in the form of DIEAP flaps. However, SIEA flaps are being abandoned in our service due to high complication rate. The DIEAP procedures are routinely performed in our institution and will likely continue to increase in number due to a better understanding of the procedures in patients and physicians.
Footnotes
Source of support: Departmental sources
References
- 1.Al-Benna S, Steinstraesser L. Postablative reconstruction is better terminology than oncoplastic surgery. Plast Reconstr Surg. 2009;124:463e–64e. doi: 10.1097/PRS.0b013e3181bf7fe3. [DOI] [PubMed] [Google Scholar]
- 2.Audretsch W, Andree C. Is mastectomy still justified – and if, in which patients? Onkologie. 2006;29:243–45. doi: 10.1159/000093477. [DOI] [PubMed] [Google Scholar]
- 3.Al-Benna S, Poggemann K, Steinau HU, Steinstraesser L. Diagnosis and Management of Primary Breast Sarcoma. Breast Cancer Res Treat. 2010;22(3):619–26. doi: 10.1007/s10549-010-0915-y. [DOI] [PubMed] [Google Scholar]
- 4.Bach AD, Kneser U, Kopp J, Andree C, et al. Possibilities for breast reconstruction following cancer surgery. MMW Fortschr Med. 2004;146:40–42. 44. [PubMed] [Google Scholar]
- 5.Al-Benna Female plastic and reconstructive surgeons’ personal decision making for breast cancer treatment and reconstruction. Arch Gynecol Obstet. 2011;284:737–41. doi: 10.1007/s00404-010-1721-9. [DOI] [PubMed] [Google Scholar]
- 6.Seidenstuecker K, Munder B, Mahajan AL, et al. Morbidity of microsurgical breast reconstruction in patients with comorbid conditions. Plast Reconstr Surg. 2011;127:1086–92. doi: 10.1097/PRS.0b013e318205f255. [DOI] [PubMed] [Google Scholar]
- 7.Alderman AK, Wilkins EG, Lowery J, et al. Determinants of patient satisfaction in post-mastectomy breast reconstruction. Plast Reconstr Surg. 2000;106:769–76. doi: 10.1097/00006534-200009040-00003. [DOI] [PubMed] [Google Scholar]
- 8.Andree C, Munder BI, Behrendt P, et al. Improved safety of autologous breast reconstruction surgery by stabilisation of microsurgical vessel anastomoses using fibrin sealant in 349 free DIEP or fascia-muscle-sparing (fms)-TRAM flaps: a two-centre study. Breast. 2008;17:492–98. doi: 10.1016/j.breast.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Langer S, Munder B, Seidenstuecker K, et al. Development of a surgical algorithm and optimized management of complications – based on a review of 706 abdominal free flaps for breast reconstruction. Med Sci Monit. 2010;16(11):CR518–22. [PubMed] [Google Scholar]
- 10.Seidenstuecker K, Munder B, Richrath P, et al. A prospective study using color flow duplex ultrasonography for abdominal perforator mapping in microvascular breast reconstruction. Med Sci Monit. 2010;16(8):MT65–70. [PubMed] [Google Scholar]
- 11.Al-Benna S, Steinstraesser L, Patani N, et al. Free flap breast reconstruction consent forms should warn against the potential loss of the internal thoracic artery for coronary artery bypass grafting. Plast Reconstr Surg. 2012;129(5):867e–68e. doi: 10.1097/PRS.0b013e31824a9e95. [DOI] [PubMed] [Google Scholar]
- 12.Al-Benna S, Al-Busaidi SS, Papadimitriou G, et al. Abdominoplasty consent forms do not caution against the potential loss of a reconstructive option for breast reconstruction. Plast Reconstr Surg. 2009;123:208e–9e. doi: 10.1097/PRS.0b013e3181a8490a. [DOI] [PubMed] [Google Scholar]
- 13.Al-Benna S. Caution note on the use of the internal mammary artery in breast reconstruction. Plast Reconstr Surg. 2007;120(1):348. doi: 10.1097/01.prs.0000264563.48652.a6. [DOI] [PubMed] [Google Scholar]
- 14.Al-Benna S, Grob M, Mosahebi A, et al. Caution note on the use of the internal mammary artery in breast reconstruction. Plast Reconstr Surg. 2006;117:1653–54. doi: 10.1097/01.prs.0000208865.31910.48. [DOI] [PubMed] [Google Scholar]
- 15.Al-Benna S, Al-Ajam Y, Tzakas E, et al. Superficial fascial system repair: an abdominoplasty technique to reduce local complications after caesarean delivery. Arch Gynecol Obstet. 2009;279:673–75. doi: 10.1007/s00404-008-0802-5. [DOI] [PubMed] [Google Scholar]
- 16.Vega SJ, Bossert RP, Serletti JM. Improving outcomes in bilateral breast reconstruction using autogenous tissue. Ann Plast Surg. 2006;56:487–90. doi: 10.1097/01.sap.0000205236.88313.10. [DOI] [PubMed] [Google Scholar]
- 17.Drazan L, Vesely J, Hyza P, et al. Bilateral breast reconstruction with DIEP flaps: 4 years’ experience. J Plast Reconstr Aesthet Surg. 2008;61:1309–15. doi: 10.1016/j.bjps.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Chun YS, Sinha I, Turko A, et al. Comparison of morbidity, functional outcome, and satisfaction following bilateral TRAM versus bilateral DIEP flap breast reconstruction. Plast Reconstr Surg. 2010;126:1133–41. doi: 10.1097/PRS.0b013e3181ea42d3. [DOI] [PubMed] [Google Scholar]
- 19.Khouri RK, Ahn CY, Salzhauer MA, et al. Simultaneous bilateral breast reconstruction with the transverse rectus abdominis musculocutaneous free flap. Ann Surg. 1997;226:25–34. doi: 10.1097/00000658-199707000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroll SS, Sharma S, Koutz C, et al. Postoperative morphine requirements of free TRAM and DIEP flaps. Plast Reconstr Surg. 2001;107:338–41. doi: 10.1097/00006534-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan JL, Allen RJ. Cost-based comparison between perforator flaps and TRAM flaps for breast reconstruction. Plast Reconstr Surg. 2000;105:943–48. doi: 10.1097/00006534-200003000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Al-Benna S. Perioperative management of morbid obesity. J Perioper Pract. 2011;21:225–33. doi: 10.1177/175045891102100701. [DOI] [PubMed] [Google Scholar]
- 23.Al-Benna S, Rajgarhia P. Blood Transfusion Requirements in Elective Breast Reconstruction Surgery. The Breast. 2010;19:475–78. doi: 10.1016/j.breast.2010.05.006. [DOI] [PubMed] [Google Scholar]