Abstract
Background
Modifications to combination antiretroviral drug therapy (CART) regimens can occur for a number of reasons, including adverse drug effects. We investigated the frequency of and reasons for antiretroviral drug modifications (ADM) during the first 3 years after initiation of CART, in a closed cohort of CART-naïve adult patients who started treatment in the period 1998–2007 in Croatia.
Material/Methods
We calculated differential toxicity rates by the Poisson method. In multivariable analysis, we used a discrete-time regression model for repeated events for the outcome of modification due to drug toxicity.
Results
Of 321 patients who started CART, median age was 40 years, 19% were women, baseline CD4 was <200 cells/mm3 in 71%, and viral load was ≥100 000 copies/mL in 69%. Overall, 220 (68.5%) patients had an ADM; 124 (56%) of these had ≥1 ADM for toxicity reasons. Only 12.7% of individuals starting CART in the period 1998–2002 and 39.4% in the period 2003–2007 remained on the same regimen after 3 years. The following toxicities caused ADM most often: lipoatrophy (22%), gastrointestinal symptoms (20%), and neuropathy (18%). Only 5% of drug changes were due to virologic failure. Female sex (hazard ratio [HR], 2.42 95%; confidence intervals, 1.39–4.24) and older age (HR, 1.42 per every 10 years) were associated with toxicity-related ADM in the first 3 months of a particular CART regimen, but after 3 months of CART they were not.
Conclusions
Less toxic and better-tolerated HIV treatment options should be available and used more frequently in Croatia.
Keywords: HIV-infected patients, HIV-infection, toxicity, adverse events, virologic failure, antiretroviral therapy
Background
Adverse effects of combination antiretroviral therapy (CART) lead to switching or interruption of HIV therapy and also may affect adherence to prescribed medications [1–14]. Antiretroviral drug modifications (ADM) due to toxicity have been reported to be associated with many factors: demographic characteristics such as female sex [4–6,13,14], age [6,14], ethnicity [15,16], genetics [17], HIV disease status [4,6], type of antiretroviral therapy [4–6,14], and co-morbidities [5]. It has also been reported that toxicity-related ADM are most frequent within the first 3 months of CART [7]. The majority of studies, however, have focused on the first event of ADM [1–3,5–8,14,18].
Croatia is a southeastern European country (population 4.3 million) with a centralized system of care for HIV since the beginning of the epidemic in 1986. This allows us to analyze ADM on a country level. Croatia has an individualized approach for prescribing CART; however, the availability of drugs and diagnostics is limited, and drugs have become unavailable at times. New drugs are also introduced slowly; for example, there is currently no single-tablet antiretroviral drug combination available, and the combination of fixed dose tenofovir and emtricitabine only became available in June 2010. The majority of southeastern European countries have also an individualized approach to CART with limited options, and currently there are no data concerning both the number of antiretroviral drug modifications and the reasons for these modifications.
The aim of this study was to investigate the frequency of and reasons for ADM, as well as how sex, age, and other risk factors affect ADM during the first 3 years after initiation of CART. We hypothesized that factors associated with drug toxicities are different in the early follow-up period (first 3 months of CART) compared to the period after 3 months of follow-up on a particular CART regimen. We examined a closed cohort of antiretroviral-naïve HIV-infected individuals who started CART in Croatia in the period 1998–2007 and analyzed all ADM occurring in the first 3 years of CART.
Material and Methods
Setting
Although a recent increase in cases of HIV infection has been observed among men who have sex with men (MSM) [19–21], Croatia is still considered a country with a low-level epidemic [19,20,22]. Health insurance is universal in Croatia and antiretroviral drug therapy became available free of charge in April 1998. An electronic database has been used at the University Hospital for Infectious Diseases (UHID) in Zagreb since 1997. This database includes all HIV-infected patients under care in Croatia, and data on their basic sociodemographic characteristics, clinical course, and antiretroviral therapy regiments, as well as laboratory findings on CD4 cell count and viral load measurements are available.
Study population
Antiretroviral-naïve patients who were not under care outside Croatia and who started CART between January 1, 1998, and December 31, 2007 and who had at least 1-month follow-up were eligible for the study. We included patients older than 18 years and excluded pregnant women. We also excluded patients in whom CART was initiated during primary infection because treatment in primary infection at the time of the study was given mainly on a theoretical basis and only for a limited time. The study was approved by the Ethics Committee of UHID.
Variables
We defined CART as an antiretroviral drug combination that was likely to suppress HIV-1 RNA to undetectable levels. These initial combinations included 2 nucleoside reverse transcriptase inhibitors (NRTI) in combination with 1 non-nucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor (PI), or 3 NRTIs. We also included patients who started CART with a PI plus NNRTI combination or with 2 PIs, with NRTIs. The type of CART in our analysis was categorized into 2NRTI plus 1PI, 2NRTI plus 1NNRTI, and other combinations. The NRTI backbone was categorized into zidovudine plus lamivudine (ZDV/3TC), stavudine plus lamivudine (D4T/3TC), abacavir plus lamivudine (ABC/3TC), and other.
Treatment modifications included a drug switch or an interruption. Switch was defined as a change of at least 1 drug, where the time between cessation of one drug combination and the initiation of another was ≤1 month and interruption when all drugs were stopped for >1 month. A switch from individual drugs such as ABC and 3TC to the single-pill co-formulation of ABC-3TC was not considered a treatment modification.
