Abstract
Regulatory T (TR) cells maintain tolerance to self-antigens and control immune responses to alloantigens after organ transplantation. Here, we show that CD4+ CD25+ human TR cells suppress virus-specific T-cell responses. Depletion of TR cells from peripheral blood mononuclear cells enhances T-cell responses to cytomegalovirus and human immunodeficiency virus antigens. We propose that chronic viral infections lead to induction of suppressive TR cells that inhibit the antiviral immune response.
CD4+ CD25+ regulatory T cells (TR cells) are a subset of circulating CD4+ T cells with suppressive properties (21, 24). They were first identified in mice as cells capable of maintaining self-tolerance by suppressing autoreactive T cells (1, 22), or suppressing alloreactive immune responses after organ transplantation (8, 15, 23). Human TR cells have been identified and characterized in peripheral blood and the thymus (2, 13, 18, 25). Although the phenotypic characterization of TR cells is still incomplete, CD25 has been used to distinguish a functionally relevant suppressive T-cell subpopulation.
TR cells develop in the thymus as cells that recognize antigens with high affinity yet escape negative selection (14). TR cells can also be induced in the periphery after antigen activation and are termed adaptive TR (4). These adaptive TR cells can be induced in vitro by cytokine priming and coculture with immature dendritic cells (6, 12, 29) and in vivo after repeated exposure to superantigen (7). In addition to suppressing auto- and alloreactive T cells, TR cells can also suppress immune responses to human tumors and to bacterial and acute viral infections in animal models (3, 26, 28). However, it is not known whether TR cells suppress immune responses in human chronic viral infections. Here we show that depletion of CD25+ T cells from peripheral blood mononuclear cells (PBMC) augments T-cell immune responses to cytomegalovirus (CMV) and human immunodeficiency virus (HIV) antigens. TR cells may play an important role in controlling and suppressing the immune responses in chronic viral diseases.
MATERIALS AND METHODS
Study samples.
Blood samples from healthy blood donors were obtained under approved University of California—San Francisco Committee on Human Research Institutional Review Board protocols. Blood samples from HIV-infected patients were obtained from 16 adult patients recruited after informed consent from the UCSF Options cohort (median CD4 T-cell count = 544 cells/μl; range, 483 to 799 cells/μl; and median viral load, 26,700 copies/ml; range, 1945 to >500,000 copies/ml). All but one of the patients were infected within 1 year prior to blood sampling, and none of the patients were currently under antiretroviral treatment. PBMC from healthy seronegative donors and HIV-infected individuals were isolated from heparinized whole blood by Ficoll-Paque PLUS density gradient centrifugation (Amersham Pharmacia, Uppsala, Sweden). The cells were washed twice in RPMI 1640 (MediaTech, Herndon, Va.) supplemented with 15% fetal calf serum (FCS) (Gemini BioProducts, Woodland, Calif.). PBMC not assayed immediately were frozen in FCS containing 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, Mo.). Frozen PBMC were thawed, washed twice in RPMI 1640 supplemented with 15% FCS, and incubated overnight at 37°C prior to use.
Phenotyping of CD25+ regulatory T cells (TR).
Briefly, fresh or frozen PBMC were washed once in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) (Sigma-Aldrich, St. Louis, Mo.) and stained with fluorescently labeled antibodies for CD3-peridinin chlorophyll protein (PerCP), CD4-fluorescein isothiocyanate (FITC), CD8-allophycocyanin (APC), CD25-FITC or -phycoerythrin (PE), CD38-APC or -PE, and/or HLA-DR—APC (BD BioSciences, San Jose, Calif., and BD BioSciences PharMingen, San Diego, Calif.) for 20 min at 4°C. The cells were then washed twice with PBS containing 1% BSA, fixed in 1% paraformaldehyde, acquired on a flow cytometer (FACSCalibur; BD BioSciences), and analyzed using FlowJo software (Tree Star, San Carlos, Calif.).
Depletion of CD25+ cells.
