Abstract
The use of synthetic methcathinones, components of “bath salts,” is a world-wide health concern. These compounds, structurally similar to methamphetamine (METH) and 3,4-methylendioxymethamphetamine (MDMA), cause tachycardia, hallucinations and psychosis. We hypothesized that these potentially neurotoxic and abused compounds display differences in their transporter and receptor interactions as compared to amphetamine counterparts. 3,4-Methylenedioxypyrovalerone and naphyrone had high affinity for radioligand binding sites on recombinant human dopamine (hDAT), serotonin (hSERT) and norepinephrine (hNET) transporters, potently inhibited [3H]neurotransmitter uptake, and, like cocaine, did not induce transporter-mediated release. Butylone was a lower affinity uptake inhibitor. In contrast, 4-fluoromethcathinone, mephedrone and methylone had higher inhibitory potency at uptake compared to binding and generally induced release of preloaded [3H]neurotransmitter from hDAT, hSERT and hNET (highest potency at hNET), and thus are transporter substrates, similar to METH and MDMA. At hNET, 4-fluoromethcathinone was a more efficacious releaser than METH. These substituted methcathinones had low uptake inhibitory potency and low efficacy at inducing release via human vesicular monoamine transporters (hVMAT2). These compounds were low potency 1) h5-HT1A receptor partial agonists, 2) h5-HT2A receptor antagonists, 3) weak h5-HT2C receptor antagonists. This is the first report on aspects of substituted methcathinone efficacies at serotonin (5-HT) receptors and in superfusion release assays. Additionally, the drugs had no affinity for dopamine receptors, and high- mid-micromolar affinity for hSigma1 receptors. Thus, direct interactions with hVMAT2 and serotonin, dopamine, and hSigma1 receptors may not explain psychoactive effects. The primary mechanisms of action may be as inhibitors or substrates of DAT, SERT and NET.
Keywords: Methcathinone, Dopamine transporter, Serotonin transporter, Norepinephrine transporter, Serotonin receptor
1. Introduction
Psychoactive substituted methcathinones are a health concern, as reports of admissions to emergency departments due to overdose continue [1;2]. Adverse events include cardiac complications, anxiety, psychosis and death [3]. These compounds have been marketed singly or in mixtures collectively referred to as “bath salts” [1]. Herein we report the pharmacology of six substituted methcathinones: butylone, 4-fluoromethcathinone (4-FMC), 3,4-methylenedioxypyrovalerone (MDPV), 4-methyl-N-methylcathinone (mephedrone), 3,4-methylenedioxy-N-methylcathinone (methylone) and naphyrone. The compounds have structural similarities to METH and MDMA but have a ketone in the β-position as do methcathinone (Fig 1) and cathinone, the active ingredient in the plant product, khat (reviewed in [4]). Butylone, MDPV, and methylone contain a 3,4-methylenedioxy moiety, like MDMA. Mephedrone and 4-FMC differ from methcathinone by a methyl- or fluoro- addition on the phenyl ring. Naphyrone has high affinity and potency at hDAT, hSERT and hNET and produces acute sympathomimetic toxicity [5–8].
Figure 1.
Structures of substituted methcathinones, MDMA, and METH.
In drug discrimination assays, methcathinone substitutes for cocaine or amphetamine and stimulates non-exocytotic dopamine (DA) release [9;10]. Dopaminergic deficits following methcathinone exposure are prevented by pretreatment with DA receptor antagonists [11]. In rat drug discrimination studies, methylone substitutes maximally for amphetamine and MDMA but not the psychoactive substituted phenethylamine 2,5-dimethoxy-4-methylamphetamine [12], suggesting distinct neurochemical mechanisms of action. Butylone, MDPV and methylone increase locomotor activity, similar to METH, while mephedrone has been reported to both increase and decrease activity (similar to MDMA); locomotor stimulatory effects are partially or fully inhibited by DA receptor or 5-HT2A receptor antagonists [13;14]. Stimulants can increase or decrease locomotor activity, depending on dose (for examples, see [15;16]). Thus, MDPV has similarities to METH, methylone to both METH and MDMA, and mephedrone primarily to MDMA.