We reviewed all records of patients with treatment modifications and classified causes of treatment modification as toxic effects or intolerance, physician’s choice, patient’s choice, treatment failure, availability of drugs, and other reasons. Toxic effects or intolerance were categorized as gastrointestinal, hepatic, hypersensitivity, CNS, neuropathy, lipoatrophy, lipohypertrophy, and other. Lipoatrophy and lipohypertrophy were assessed subjectively if noticed by the patient and confirmed by the physician. Virologic suppression was defined as achieving a viral load of <400 copies/mL after 12, 24, and 36 months of CART.
Statistical methods
We described the baseline characteristics of our patients by the median and interquartile range for continuous variables and as frequencies for categorical variables. The baseline data on patients are presented according to the type of ADM: ADM for toxicity reasons, ADM for other reasons, and no ADM. We compared data on basic sociodemographic characteristics (age, sex, distance from HIV center, urban or rural residence, and HIV transmission risks), HIV disease factors (CD4 cell count, HIV-1 RNA viral load, and prior or concomitant AIDS), type of CART, calendar period of CART initiation, and coinfection with hepatitis B or C virus, using the chi-square or Fisher’s exact test for categorical variables and the Kruskal-Wallis test for continuous variables.
We calculated the follow-up time in several ways. The follow-up time ended 3 years after CART initiation for patients who were on CART at that date. If a patient who stopped all medications did not restart CART before 3 years after CART initiation, the period of follow-up ended with the date of interruption. Patients who died or were lost to follow-up were followed from the date of starting CART until the date of death or date of becoming lost to follow-up.
We used Poisson analysis to compute the rate of toxicity, and rate ratios and associated 95% confidence intervals (CI) for different individual drugs, and compared those according to sex. The total number of days on each individual antiretroviral drug was used as a denominator.
We compared treatment success in patients who had no treatment modification to those who had only a switch type or an interruption type of drug modification. It was assessed by the frequency of the viral loads less than 400 copies/ml and the median absolute CD4 cell count at 12, 24, and 36 months; and by the median increase in the CD4 cell count between baseline and 12, 24, and 36 months of CART. The nearest CD4 counts and VL measurements within 3 months of 12, 24, and 36 months after starting CART were identified and used in this analysis. We conducted a generalized estimation equation model analysis to compare treatment success in the 3 above-mentioned groups.
The main outcome in our study was a drug treatment modification because of toxicity or intolerance. The outcome was initially divided into 3 categories (no drug modification, modification due to toxicity or intolerance, and other modification). We used discrete-time regression analysis to model competing risks and repeated events with a month as the interval of time. Thus, when an event occurred, a new episode (sequence) began, and time was reset according to the outcome of the new drug combination. If the new drug combination was not modified further, time (in months) was reset to 1; otherwise, time was reset according to the number of months on treatment with the drug or drug combination that was subsequently changed. The expanded file for discrete-time analysis had 10 615 observations. We compared modifications due to drug toxicity to no treatment modifications in a binary model in which we censored the event of drug modification due to non-toxic reasons. Crude analysis was done including the sequence and time of event (in months) and 1 fixed or time-varying explanatory variable. Fixed explanatory variables were sex, baseline age, HIV transmission group, distance from HIV center (dichotomized at 160 km), and place of residence (rural vs. urban, based on a population level of below or above 40 000 inhabitants), clinical AIDS before or concomitant with CART, calendar year of CART initiation (1998–2002 vs. 2003–2007) and seropositivity for hepatitis C and positivity for hepatitis B antigen. Time-varying covariates were type of CART regimen and CD4 cell counts. The CD4 cell count in the discrete time model was matched with the months of measurements, and missing data were extrapolated by carrying the last observation forward. Covariates with a p<0.25 in crude analysis were considered as candidates for inclusion in the multivariable model. Sex, age, and antiretroviral drug combinations were included in all models. We constructed separate models for different follow-up times (0–3 and 3–36 months) on a particular drug regimen. We estimated a complementary log-log model, which is a proportional hazards model allowing interpretation of coefficients in terms of hazard ratios (HR). To assess the proportionality assumption, we examined the interaction of time with each independent variable in all our models and, if significant (p <0.05), the interaction was kept in the model. The dependency among repeated observations was accounted for by robust standard errors. The analysis was done using the statistical software package SAS version 9.3.1 (SAS institute Inc, Cary, North Carolina, USA); the level of significance was set at 0.05.
Results
Baseline characteristics
A total of 321 treatment-naïve patients who started ART in the period 1997 to 2007 were included in our study. The median age at baseline was 40 years, 19% were female, baseline CD4 was <200 cells/mm3 in 71%, and viral load was ≥100 000 copies/mL in 69% of patients. Baseline characteristics according to type of ADM are shown in Table 1. At 3 years after initiation of CART, 274 (85%) individuals were still taking CART, 19 (6%) were known to be alive and not taking CART, 24 (7%) were dead, 2 (1%) had moved, and 2 (1%) were considered lost to follow-up.
Table 1.