CD25+ cells were purified with MACS CD25 MicroBeads (Miltenyi Biotec, Auburn, Calif.). Briefly, fresh or frozen PBMC were washed twice in PBS containing 0.5% BSA and 2 mM EDTA, resuspended in 80 μl of PBS containing 0.5% BSA-2 mM EDTA and 20 μl of MACS CD25 MicroBeads per 107 total PBMC, and incubated for 15 min at 6 to 12°C. PBMC were washed twice in PBS containing 0.5% BSA and 2 mM EDTA and applied to a magnetic column on a MidiMACS separation unit (Miltenyi Biotec). CD25+ and CD25− T-cell fractions were collected. The CD25+ cell fraction contained >90% CD4+ T cells. In some experiments the CD25+ cell fraction was purified to >99% CD4+ T cells by cell sorting after staining with monoclonal antibodies to CD3 and CD4 (FACSvantage; BD Biosciences).
Tetramer staining.
PBMC were washed once in PBS containing 1% BSA and stained with CD8-FITC, CD3-PE, and APC-conjugated HLA A*02 CMV pp65 Tetramer (NLVPMVATV) (Beckman Coulter Immunomics, San Diego, Calif.). After staining, the cells were washed twice in PBS containing 1% BSA and fixed in 1% paraformaldehyde.
Cytokine flow cytometry.
PBMC, PBMC depleted of CD25+ T cells, or PBMC cocultured with CD25+ T cells were stimulated with staphylococcal enterotoxin B (SEB) (Sigma-Aldrich), CMV pp65 peptide (NLVPMVATV) (Resgen Invitrogen, Carlsbad, Calif.), HIV type 1 (HIV-1) Gag p24 (Protein Sciences, Meriden, Conn.), HIV-1 SF2 p55 Gag (National Institutes of Health AIDS Research and Reference Reagent Program), HIV Gag p55 peptide mix (BD BioSciences PharMingen), Human CMV viral lysate (Advanced Biotechnologies, Inc., Columbia, Md.), or with RPMI 1640 supplemented with 15% FCS and incubated for 18 h. All antigens were used at a final concentration of 2.5 to 5 μg/ml. Brefeldin A (Sigma-Aldrich) was added at a final concentration of 5 μg/ml for the last 6 h of incubation. Cells were washed in PBS containing 2 mM EDTA, fixed in 1% paraformaldehyde, and permeabilized in FACS permeabilizing solution (BD BioSciences) for 10 min prior to being stained with CD8-APC, CD3-PerCP, gamma interferon (IFN-γ)-PE, and tumor necrosis factor alpha (TNF-α)-FITC (BD BioSciences). The cells were washed twice in PBS containing 1% BSA and fixed in 1% paraformaldehyde before being acquired on a flow cytometer (FACSCalibur; BD Biosciences) and analyzed using FlowJo software (Tree Star).
RESULTS AND DISCUSSION
CD4+ CD25+ TR cells inhibit superantigen-induced IFN-γ expression in both CD4 and CD8 T cells.
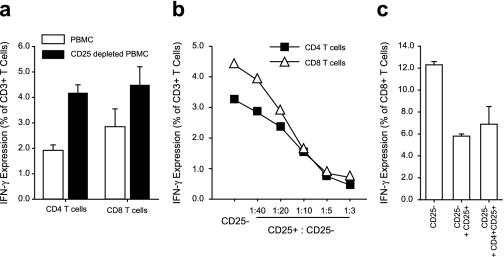
To assess the potential inhibitory capacity of TR cells, we compared IFN-γ expression in PBMC after superantigenic stimulation with or without CD25+ T cells. Depletion of CD25+ cells dramatically augmented the IFN-γ expression in both the CD4 and CD8 T-cell compartments (Fig.1a). When autologous CD25+ cells were added back to a culture of PBMC depleted of CD25+ T cells, the IFN-γ expression was suppressed in a dose-dependent manner (Fig. 1b). The suppressive activity was found in the CD4+ T-cell compartment of the CD25+ cell fraction (Fig. 1c), and the suppression of cytokine expression was not due to dilution of responding cells (data not shown). Similar results were obtained by analyzing TNF-α expression (data not shown).