Pharmacological effects of several substituted methcathinones have been reported. Methylone inhibits neurotransmitter uptake with higher potency at DAT and NET compared to SERT and increases release of neurotransmitter in cells heterologously expressing the transporters, and in platelets and rat synaptosomes [6;8;17–19]. Mephedrone causes neurotransmitter release from rat brain synaptosomes at nanomolar concentrations, and with higher potency at DAT and NET than SERT [20]. Mephedrone, methylone and butylone have micromolar potencies at the VMAT2 in bovine chromaffin granules and rat striatal vesicles [14;17]. However, a detailed description of interactions of the six substituted methcathinones with NET release, VMAT2 and 5-HT receptor function and sigma1 receptors has not been reported.
To better understand the pharmacology of the substituted methcathinones, we tested their affinities and potencies at targets of stimulants such as METH, including the hDAT, hSERT, hNET and hVMAT2 in radioligand binding, uptake, and superfusion release assays. To determine if direct interactions with 5-HT receptors play a role in psychotomimetic sequelae to drug exposure, we tested drug affinity and efficacy at h5-HT1A, h5-HT2A and h5-HT2C receptors, known targets of hallucinogens like LSD. Because these drugs have a phenalkylamine basic structure, we measured their affinity for DA D1, D2, D3 and D4.4 receptors. Finally, we tested the affinity of the substituted methcathinones at hSigma1 receptors, an intracellular receptor for which METH has low micromolar affinity [21].
2. Materials and Methods
2.1. Materials
[125I]RTI-55, [3H]DA, [3H]5-HT, [3H]norepinephrine (NE), [3H]8-OH-DPAT, [125I]DOI, [3H]SCH23390, [3H]YM-09151-2, [3H](+)pentazocine and [35S]GTPγS were purchased from Perkin Elmer Life and Analytical Sciences (Boston, MA). [3H]DHTB and DHTB were purchased from American Radiolabeled Chemicals (St. Louis, MO). Cocaine, LSD, METH, MDMA, and methcathinone were generously supplied by the NIDA Drug Supply Program (Bethesda, MD). 4-FMC, butylone, mephedrone, methylone, MDPV, and naphyrone were supplied by NIDA (Bethesda, MD). FetalClone and bovine calf serum (BCS) was purchased from HyClone (Logan, UT) and fetal calf serum was purchased from Atlas Biologicals (Fort Collins, CO). The IP-1 kit was purchased from Cisbio (Bedford, MA) and SB242084 was purchased from R&D Systems (Minneapolis, MN). Most other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Culture and binding assay conditions are summarized in Table 1. The Kd and Bmax values listed in Table 1 were generated by the authors using the cell lines and conditions described in the text. All growth media included 100 units/ml penicillin and 100 µg/ml streptomycin.
Table 1.
Cell culture and binding assay conditions with Kd and Bmax values.
| Cell line | Medium | Medium supplements | Radioligand Concentration in assay |
Nonspecific binding | Kd (nM) | Bmax fmol/mg protein |
|---|---|---|---|---|---|---|
| HEK-hDAT | DMEM | 5% Fetal Clone 5% bovine calf serum 2 µg puromycin/mL |
[125I]RTI-55 0.04 nM |
5 µM mazindol | 1.83 ± 0.21a | 7900 ± 1300 a |
| HEK-hSERT | DMEM | 5% Fetal Clone 5% bovine calf serum 2 µg puromycin/mL |
[125I]RTI-55 0.04 nM |
5 µM imipramine | 0.98 ± 0.16 a | 850 ± 80 a |
| HEK-hNET | DMEM | 10% Fetal Clone 300 µg G418/mL |
[125I]RTI-55 0.04 nM |
5 µM mazindol | 12.1 ± 1.9 a | 3600 ± 500 a |
| HEK-hVMAT2 | DMEM | 5% Fetal Clone 5% bovine calf serum 300 µg G418/mL |
[3H]DHT B 7–10 nM |
10 µM Ro4-1284 | 38.0 ± 6.5 | 6.91 ± 0.49 |
| HEK-h5HT1A | DMEM | 10% Fetal Clone 300 µg G418/mL |
[3H]8-OH-DPAT 0.5 nM |
1 µM WAY-100635 | 5.0 ± 1.7 | 1670 ± 390 |