Baseline characteristics of 321 individuals according to reason for CART modification.
| Characteristics | Reason for CART modification | No CART modification | ||
|---|---|---|---|---|
| Toxicity (n=124)* | Other (n=96) | No (n=101) | p- value | |
| Age, years | 42.3 (35–50.9) | 38.2 (31.5–45.8) | 38.1 (30.9–48.9) | 0.005 |
| CD4 cell, count/μL | 85.0 (31–212) | 71.5 (26–228.5) | 151.0 (62.0–216.0) | 0.027 |
| HIV-1 RNA, log10 copies/mL | 5.3 (4.8–5.9) | 5.4 (4.9–5.8) | 5.3 (4.8–5.7) | 0.139 |
| Female gender | 33 (26.6) | 14 (14.6) | 15 (14.9) | 0.032 |
| Distance from HIV center <160 km | 47 (37.9) | 38 (39.6) | 56 (55.4) | 0.018 |
| Urban place of living | 45 (36.3) | 25 (26.0) | 38 (37.6) | 0.166 |
| Prior or concomitant AIDS | 49 (39.5) | 47 (49.0) | 24 (23.8) | 0.001 |
| Calendar year of CART initiation | <0.001 | |||
| 1998–2002 | 62 (50.0) | 45 (46.9) | 21 (20.8) | |
| 2003–2007 | 62 (50.0) | 51 (53.1) | 80 (79.2) | |
| HBsAg positive | 4 (3.2) | 5 (5.2) | 7 (6.9) | 0.423 |
| Antibody to HCV | 16 (12.9) | 13 (13.5) | 7 (6.9) | 0.254 |
| Mode of transmission | 0.379 | |||
| MSM | 41 (33.1) | 39 (40.6) | 49 (48.5) | |
| Heterosexual | 67 (54.0) | 43 (44.8) | 40 (39.6) | |
| Intravenous drug use | 8 (6.5) | 7 (7.3) | 5 (5.0) | |
| Other/unknown | 8 (6.5) | 7 (7.3) | 7 (6.9) | |
| Initial NRT backbone | <0.001 | |||
| D4T3TC | 70 (56.5) | 51 (53.1) | 16 (15.8) | |
| ZDV3TC | 42 (33.9) | 36 (37.5) | 79 (78.2) | |
| Other/none | 12 (9.7) | 9 (9.4) | 6 (5.9) | |
| Type of initial CART | 0.149 | |||
| 2NRTI plus 1PI | 65 (52.4) | 53 (55.2) | 45 (44.6) | |
| 2NRTI plus NNRTI | 53 (42.7) | 39 (40.6) | 56 (55.4) | |
| Other | 6 (4.8) | 4 (4.2) | ||
26 of 124 patients had also a modification for other reasons. Values are medians with interquartile ranges or frequencies and percentages. CART – combination antiretroviral therapy; HBsAg – hepatits B surface antigen; HCV – hepatitis C virus; MSM – men who have sex with men; D4T – stavudine; 3TC – lamivudine; ZDV – zidovudine; NRTI – nucleoside reverse-transcriptase inhibitor; PI – protase inhibitor; NNRTI – nonnucleoside reverse-transcriptase inhibitor.
Drug modifications
A total of 387 ADM were observed. The reasons for ADM were toxicity (including intolerance) in 176 (45.5%), physician’s decision in 117 (30.2%), patient’s choice in 37 (9.6%), treatment failure in 19 (4.9%), availability in 36 (9.3%), and miscellaneous in 2 (0.5%). Three hundred sixteen (81.7%) of the ADM episodes were switches, and 71 (18.3%) were interruptions. The total follow-up time of persons on CART was 852.4 years, and the median follow-up time per patient was 3 (IQR, 2.9–3.0) years. During the 3-year follow-up, there were 50 instances of restarting an interrupted antiretroviral regiment. The 387 ADM involved 220 (68.5%) of the 321 patients in our study (1.8 ADM per patient); 124 (39%) had ≥1ADM for toxicity reasons. Lipoatrophy (22%), gastrointestinal symptoms (20%), and neuropathy (18%) were the most common causes of toxicity-related ADM.
Of 852.4 years follow-up on CART, patients spent the most time on the following drugs or drug combinations: ZDV plus 3TC (450.1 years, 52.8%, n=212), d4T plus 3TC (193.5 years, 22.7%, n=162), ABC plus 3TC (132.3 years, 15.5%, n=81), efavirenz (EFV) (359.4 years, 42.2%, n=172), nevirapine (NVP) (83.7 years, 9.8%, n=56), lopinavir (LPV) (241.4 years, 28.3%, n=116), and indinavir (IND) or indinavir plus ritonavir (IND/r) (146.3 years, 17.2%, n=84). We calculated the rate of ADM for toxicity reasons per 100 patient-years for each individual drug. As expected, d4T was associated with the highest rate of toxicity-ADM. There were also differences in toxicity-related drug modifications between males and females (Table 2). Of individual drugs, ZDV and NVP were more frequently switched or interrupted for toxicity reasons in women than in men (Table 2).
Table 2.
Observed toxicity rate of CART modifications of per 100 patient years for selected antiretrovirals according to gender.
| Males | Females | p-value | |
|---|---|---|---|
| NRTI | |||
| Zidovudine | 5.1 (3.3–8.0) | 13.2 (6.9–25.4) | 0.019 |
| Stavudine | 32.0 (24.3–42.3) | 28.5 (16.5–49.0) | 0.703 |
| Abacavir | 2.7 (0.9–8.2) | 2.7 (0.9–8.2) | |
| Didanosine | 13.9 (5.2–37.1) | 22.3 (5.6–89.2) | 0.586 |
| NNRTI | |||
| Nevirapine | 3.0 (0.7–11.9) | 24.5 (9.2–65.2) | 0.015 |
| Efavirenz | 6.9 (4.4–10.7) | 13.1 (6.8–25.1) | 0.111 |
| PI | |||
| Indinavir | 20.8 (14.0–30.7) | 27.4 (17.7–42.4) | 0.657 |
| Lopinavir | 4.7 (2.6–8.8) | 9.8 (3.2–30.4) | 0.27 |
Values are rates and 95% confidence intervals. NRT – nucleoside reverse-transcriptase inhibitor; NNRT – nonnucleoside reverse-transcriptase inhibitor; PI – protase inhibitor.