FIG. 1.
TR cells suppress superantigen-induced cytokine production. IFN-γ expression in T cells in PBMC was compared with that of T cells in PBMC depleted of CD25+ cells (a). The cultures were stimulated for 18 h with SEB. Brefeldin A was added for the last 5 h to promote cytokine accumulation. Means ± standard errors of the mean are shown (n = 3). (b) CD25+ T cells were added back into PBMC depleted of CD25+ cells at increasing ratios. The CD25+ T cells were stained with CFSE before being added back and were gated out of the analysis. (c) CD25+ T cells and sorted CD25+ CD4+ T cells (>99% pure) were added back into PBMC depleted of CD25+ cells. Means ± standard errors of the mean are shown (n = 2).
Suppressive CD25+ T cells can be induced from CD25− T cells by activation with superantigen alone.
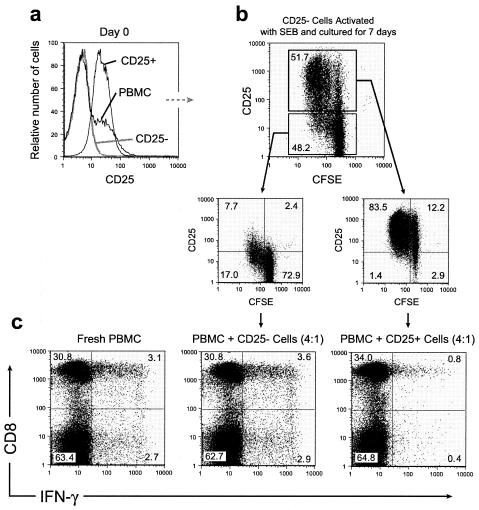
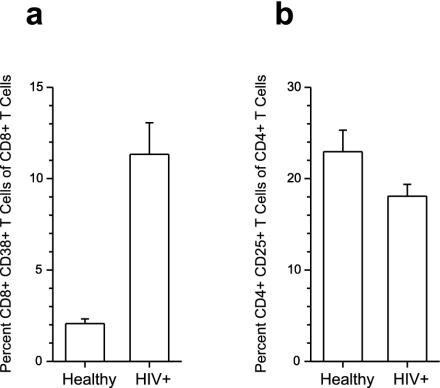
The CD4+ T-cell population in peripheral blood of healthy blood donors contains about 10 to 15% CD4+ CD25+ T cells (5). The role of these cells in controlling and suppressing autoreactive T cells is now well established, and the induction of the TR cells has been shown to take place in the thymus (10, 14, 19). However, induction of suppressive T cells specific for exogenous antigens that are not expressed in the thymus is likely to take place in the periphery. Therefore, we hypothesized that TR cells can be generated in vitro from peripheral CD25− cells. We depleted CD25+ T cells from PBMC (Fig. 2a), labeled the remaining cells with 6-carboxyfluorescein succinimidyl ester (CFSE), and cultured them for 7 days in medium containing SEB. At day 7, 40 to 60% of the T cells expressed CD25, as did a large majority of the proliferating cells (Fig. 2b). Next, we wanted to test whether the induced CD25+ cells had suppressive properties. When the CD25+ cells were added to PBMC stimulated with SEB, they suppressed IFN-γ expression in the responding T cells in a dose-dependent manner. No suppression was observed when the CD25− cells were added to the culture (Fig. 2c). From these results, we hypothesized that there may be an expansion of TR cells in conditions with persistent immune activation. HIV infection leads to chronic immune activation and is associated with increased frequency of CD38+ CD8+ T cells (Fig. 3a). However, in a cross-sectional study, we did not observe a higher percentage of CD4+ CD25+ T cells in peripheral blood in HIV-infected individuals (n = 10) than in healthy blood donors (n = 10) (Fig. 3b). This may be due to redistribution of CD4+ CD25+ T cells to lymphoid tissues. Alternatively, the suppressive T cells occurring in peripheral blood may represent a heterogenic population of CD4 T cells. In mice, CD4+ CD69+ T cells have been shown to possess suppressive activity (11). This population only partly overlapped with the CD4+ CD25+ T-cell subset. It is, therefore, possible that using CD25 expression to identify suppressive regulatory CD4+ T cells underestimates the real frequency of suppressive T cells in healthy as well as HIV-infected individuals.