| HEK-h5HT2A | DMEM | 5% Fetal Clone 5% bovine calf serum 300 µg G418/mL |
[125I]DOI 0.1 nM |
10 µM 5-HT | 3.62 ± 0.59 | 612 ± 19 |
| HEK-H5HT2C | DMEM | 5% Fetal Clone 5% bovine calf serum 300 µg G418/mL |
[125I]DOI 0.07 nM |
10 µM 5-HT | 4.18 ± 0.46 | 900 ± 170 |
| LhD1 | DMEM | 10% Fetal Clone 400 µg G418/mL |
[3H]SCH-23390 0.18 nM |
1 µM SCH-23390 | 0.62 ± 0.29 | 1450 ± 220 |
| CHOp-D2 | alpha- MEM |
10% fetal calf serum 200 µg G418/mL |
[3H]YM-01915-2 0.2–0.5 nM |
1 µM chlorpromazine |
0.060 ± 0.010 | 624 ± 61 |
| CHOp-D3 | alpha- MEM |
10% fetal calf serum 200 µg G418/mL |
[3H]YM-01915-22 0.2– 0.5 nM |
1 µM chlorpromazine |
0.120 ± 0.020 | 2800 ± 210 |
| HEK-D4.4-AC1 | DMEM | 5% FetalClone 5% bovine calf serum 2 µg puromycin/ml 200 µg hygromycin/ml |
[3H]YM-01915-22 0.2– 0.5 nM |
1 µM haloperidol | 1.97 ± 0.19 | 5400 ± 600 |
| Cos7-hSigma1 | DMEM | 7% Fetal Clone 3% bovine calf serum |
[3H](+)-pentazocine 1.3 nM |
10 µM haloperidol | 3.63 ± 0.23 | 3190 ± 230 |
Values from Eshleman et al., 1999, with permission.
Radioligand binding assays were conducted with duplicate determinations. Assays were terminated by filtration using a Wallac 96-well harvester through Perkin Elmer filtermat A filters that for some assays were presoaked in 0.05% polyethylenimine ([3H]neurotransmitter uptake assays and binding assays for VMAT2, GTPγS, h5-HT1A, h5-HT2A, h5-HT2C, DA D1, DA D2, DA D3, DA D4.4). Tris (50 mM, pH 7.4 at 4°C, for h5-HT1A, h5-HT2A, h5-HT2C, hSigma1 binding assays) or saline (0.9% NaCl for all other filtration assays) was used during filtration. Scintillation fluid was added, and radioactivity retained on the filters was determined using a Perkin Elmer microbeta plate counter.
2.2. Biogenic amine transporters
2.2.1. Inhibition of [125I]RTI-55 binding to, and [3H]neurotransmitter uptake by, hDAT, hSERT or hNET in Clonal Cells
The methods for characterizing radioligand binding and functional uptake assays have been described previously [22]. Human embryonic kidney (HEK-293) cells expressing the recombinant hDAT (HEK-hDAT), hSERT (HEK-hSERT) or hNET (HEK-hNET) were used. Culture and binding assay conditions are summarized in Table 1. Binding assays were conducted with a total particulate membrane preparation. The uptake assay was conducted in triplicate and initiated by the addition of [3H]DA, [3H]5-HT, or [3H]NE (20 nM final concentration) to intact detached cells.
2.2.2. Biogenic amine transporters: [3H]Neurotransmitter release
The methods for characterizing drug-induced release of pre-loaded [3H]neurotransmitter from HEK-hDAT, HEK-hSERT and HEK-hNET cells have been described previously [23]. In brief, cells were loaded with 120 nM [3H]DA for 15 min at 30°C (HEK-hDAT), 20 nM [3H]5-HT for 30 min at 25°C (HEK-hSERT) or 120 nM [3H]NE for 15 min at 30°C (HEK-hNET). Cells were centrifuged, resuspended in Krebs HEPES buffer (pH 7.4; 122 mM NaCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 10 µM pargyline, 100 µM tropolone, 0.2% glucose and 0.02% ascorbic acid, buffered with 25 mM HEPES), and added to the superfusion device (Brandel, Gaithersburg, MD). Buffer was perfused for 12–15 min, and the last 6 min (3 fractions) were collected. Drug was added, and 24 min (12 fractions) of effluent were collected. SDS (1%) was then perfused, and 4 fractions were collected over a 10 min period. Data were normalized to the maximal effects of the positive controls METH (hDAT and hNET) and p-chloroamphetamine, (hSERT). Radioactivity in the samples was determined using conventional liquid scintillation spectrometry. Fractional release was the amount of radioactivity in a fraction divided by the total radioactivity remaining in the sample.