Of 176 drug modifications due to toxicity, 165 (94%) were single-drug changes. More frequent single-drug substitutions because of toxicity included d4T (59 events), ZDV (25 events), IND (25 events), and EFV (24 events). Of 117 drug modifications due to physicians’ suggestion, 98 (84%) were single-drug changes; the most frequent single-drug changes involved d4T (45 events), IND (17 events), and zalcitabine (ddC) (13 events). Drug changes because of patient’s decision (37 events) were more frequently interruptions (32, 86%) and hence involved more than 1 drug 31 times. Changes because of drug availability occurred 36 times and included single-drug substitutions in 27 (75%) instances. Most of those changes occurred in 1999 (24 events) and included substitutions for 3TC (16 events).
Factors related to drug toxicity
Results from crude and multivariable discrete time recurrent event regression analyses of predictors of ADM are presented in Tables 3 and 4. Of the 176 toxicity-related ADM, 55 (31.3%) occurred during the first 3 months of a particular CART regimen involving 52 patients, and 121 (68.7%) events among 90 patients occurred after more than 3 months of CART. Female sex and older age were associated with toxicity-related drug modifications in the first 3 months of a particular CART regiment, but afterwards they were not. As expected, nucleoside backbones such as ABC+3TC and ZDV+3TC had an overall lower risk of toxicity-related modification; however, this was not the case during the first 3 months of therapy on a particular drug regimen. Because of concerns about collinearity, the period of CART initiation (1998–2002 vs. 2003–2007) was not included in the model together with the type of nucleoside backbone. When a separate multivariable model was built (including the period of study, but not the type of nucleosides) for 3 to 36 month follow-up, there was a significant interaction of time with the period of CART initiation. This suggests that those who started CART in the period 1998–2002 had a higher hazard of toxicity-related drug modifications at 9 months (HR 1.63, 95% CI 1.05–2.51) and later, whereas coefficients of other variables in the model remained very similar. Only 12.7% of individuals starting CART in the period 1998–2002 were on the same regimen after 3 years, whereas this was the case in 39.4% of individuals in the period 2003–2007 (Figure 1).
Table 3.
Crude analysis of factors related to toxicity drug modicifactions. Comparison of toxicity drug modifications to no drug modifications.
| Months of follow-up | ||
|---|---|---|
| 0 to 3 | 3 to 36 | |
| p-value Crude HR (95% CI) | ||
| Gender | 0.001 | 0.300 |
| Female vs. male | 2.58 (1.50–4.45) | 1.3 (0.79–2.16) |
| Age, per 10-year increase | <0.001 | 0.180 |
| Age | 1.47 (1.2–1.81) | 1.12 (0.95–1.32) |
| Distance from HIV center | 0.659 | 0.05 |
| <160 km vs. ≥160 km | 0.89 (0.53–1.5) | 0.63 (0.40–1.00)* |
| Place of living | 0.462 | 0.822 |
| Urban vs. rural | 0.81 (0.47–1.41) | 0.95 (0.63–1.43) |
| CART initiation period | 0.587 | 0.007 |
| 1998–2002 vs. 2003–2007 | 0.86 (0.51–1.47) | 1.83 (1.79–2.83)** |
| Risk group | 0.085 | 0.851 |
| Non-MSM vs. MSM | 1.63 (0.93–2.85) | 0.96 (0.64–1.44) |
| Clinical AIDS concomitant or before CART | 0.971 | 0.304 |
| No vs. yes | 1.01 (0.58–1.75) | 0.8 (0.52–1.22) |
| Nucleoside backbone | 0.850 | 0.006 |
| ABC3TC vs. D4T3TC | 0.69 (0.22–2.21) | 0.21 (0.07–0.61)*** |
| ZDV3TC vs. D4T3TC | 0.86 (0.47–1.58) | 0.34 (0.19–0.63)*** |
| Other/none vs. D4T3TC | 1.11 (0.45–2.73) | 1.01 (0.50–2.04)*** |
| Type of CART | 0.013 | 0.012 |
| 2NRT + 1PI vs. 2NRT + 1NNRT | 0.51 (0.29–0.90) | 1.50 (1.02–2.20)# |
| Other vs. 2NRT+ 1NNRT | 1.98 (0.75–5.20) | 2.83 (1.39–5.76)# |
| CD4 cell count/μL, per 100 cells | 0.452 | 0.005 |
| CD4 cell count/μL | 1.09 (0.87–1.37) | 0.84 (0.75–0.95) |
| Viral load, log10 copies/mL | 0.657 | 0.806 |
| <5 vs. ≥5 | 1.13 (0.65–1.98) | 1.06 (0.69–1.63) |
| Hepatitis B surface antigen positivity | 0.313 | 0.108 |
| No vs. yes | 0.65 (0.28–1.51) | 2.86 (0.79–10.27) |
| Hepatitis C antibody positivity | 0.909 | 0.879 |
| No vs. yes | 0.95 (0.42–2.17) | 1.04 (0.62–1.74) |
Analysis included the variable of interest, time, sequence and, if significant, the interaction of time with the variable of interest. HR, hazard ratio, CI, confidence interval. ABC – abacavir; 3TC – lamivudine; D4T – stavudine; ZDV – zidovudine; NRT – nucleoside reverse-transcriptase inhibitor; NNRT – nonnucleoside reverse-transcriptase inhibitor; PI – protease inhibitor.