FIG. 2.
Suppressive CD25+ T cells can be induced from PBMC depleted of CD25+ T cells after activation with SEB. PBMC were depleted of CD25+ cells (a), labeled with CFSE, and cultured in the presence of SEB for 7 days (b). At day 7 (c), the CD25+ and CD25− cell fractions were added into fresh PBMC cultures from the same donor. The cocultures were stimulated for 18 h with SEB. Brefeldin A was added for the last 5 h to promote cytokine accumulation. The CFSE-stained cells added into the fresh PBMC at day 7 were gated out of the analysis. Representative data are shown.
FIG. 3.
The frequency of TR cells in HIV patients is unaltered. PBMC from HIV patients (n = 10) and healthy subjects (n = 10) were stained with fluorochrome-labeled monoclonal antibodies and analyzed for the frequencies of CD4+ CD25+ T cells and CD8+ CD38+ T cells. Means ± standard errors of the mean are shown.
CD4+ CD25+ TR cells suppress antiviral immune responses.
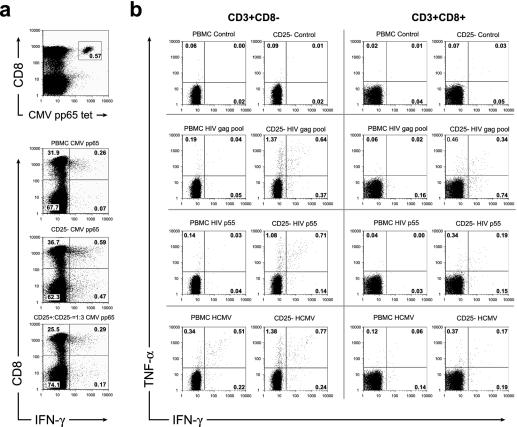
To investigate whether circulating CD25+ T cells suppress immune responses to viral antigens, we compared the IFN-γ expression of CMV-pp65-specific CD8+ T cells with or without depletion of CD25+ T cells. The frequency of HLA-A2/CMV pp65 tetramer+ CD8+ T cells was 0.57%, whereas the percentage of responding IFN-γ+ CD8+ T cells in the PBMC culture was 0.26% (Fig. 4a). Thus, less than 50% of the CD8+ T cells specific for this antigen responded with IFN-γ expression in this individual. However, when the PBMC culture was depleted of CD25+ T cells, the frequency of IFN-γ+ CD8+ T cells increased to 0.59%, similar to the frequency identified with the CMV pp65 tetramer. When CD25+ T cells were added back, the IFN-γ expression was suppressed to the level observed before depletion.
FIG. 4.
TR cells suppress antiviral immune responses. PBMC from a healthy CMV-infected individual were stained with a CMV pp65 tetramer and with cell surface markers (a). The frequency of tetramer-positive CD8+ T cells was compared with the frequency of IFN-γ-expressing T cells in PBMC, PBMC cultures depleted of CD25+ cells, and PBMC cultures depleted of CD25+ cells to which the depleted cells were added back in a 1:3 ratio. PBMC from an HIV-infected subject were stimulated with HIV antigens (b). The frequencies of IFN-γ- and TNF-α-expressing T cells in PMBC cultures and PBMC cultures depleted of CD25+ cells were compared. Representative data are shown.