2.3. hVMAT2
2.3.1 Inhibition of [3H]DHTB Binding to, and [3H]5-HT uptake by, the human vesicular monoamine transporter 2 (hVMAT2) in Clonal Cells
The hVMAT2 cDNA was a generous gift from Robert Edwards (University California San Francisco, CA). The hVMAT2 cDNA was subcloned into the vector pcDNA3.1-neo, and orientation and sequence of the insert were verified as described previously [24]. HEK cells were transfected with the hVMAT2 vector using Lipofectamine (Invitrogen, Carlsbad, CA) and selected with G418 (700 µg/ml). Membrane preparation: HEK-hVMAT2 cells were grown until confluent on 15 cm plates. The membrane preparation was an adaptation of the method of [25]. Medium was removed from the plate, solution A [sucrose (0.32 M) with protease inhibitors] was added, and the cells were scraped from the plate. Cells were homogenized with 12 strokes of a glass/glass homogenizer. The homogenate was centrifuged at 800xg for 10 min. The supernatant was removed and saved, and the pellet was resuspended, homogenized and centrifuged as described above. The supernatants were combined and centrifuged at 10,000xg for 20 min. The pellet was resuspended in solution A (0.75 ml). The membranes were osmotically shocked by addition of 3.5 volumes ice cold water and homogenized by 5 strokes of a glass/Teflon homogenizer. The osmotic shock disrupts membrane integrity and facilitates the release of the contents of membranous structures. The osmolarity was reestablished with addition of Tris (25 mM final, pH 7.4 at 4°C), potassium tartrate (100 mM), and MgSO4 (0.9 mM final). The homogenate was centrifuged at 20,000xg for 20 min.
[3H]DHTB binding assay: The pellet was resuspended in VMAT2 buffer (100 mM potassium tartrate, 25 mM Tris, 4 mM KCl, 2 mM MgSO4, 1.7 mM ascorbic acid, 100 µM tropolone and 1 µM pargyline, pH 7.4 at 25°C). The binding assay was conducted in a final volume of 0.25 ml. This buffering system was developed in part because buffers containing high concentrations of chloride ions may disrupt proton electrochemical gradients if chloride channels are present in vesicular membranes [26]. Following 60 min incubation in the dark at room temp, binding was terminated by filtration as described above.
[3H]5-HT uptake assay: The hVMAT2 transports DA, NE and 5-HT; [3H]5-HT was used for the uptake assay because it had slightly higher potency than DA or NE in preliminary experiments (data not shown). The final pellet was resuspended in VMAT2 buffer supplemented with MgATP (2 mM) using 2 gentle strokes of a Teflon/glass homogenizer. The uptake assay was conducted in duplicate and included membrane preparation, drug, [3H]5-HT (40 nM), and VMAT2 buffer with 2 mM MgATP in a final volume of 0.25 ml. Membranes were preincubated with drugs for 10 minutes at 30°C. After addition of [3H]5-HT, uptake was conducted at 30°C for 6 min and terminated by filtration as described above. Specific uptake was defined as the difference in uptake observed in the absence and presence of reserpine (1 µM).
2.3.2. hVMAT2: [3H]NE release assay
Two 15 cm plates of HEK-hVMAT2 cells were preincubated with VMAT2 buffer for 10 min at 37°C. The buffer was removed, and cells were gently scraped into VMAT2 buffer with 2 mM ATP. [3H]NE was used for the release assays because it had less non-transporter-mediated leakage due to its lower membrane solubility compared to DA and 5-HT. [3H]NE (125 nM) was added, and uptake was conducted for 20 min at 30°C. Drug serial dilutions were prepared in VMAT2 buffer with 1 mM ATP. Cells (280 µl) were added to the superfusion device. Release was conducted at 30°C. Buffer was perfused for 12 min, with the last 6 min (3 fractions) collected for a baseline. Drug was added, and 22 min (11 fractions) of effluent were collected. Ultrapure water was then perfused to lyse the cells, and 4 fractions (10 min) were collected. Radioactivity in each fraction was expressed as a percent of the radioactivity remaining in the cells at that time (the sum of that fraction and all subsequent fractions). Baselines were determined for each time course, defined as the radioactivity in the two fractions with lowest cpms.