At month 18;
at month 9;
at month 6, p=0.004 for the comparison ABC3TC vs. D4T3TC, p<0.001 for the comparison ZDV3TC vs. D4T3TC, p=0.984 for the comparison other/none vs. D4T3TC;
at month 12, p=0.020 for the comparison 2NRT+1PI vs. 2NRT+1NNRT, p=0.004 for the comparison other vs. 2NRT+1NNRT.
Table 4.
Multivariate analysis of factors related to toxicity drug modifications.
| Months of follow up | ||
|---|---|---|
| 0 to 3 | 3 to 36 | |
| p-value HR (95% CI) | ||
| Gender | 0.002 | 0.870 |
| Female vs. Male | 2.42 (1.39–4.24) | 1.05 (0.59–1.88) |
| Age, per 10-year increase | <0.001 | 0.188 |
| Age | 1.42 (1.16–1.74) | 1.13 (0.94–1.36) |
| Nucleoside backbone | 0.69 | |
| ABC3TC vs. D4T3TC | 0.56 (0.16–1.97) | 0.23 (0.08–0.68)* |
| ZDV3TC vs. D4T3TC | 1.06 (0.58–1.95) | 0.38 (0.20–0.72)* |
| Other/none vs. D4T3TC | 0.66 (0.21–2.11) | 1.24 (0.49–3.10)* |
| Type of CART | 0.061 | |
| 2NRT + 1PI vs. 2NRT + 1NNRT | 0.60 (0.33–1.12) | 1.49 (0.99–2.26)** |
| Other vs. 2NRT+ 1NNRT | 2.89 (0.77–10.81) | 3.41 (0.95–12.28)** |
Adjusted for time sequence and in the 3 to 36 months follow-up model also for the CD4 cell count (not significant) and distance from HIV center (not significant). HR – hazard ratio; CI – confidence interval; ABC – abacavir; 3TC – lamivudine; D4T – stavudine; ZDV – zidovudine; NRT – nucleoside reverse-transcriptase inhibitor; NNRT – nonnucleoside reverse-transcriptase inhibitor; PI – protease inhibitor.
At month 6; p=0.008 for the comparison ABC3TC vs. D4T3TC, p=0.003 for the comparison ZDV3TC vs. D4T3TC, p=0.648 for the comparison other/none vs. D4T3TC.
at month 15; p=0.06 for the comparison 2NRT+1PI vs. 2NRT+ 1NNRT, p=0.06 for the comparison other vs. 2NRT+1NNRT.
Figure 1.
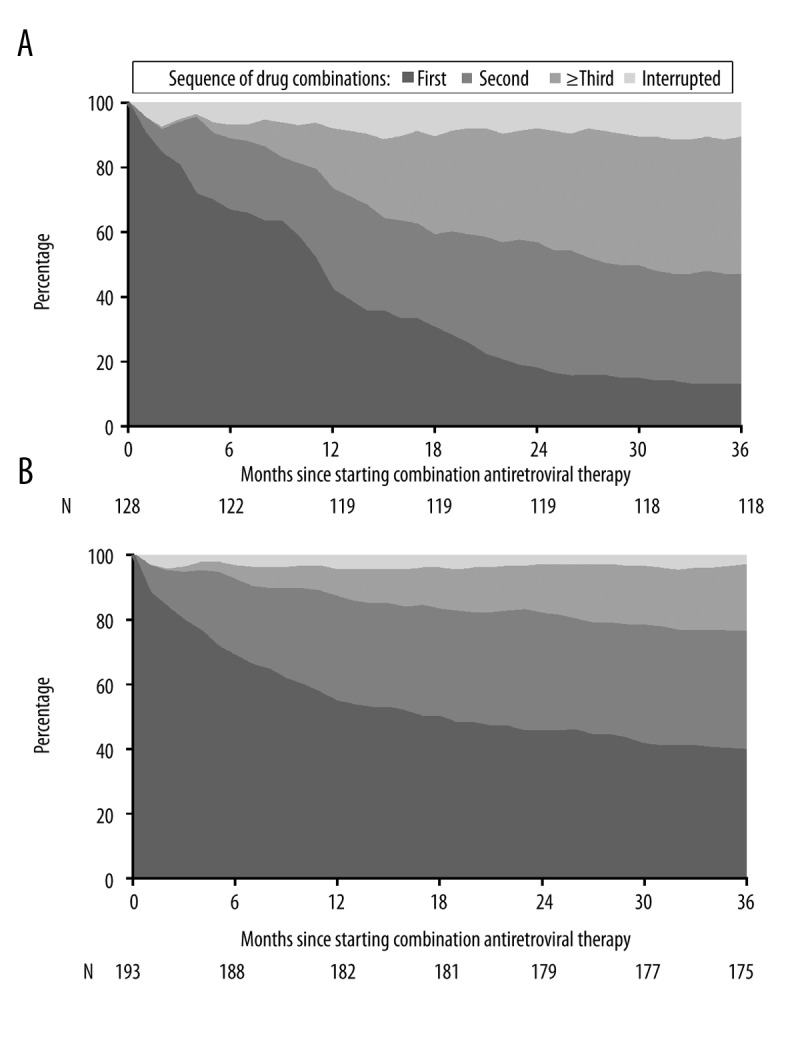
Durability of first and subsequent antiretroviral drug combination therapy in patients starting therapy between 1998 and 2002 (A) and 2003 to 2007 (B).