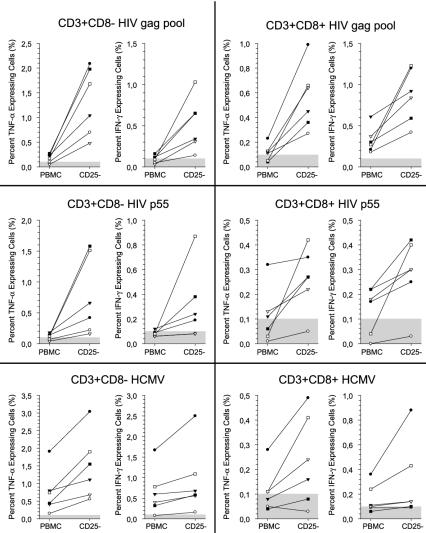
To further assess the degree of immunosuppression of antiviral immune responses, we measured intracellular IFN-γ and TNF-α expression in T cells in response to several HIV antigens in PBMC cultures and in PBMC cultures depleted of CD25+ T cells. In all HIV-infected subjects, the anti-HIV immune response was considerably increased in both the CD4 and CD8 T-cell populations after depletion of CD25+ T cells (Fig. 4b and 5). These results indicate that suppressive CD25+ T cells suppress the immune response to chronic viral antigens.
FIG. 5.
Depletion of TR cells enhances the antiviral immune response to HIV. PBMC from HIV-infected subjects (n = 6) were stimulated with HIV and CMV antigens. The frequencies of IFN-γ- and TNF-α-expressing T cells in PMBC cultures and PBMC cultures depleted of CD25+ cells were compared. The cells were gated on the CD3+ CD8− and CD3+ CD8+ T cells. The shaded area in each graph represents the level of detection. Note: the scale on the y axis differs in the panels.
Based on these results, we propose that chronic viral infection leads to induction in the periphery of a TR cell population which is involved in the suppression of antiviral immune responses. TR cells may thereby impair an otherwise successful immune response. Our data support a mechanism of antigen-specific induction of TR cells, which then exert suppression in a nonspecific manner (9, 27). In HIV infection, HIV-induced TR cells could potentially contribute to the generalized immunosuppression (16, 17, 20), and it is possible that manipulation of TR cells could help restore antigen-specific immune responsiveness in chronic viral infections, such as HIV infection.
Acknowledgments
We gratefully acknowledge assistance from Gerald Spotts and Paul Bradley in obtaining samples.
This work was supported by NIH grant U01 AI41531 (to D.F.N. and F.M.H.). D.F.N. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation. J.M. is supported by a scholarship from the Swedish Research Council and the Swedish Foundation for International Cooperation in Research and Higher Education (STINT).
REFERENCES
- 1.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245-1253. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 4.Bluestone, J. A., and A. K. Abbas. 2003. Opinion-regulatory lymphocytes: natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3:253-257. [DOI] [PubMed] [Google Scholar]
- 5.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J. Exp. Med. 193:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groux, H., A. O'Garra, M. Bigler, M. Rouleau, S. Antonenko, J. E. de Vries, and M. G. Roncarolo. 1997. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389:737-742. [DOI] [PubMed] [Google Scholar]
- 7.Grundstrom, S., L. Cederbom, A. Sundstedt, P. Scheipers, and F. Ivars. 2003. Superantigen-induced regulatory T cells display different suppressive functions in the presence or absence of natural CD4(+)CD25(+) regulatory T cells in vivo. J. Immunol. 170:5008-5017. [DOI] [PubMed] [Google Scholar]
- 8.Hara, M., C. I. Kingsley, M. Niimi, S. Read, S. E. Turvey, A. R. Bushell, P. J. Morris, F. Powrie, and K. J. Wood. 2001. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 166:3789-3796. [DOI] [PubMed] [Google Scholar]
- 9.Homann, D., A. Holz, A. Bot, B. Coon, T. Wolfe, J. Petersen, T. P. Dyrberg, M. J. Grusby, and M. G. Von Herrath. 1999. Autoreactive CD4+ T cells protect from autoimmune diabetes via bystander suppression using the IL-4/Stat6 pathway. Immunity 11:463-472. [DOI] [PubMed] [Google Scholar]