2.4. 5-HT receptors
2.4.1 h5-HT1A Receptor: [3H]8-OH-DPAT binding and [35S] GTPγS binding
Human embryonic kidney cells expressing the human 5-HT1A receptor (HEK-h5-HT1A) were used. The methods for transfection of HEK cells, cell membrane preparation, [3H]8-OH-DPAT binding, and data analysis have been described previously [23]. Culture and binding assay conditions are summarized in Table 1.
The method for [35S]GTPγS binding has been described [23] and was adapted from [27]. In brief, cell membranes (40–75 µg protein) were preincubated (10 min, room temperature) with test compound in duplicate in assay buffer. The reaction was initiated by addition of GDP (3 µM) and [35S]GTPγS (~150,000 cpm, 1350 Ci/mmol) in a final volume of 1 ml. The reaction was incubated for 60 min at room temperature on a rotating platform and terminated as described above. Agonist efficacy is expressed relative to that of 100 nM 5-HT.
2.4.2. h5-HT2A and h5-HT2C Receptors: [125I]DOI binding
Binding to 5-HT2A and 5-HT2C receptors was tested in HEK-293 cells expressing either the h5-HT2A receptor (HEK-h5-HT2A cells) or the h5-HT2C receptor (HEK-5-HT2C cells) adapting methods described earlier [23;28]. The cDNAs for the h5-HT2A and h5-HT2C receptors were purchased from Missouri S&T cDNA Resource Center (Rolla, MO). Culture and binding assay conditions are summarized in Table 1.
2.4.3. h5-HT2A and h5-HT2C Receptors: Inositol monophosphate (IP-1) formation
Activation of h5-HT2A and h5-HT2C receptors was tested by measuring the accumulation of inositol monophosphate using the IP-1 Elisa kit (Cisbio, Bedford, MA) as described previously [23]. Briefly, cells were plated at a density of 400,000 cells per well in 24 well plates in DMEM supplemented with 10% charcoal-stripped FetalClone and, for h5-HT2C cells, 450 µg G418/ml. The next day, medium was removed, cells were rinsed and preincubated for 60 min with DMEM, supplemented with 450 µg G418/ml for h5-HT2C cells. After removal of medium, stimulation buffer without or with antagonists was added. After 10 min incubation, agonists were added and plates were incubated for 60 min. Cells were lysed for 30 min, and 50 µl aliquots of the lysates were added to the IP-1 plate. The assay was conducted according to kit instructions. Stimulated IP-1 formation was normalized to the maximal effect of 5-HT, which was determined in each assay. To determine inhibitory potency and efficacy, compounds were tested in the presence of 100 nM 5-HT and normalized to the inhibitory efficacy of 10 µM ketanserin (h5-HT2A receptors) or 100 nM SB242084 (h5-HT2C receptors).
2.5. DA D1, D2, D3 and D4.4 receptors: [ 3 H]SCH-23390 and [ 3 H]YM-09151-2 binding
DA D1 [3H]SCH23390 Binding: Mouse fibroblast cells expressing the human DA D1 receptor at high density (LhD1 cells) were obtained from Stanford Research Institute (SRI, Menlo Park, CA). The assay was conducted as described previously [29]. Cells were scraped from culture plates and centrifuged at 500 × g for 5 min. The pellet was overlaid with assay buffer (50 mM Tris, pH 7.4 at 25°C) containing 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, and 1 mM MgCl2) and frozen at −70°C. On the day of the experiment, the pellet was homogenized in assay buffer with a Polytron. Cell homogenate (10–15 µg protein) was added to wells containing test drug or buffer. After 10 min preincubation, [3H]SCH-23390 for a final assay volume of 1 ml. After incubation at 25°C for 60 min, the reaction was terminated by filtration as described above.