Virologic and immunological outcomes
Virologic efficacy (defined as achieving an HIV-1 RNA load of less than 400 copies/mL) was highest among patients with no CART changes and lowest among patients with interruption of CART (Table 5). Immunological responses (defined as the increase in the CD4 cell count from baseline and the absolute CD4 cell count) significantly improved between 12 and 36 months in patients with no CART change and those who had only switches, but there was no improvement in patients interrupting CART. The increase in CD4 cell count was greater in patients with no CART change compared to patients with switch-type only drug modifications.
Table 5.
Virological and immunological outcomes 12, 24 and 36 months after initiation of combination antiretroviral therapy according to type of drug modifications.
| Drug modifications | Group | Time | Interaction | |||
|---|---|---|---|---|---|---|
| Switch type only | Interruption type | None | ||||
| CD4 cell count, per mm3 | ||||||
| Baseline | 65 (27–196) | 177.5 (38–246) | 151 (63.5–217.5) | |||
| 12 months | 266 (185–407) | 276 (187–378) | 363 (234–491) | <0.001 | <0.001 | <0.001 |
| 24 months | 341 (226–443.5) | 330 (213–439) | 482.5 (326–639) | |||
| 36 months | 369.5 (258–530) | 291 (191–450) | 480.5 (350–665.5) | |||
| Increase from baseline, CD4 cell count, per mm3 | ||||||
| 12 months | 173 (101–256) | 138 (59–226) | 189.5 (108–299) | |||
| 24 months | 225.5 (146.5–335.5) | 146 (38–283) | 300 (179–459) | <0.001 | <0.001 | <0.001 |
| 36 months | 281.5 (186–401) | 138 (11–288) | 329 (181–468.5) | |||
| Viral load,% <400 c/ml | ||||||
| 12 months | 143/155 (92.3) | 30/53 (56.6) | 89/90 (98.9) | |||
| 24 months | 143/152 (94.1) | 37/53 (69.8) | 81/82 (98.8) | <0.001 | 0.159 | 0.179 |
| 36 months | 135/152 (88.8) | 32/49 (65.3) | 82/84 (97.6) | |||
Values are medians with interquartile ranges in parenthesis or number of patients with the characteristic/number analyzed and (%). P-values are obtained by generalized estimating equation analysis.
Discussion
We found that CART modifications were frequent in Croatia; more than two-thirds of patients changed CART during the first 3 years of therapy. In the Eurosida study, which included patients starting CART predominantly between 1999 and 2002, 70% of patients remained on their original regimen at 1 year after starting CART [3], whereas in our study only 50% (42% for the study period 1998–2002 and 54% in years 2003–2007) were on the initial CART regiment after 1 year of therapy. The 1-year probability of drug change in the Swiss cohort was 37% in CART-naïve patients starting therapy during 1995–1998 and between 43.8% and 48.8% in the period 2000–2005 [1]. Higher short-term CART modification rates have also been reported in vulnerable populations such as younger individuals, African-Americans, injection drug users, and patients lacking private health insurance [14]. In our study only 5% of drug changes were due to virologic failure; hence, we confirmed previous findings from developed countries that the major cause for drug modifications is toxicity and not virologic failure [1–6,8,23]. In resource-limited settings, rates of drug modifications are lower and more frequently driven by virologic failure [24,25].
Thirty-nine percent of our patients had toxicity-related ADM during the first 3 years of CART; 25% had a change during the first year of follow-up. This is higher than in individuals from the Swiss cohort starting therapy between 2005 and 2008, in which 15.8% of individuals modified their treatment because of drug intolerance/toxicity during the first year of CART, [6] and is also higher than the 16% reported in the Eurosida cohort [3]. Data from the ICONA cohort from Italy, including patients starting therapy between 1997 and 1999, found treatment discontinuation caused by toxicity in 21% of individuals [4]. The follow-up study from the same cohort included patients from 1997 to 2007 and reported a probability of discontinuation because of intolerance/toxicity of 23.2% at 1-year of follow-up [5]. In resource-limited settings, ADM because of toxicity are less frequently done. For example, the cumulative probability of changes at 2 years due to toxicity in first-line regimens in Switzerland was 23.8%, compared to 11.7% in the townships of Khayelitsha and Gugulethu in Cape Town, South Africa [25].
Several observational studies have found that females have a higher toxicity rate of CART modifications than males [3–6,14]. Our findings were similar, but we also showed that there is a time-dependent effect and that the average hazard rate of treatment changes due to toxicity after 3-months on a particular CART regiment is similar in males and females. Regimens that contained ZDV or NVP were associated with higher risk of modification for toxicity reasons in women than in men, which is also in accordance with previous findings [26–29]. The effect of age on toxicity-related CART modifications has been examined in a number of studies, which have reported various findings, possibly because of the diverse populations analyzed. A single-center study from London in 2001 showed that older patients were less likely to modify CART [30]. A study in vulnerable populations from Birmingham, Alabama, USA also found that younger age was a risk factor for treatment discontinuation due to non-gastrointestinal toxicity [14]. Similar to our findings, in the Swiss cohort older age was associated with higher risks of toxicity-related ADM during the first year of CART [6]. There have also been studies that did not find that age was a factor for toxicity-driven CART modifications [5]. In our study, older patients had a higher risk of treatment changes due to toxicity in the first 3 months on a particular CART regiment; however, afterwards the average hazard ratio of treatment changes due to toxicity was not associated with age (Tables 3 and 4).