- 10.Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317-5326. [PubMed] [Google Scholar]
- 11.Iwashiro, M., R. J. Messer, K. E. Peterson, I. M. Stromnes, T. Sugie, and K. J. Hasenkrug. 2001. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc. Natl. Acad. Sci. USA 98:9226-9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonuleit, H., E. Schmitt, G. Schuler, J. Knop, and A. H. Enk. 2000. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 192:1213-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonuleit, H., E. Schmitt, M. Stassen, A. Tuettenberg, J. Knop, and A. H. Enk. 2001. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193:1285-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan, M. S., A. Boesteanu, A. J. Reed, A. L. Petrone, A. E. Holenbeck, M. A. Lerman, A. Naji, and A. J. Caton. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2:301-306. [DOI] [PubMed] [Google Scholar]
- 15.Kingsley, C. I., M. Karim, A. R. Bushell, and K. J. Wood. 2002. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J. Immunol. 168:1080-1086. [DOI] [PubMed] [Google Scholar]
- 16.Miedema, F., A. J. Petit, F. G. Terpstra, J. K. Schattenkerk, F. de Wolf, B. J. Al, M. Roos, J. M. Lange, S. A. Danner, J. Goudsmit, et al. 1988. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J. Clin. Investig. 82:1908-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musey, L. K., J. N. Krieger, J. P. Hughes, T. W. Schacker, L. Corey, and M. J. McElrath. 1999. Early and persistent human immunodeficiency virus type 1 (HIV-1)-specific T helper dysfunction in blood and lymph nodes following acute HIV-1 infection. J. Infect. Dis. 180:278-284. [DOI] [PubMed] [Google Scholar]
- 18.Ng, W. F., P. J. Duggan, F. Ponchel, G. Matarese, G. Lombardi, A. D. Edwards, J. D. Isaacs, and R. I. Lechler. 2001. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood 98:2736-2744. [DOI] [PubMed] [Google Scholar]
- 19.Papiernik, M., M. L. de Moraes, C. Pontoux, F. Vasseur, and C. Penit. 1998. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 10:371-378. [DOI] [PubMed] [Google Scholar]
- 20.Roos, M. T., F. Miedema, M. Koot, M. Tersmette, W. P. Schaasberg, R. A. Coutinho, and P. T. Schellekens. 1995. T cell function in vitro is an independent progression marker for AIDS in human immunodeficiency virus-infected asymptomatic subjects. J. Infect. Dis. 171:531-536. [DOI] [PubMed] [Google Scholar]
- 21.Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101:455-458. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151-1164. [PubMed] [Google Scholar]
- 23.Sanchez-Fueyo, A., M. Weber, C. Domenig, T. B. Strom, and X. X. Zheng. 2002. Tracking the immunoregulatory mechanisms active during allograft tolerance. J. Immunol. 168:2274-2281. [DOI] [PubMed] [Google Scholar]
- 24.Shevach, E. M. 2000. Regulatory T cells in autoimmmunity*. Annu. Rev. Immunol. 18:423-449. [DOI] [PubMed] [Google Scholar]
- 25.Stephens, L. A., C. Mottet, D. Mason, and F. Powrie. 2001. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 31:1247-1254. [DOI] [PubMed] [Google Scholar]
- 26.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton, A. M., and E. M. Shevach. 2000. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164:183-190. [DOI] [PubMed] [Google Scholar]
- 28.Woo, E. Y., H. Yeh, C. S. Chu, K. Schlienger, R. G. Carroll, J. L. Riley, L. R. Kaiser, and C. H. June. 2002. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J. Immunol. 168:4272-4276. [DOI] [PubMed] [Google Scholar]
- 29.Yamagiwa, S., J. D. Gray, S. Hashimoto, and D. A. Horwitz. 2001. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J. Immunol. 166:7282-7289. [DOI] [PubMed] [Google Scholar]