Chinese hamster ovary (CHO) cells expressing the human DA D2 or D3 receptors (CHOp-D2 or CHOp-D3, provided by SRI) and HEK cells coexpressing the human D4.4 receptor and adenylate cyclase type I (HEK-D4.4-AC1, a generous gift from Dr. Kim Neve, Oregon Health and Science University, Portland, OR) were used. The assay was conducted as described previously [29]. Membranes were prepared according to the procedures described for D1 cells, using D2/D3/D4.4 binding buffer (50 mM Tris containing 120 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 4 mM MgCl2, and 1 mM EDTA, pH 7.4). Cell homogenate (10–15 µg protein for D2, 7–10 µg protein for D3 and D4.4) was added to wells containing test drug or buffer. After 10 min, [3H]YM-09151-2 was added. After incubation at 25°C for 60 min, the reaction was terminated as described above.
2.6. hSigma1 receptors: [3H]Pentazocine binding
The full length coding region of the human sigma-1 receptor cDNA was obtained from OriGene (Rockville, MD). Sigma1 receptor cDNA was prepared using Qiagen (Chatwsorth, CA) and Invitrogen Maxiprep kits following transformation of XL10-Gold Ultracompetent E. coli cells (Agilent, Santa Clara, CA) and the sequence was confirmed. COS-7 cells were transfected with 24 µg hSigma1 receptor cDNA using Lipofectamine 2000 (Invitrogen). Cell membrane preparation methods were adapted from [21]. In brief, cells were scraped from the plate in phosphate-buffered saline and pelleted, the pellet was resuspended in 5 mM Tris (pH 7.4, 4°C) with 5 mM MgCl2, homogenized with a Polytron and centrifuged at 35,000xg for 60 min. The pellet was resuspended in 50 mM Tris buffer (pH 7.4, 4°C), and centrifuged as above. The final pellet was resuspended in binding buffer (50 mM Tris, pH 8.0, 37°C) and homogenized immediately prior to use. Each assay tube contained test compound or vehicle control, [3H](+)-pentazocine, membrane suspension (~ 13 µg protein), and binding buffer for a final volume of 1 ml. Preliminary experiments determined that radioligand binding was linear over the range of 2–13 µg protein, and that binding reached equilibrium in 3 h at 37°C. Little to no specific binding was detected in non-transfected COS-7 cells (data not shown). Reactions were terminated by filtration as described above.
2.7. Data analysis
For competition binding results, data were normalized to the specific binding in the absence of drug. Three or more independent competition experiments were conducted with duplicate determinations. GraphPAD Prism (La Jolla, CA) was used to analyze the ensuing data, with IC50 values converted to Ki values using the Cheng-Prusoff equation (Ki=IC50/(1+([drug*]/Kd drug*))), where drug* was the radioligand used in the binding assays [30], and was determined using the described assay conditions. The Kd values used in the equations are listed in Table 1 for each receptor. Differences in affinities were assessed by one way ANOVA using the logarithms of the Ki values for test compounds and standards. Dunnett’s multiple comparison test was used to compare test compounds to a drug standard, typically METH.
For release assays, area under the curve (AUC) for fractional release in the absence or presence of test compound over time was calculated using GraphPad Prism, and EC50 values were determined using logarithms of drug concentrations and sigmoidal dose-response nonlinear regression. For functional assays, GraphPAD Prism was used to calculate either EC50 (agonist) or IC50 (antagonist) values using data expressed as % total specific uptake for transporters, % 5-HT-stimulation for 5-HT1A-stimulated GTPγS binding and 5-HT2A- and 5-HT2C-receptor-mediated IP-1 formation. For functional assays, differences in potencies were assessed by one way ANOVA using the logarithms of the EC50 values for test compounds and standards. Dunnett’s multiple comparison test was used to compare test compounds to a drug standard, typically METH.
3. Results
3.1. hDAT, hSERT and hNET: Inhibition of [125I]RTI-55 binding and [3H]neurotransmitter uptake
The affinities of the six substituted methcathinones, as well as METH, MDMA and methcathinone and the standard compounds cocaine and mazindol for the hDAT, hSERT and hNET were assessed (Table 2). In the radioligand binding assays, there were similarities and differences in the rank order of affinities of compounds among the transporters. For instance, the four compounds with the highest affinities for hDAT, hSERT and hNET were naphyrone, MDPV, mazindol, and cocaine, although their rank orders differed among the transporters.