Results of our study in the crude analysis suggest that patients who started CART with an NNRTI-based regimens compared to a PI-based regimen had a higher risk of treatment modification within the first 3 months after CART initiation. This is in concordance with the adverse effects of EFV (central nervous toxicity) and NVP (rash and hepatotoxicity), which typically occur more frequently at initiation of CART. After about 12 months of therapy, patients on a PI-based regimen tended to have a higher risk of treatment change because of toxicity (Tables 3 and 4). PI-based regimens in our study population were mainly the use of IND with or without ritonavir and LPV, and to a lesser extent nelfinavir. We were not able to find a difference between different nucleoside backbones during the first 3 months of therapy. However, afterwards, as expected, d4T use was associated with a higher toxicity rate compared to ABC and ZDV use.
The toxicity rates causing treatment modifications of individual antiretrovirals has not been widely reported. We found a toxicity rate in men for d4T and LPV modifications (32 events per 100 years of follow-up, and 4.7 events per 100 years of follow-up, respectively) similar to cohorts from Chelsea and Westminster in London (30.5 events per 100 years of follow-up and 4.7 events per 100 years of follow-up, respectively) [23]. The toxicity rate of drug modifications for other antiretrovirals (ABC, ddI, NVP, EFV) was somewhat higher in our study populations. The largest difference was in the toxicity rate drug modifications due to ZDV, which was much more frequently changed in men from London (rate 22.7 per 100 years of follow-up) compared to men from Croatia (5.1 per 100 years of follow-up). However, in the study from London, it could not be assessed whether patients switched ZDV because they actually had lipoatrophy or patients switched to prevent lipoatrophy. Also, patients in Croatia are usually not switched if mild anemia is present, which might not have been the case in London.
Since the majority of patients in our study had a low baseline CD4 cell count and had generally advanced HIV infection, it is not surprising that patients who interrupted CART had worse virologic and immunologic outcomes compared to patients with no treatment modifications or switch-type modifications. When patients with no treatment modification were compared to patients with only switch-type modifications, we observed slightly better outcomes in patients with no treatment changes at 36 months of follow-up. However, because of the relatively small number of patients under study, we should be cautious in making a firm conclusion. Many switches and simplification strategies are available and are generally considered safe in virologically-suppressed individuals [31].
Our study has limitations. For example, the reason for ADM may be multifactorial, but we selected the one considered predominant. We investigated the toxic effects of CART by the occurrence of treatment modification, and some patients choose to stay on treatment despite adverse effects, so the true incidence of toxicities of a particular drug was not estimated. Whether patients stop or change their CART may be influenced by the number and availability of drugs. Our study included only a few patients with a nucleoside backbone of TDF plus FTC, a backbone that is currently widely used in many countries. However, in southeastern European countries such as Croatia and Serbia, TDF and TDF plus FTC have been introduced only after a long delay and are still not widely used. We used a repeated time-to-event analysis, which may provide more power and more efficient coefficients. However, setting the follow-up time is challenging because out of 3 antiretroviral drugs used, only 1 is usually changed. The strength of this single-center clinic-based study is that all patients in Croatia were included and that the collected data were complete, with almost no patients lost to follow-up.
Conclusions
More and better-tolerated treatment options should be available and more frequently used in Croatia. In times when many national health insurance programs, especially those in middle-income countries, are concerned about the rising cost of treating increasing number of patients with HIV, we should keep in mind that older drug formulations are associated with higher toxicity rates.
Acknowledgments
This study was partially presented at the IUSTI Europe Congress 6–8 September 2012, Antalya-Turkey.
Footnotes
Source of support: This study was supported in part by grants from the Croatian Ministry of Science, Education and Sports to Josip Begovac (Grant 108-1080116-0098). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Vo TT, Ledergerber B, Keiser O, et al. Durability and outcome of initial antiretroviral treatments received during 2000–2005 by patients in the Swiss HIV Cohort Study. J Infect Dis. 2008;197:1685–94. doi: 10.1086/588141. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien ME, Clark RA, Besch CL, et al. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. J Acquir Immune Defic Syndr. 2003;34:407–14. doi: 10.1097/00126334-200312010-00008. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Phillips AN, Soriano V, et al. Reasons for stopping antiretrovirals used in an initial highly active antiretroviral regimen: increased incidence of stopping due to toxicity or patient/physician choice in patients with hepatitis C coinfection. AIDS Res Hum Retroviruses. 2005;21:527–36. doi: 10.1089/aid.2005.21.527. [DOI] [PubMed] [Google Scholar]
- 4.d’Arminio Monforte A, Lepri AC, Rezza G, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. I.CO.N.A. Study Group. Italian Cohort of Antiretroviral-Naive Patients. AIDS. 2000;14:499–507. doi: 10.1097/00002030-200003310-00005. [DOI] [PubMed] [Google Scholar]
- 5.Cicconi P, Cozzi-Lepri A, Castagna A, et al. Insights into reasons for discontinuation according to year of starting first regimen of highly active antiretroviral therapy in a cohort of antiretroviral-naive patients. HIV Med. 2010;11:104–13. doi: 10.1111/j.1468-1293.2009.00750.x. [DOI] [PubMed] [Google Scholar]
- 6.Elzi L, Marzolini C, Furrer H, et al. Treatment modification in human immunodeficiency virus-infected individuals starting combination antiretroviral therapy between 2005 and 2008. Arch Intern Med. 2010;170:57–65. doi: 10.1001/archinternmed.2009.432. [DOI] [PubMed] [Google Scholar]
- 7.Cesar C, Shepherd BE, Krolewiecki AJ, et al. Rates and reasons for early change of first HAART in HIV-1-infected patients in 7 sites throughout the Caribbean and Latin America. PLoS One. 2010;5(6):e10490. doi: 10.1371/journal.pone.0010490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan Y, L’Italien G, Mukherjee J, Iloeje UH. Determinants of discontinuation of initial highly active antiretroviral therapy regimens in a US HIV-infected patient cohort. HIV Med. 2006;7:156–62. doi: 10.1111/j.1468-1293.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 9.Dieleman JP, Jambroes M, Gyssens IC, et al. Determinants of recurrent toxicity-driven switches of highly active antiretroviral therapy. The ATHENA cohort. AIDS. 2002;16:737–45. doi: 10.1097/00002030-200203290-00009. [DOI] [PubMed] [Google Scholar]
- 10.Glass TR, De Geest S, Weber R, et al. Correlates of self-reported nonadherence to antiretroviral therapy in HIV-infected patients: the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2006;41:385–92. doi: 10.1097/01.qai.0000186371.95301.52. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Margolick JB, Conover CS, et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2005;38:320–28. [PubMed] [Google Scholar]
- 12.Keiser O, Fellay J, Opravil M, et al. Adverse events to antiretrovirals in the Swiss HIV Cohort Study: effect on mortality and treatment modification. Antivir Ther. 2007;12:1157–64. [PubMed] [Google Scholar]
- 13.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 14.Robison LS, Westfall AO, Mugavero MJ, et al. Short-term discontinuation of HAART regimens more common in vulnerable patient populations. AIDS Res Hum Retroviruses. 2008;24:1347–55. doi: 10.1089/aid.2008.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stohr W, Back D, Dunn D, et al. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther. 2008;13:675–85. [PubMed] [Google Scholar]
- 16.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–400. [PubMed] [Google Scholar]
- 17.Mallal S, Phillips E, Carosi G, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–79. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 18.Abgrall S The Antiretroviral Therapy Cohort C. Durability of first ART regimen and risk factors for modification, interruption or death in HIV-positive patients starting ART in Europe and N. America 2002–2009. AIDS. 2013;27:803–13. doi: 10.1097/QAD.0b013e32835cb997. [DOI] [PubMed] [Google Scholar]
- 19.Bozicevic I, Lepej SZ, Rode OD, et al. Prevalence of HIV and sexually transmitted infections and patterns of recent HIV testing among men who have sex with men in Zagreb, Croatia. Sex Transm Infect. 2012;88:539–44. doi: 10.1136/sextrans-2011-050374. [DOI] [PubMed] [Google Scholar]
- 20.Kolaric B. Croatia: still a low-level HIV epidemic? – seroprevalence study. Coll Antropol. 2011;35:861–65. [PubMed] [Google Scholar]
- 21.Bozicevic I, Begovac J. The emerging HIV epidemic among men who have sex with men in southeastern Europe. Expert Rev Anti Infect Ther. 2010;8:1351–58. doi: 10.1586/eri.10.131. [DOI] [PubMed] [Google Scholar]
- 22.Begovac J, Zekan A, Skoko-Poljak D. Twenty years of human immunodeficiency virus infection in Croatia – an epidemic that is still in an early stage. Coll Antropol. 2006;30(Suppl 2):17–23. [PubMed] [Google Scholar]
- 23.Davidson I, Beardsell H, Smith B, et al. The frequency and reasons for antiretroviral switching with specific antiretroviral associations: the SWITCH study. Antiviral Res. 2010;86:227–29. doi: 10.1016/j.antiviral.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Srasuebkul P, Calmy A, Zhou J, et al. Impact of drug classes and treatment availability on the rate of antiretroviral treatment change in the TREAT Asia HIV Observational Database (TAHOD) AIDS Res Ther. 2007;4:18. doi: 10.1186/1742-6405-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keiser O, Orrell C, Egger M, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med. 2008;5(7):e148. doi: 10.1371/journal.pmed.0050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kesselring AM, Wit FW, Sabin CA, et al. Risk factors for treatment-limiting toxicities in patients starting nevirapine-containing antiretroviral therapy. AIDS. 2009;23:1689–99. doi: 10.1097/QAD.0b013e32832d3b54. [DOI] [PubMed] [Google Scholar]
- 27.Bersoff-Matcha SJ, Miller WC, Aberg JA, et al. Sex differences in nevirapine rash. Clin Infect Dis. 2001;32:124–29. doi: 10.1086/317536. [DOI] [PubMed] [Google Scholar]
- 28.Antinori A, Baldini F, Girardi E, et al. Female sex and the use of anti-allergic agents increase the risk of developing cutaneous rash associated with nevirapine therapy. AIDS. 2001;15:1579–81. doi: 10.1097/00002030-200108170-00018. [DOI] [PubMed] [Google Scholar]
- 29.van Leth F, Andrews S, Grinsztejn B, et al. The effect of baseline CD4 cell count and HIV-1 viral load on the efficacy and safety of nevirapine or efavirenz-based first-line HAART. AIDS. 2005;19:463–71. doi: 10.1097/01.aids.0000162334.12815.5b. [DOI] [PubMed] [Google Scholar]
- 30.Mocroft A, Youle M, Moore A, et al. Reasons for modification and discontinuation of antiretrovirals: results from a single treatment centre. AIDS. 2001;15:185–94. doi: 10.1097/00002030-200101260-00007. [DOI] [PubMed] [Google Scholar]
- 31.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]