Table 2.
Affinity and potency of substituted methcathinones and standard compounds at hDAT, hSERT and hNET.
| Inhibition of [125I]RTI-55 binding Ki ± sem (µM)(n) |
Inhibition of [3H]neurotransmitter uptake IC50 ± sem (µM)(n) |
|||||
|---|---|---|---|---|---|---|
| Drug | hDAT | hSERT | hNET | hDAT [3H]DA | hSERT [3H]5-HT | hNET [3H]NE |
| Butylone | 1.73 ± 0.27(7) | 10.5 ± 1.9(5) | 7.98 ± 0.55 (3) | 0.211 ± 0.054(6) | 1.97 ± 0.43(5) | 1.06 ± 0.24 (4) |
| 4-FMC | 10.4 ± 3.3(6) | >100 µM(6) | 35 ± 10(6) | 0.273 ± 0.074 (4) | >10 µM(3) | 0.127 ± 0.020(4) |
| MDPV | 0.0194 ± 0.0032 (3) | 1.25 ± 0.21 (3) | 0.107 ± 0.034 (3) | 0.0126 ± 0.0024(3) | 1.38 ± 27(3) | 0.0188 ± 0.0021 (3) |
| Mephedrone | 4.80 ± 0.75 (3) | 21.0 ± 4.8(4) | 11.8 ± 4.0(4) | 0.098 ± 0.027 (4) | 0.51 ± 0.15(5) | 0.0536 ± 0.0087 (3) |
| Methylone | 5.02 ± 0.29 (3) | 95.5 ± 4.3 (3) | 16.5 ± 4.9(6) | 0.343 ± 0.024 (4) | 1.92 ± 0.35(5) | 0.234 ± 0.055 (8) |
| Naphyrone | 0.01077 ± 0.00076 (3) | 0.0149 ± 0.0042 (3) | 0.059 ± 0.015(3) | 0.0126 ± 0.0031 (5) | 0.0593 ± 0.0097 (4) | 0.035 ± 0.014(11) |
| METH | 3.96 ± 0.92 (3) | 240.5 ± 8.9 (3) | 1.39 ± 0.12(6) | 0.026 ± 0.010(4) | 4.1 ± 1.3(7) | 0.0260 ± 0.0077 (6) |
| MDMA | 22.0 ± 5.1 (3) | 14.7 ± 2.9(4) | 30.9 ± 5.6 (3) | 0.201 ± 0.047(5) | 0.109 ± 0.017 (5) | 0.0242 ± 0.0050 (4) |
| Methcathinone | 3.3 ± 1.1 (3) | >100 µM(3) | 4.48 ± 0.49 (6) | 0.144 ± 0.032 (4) | 13.5 ± 2.9(6) | 0.0311 ± 0.0051(3) |
| Cocaine | 0.558 ± 0.082 (10) | 0.234 ± 0.036(5) | 1.38 ± 0.29 (7) | 0.370 ± 0.072 (9) | 0.257 ± 0.048 (7) | 0.217 ± 0.025(8) |
| Mazindol | 0.041 ± 0.019(9) | 0.026 ± 0.012(4) | 0.0054 ± 0.0016(7) | 0.0300 ± 0.0081 (7) | 0.0307 ± 0.0055 (3) | 0.00185 ± 0.00049 (9) |
(n) Number of independent experiments conducted in duplicate (binding) or triplicate (uptake).
Data are normalized to specific binding or specific uptake in the absence of drugs. Drugs were tested in binding assays at concentrations ranging from 1 nM to either 10 µM or 100 µM. Hill slopes for binding ranged from −0.58 to −1.23.
METH and 4-FMC had the lowest affinity for hSERT, while MDMA and 4-FMC had the lowest affinity for hDAT and hNET. In addition, the potency of these compounds to inhibit [3H]neurotransmitter uptake was determined. Rank orders of potency were very different from binding affinities. The compounds with the highest potencies were naphyrone, MDPV, mazindol and METH at hDAT; mazindol, naphyrone, MDMA and mephedrone at hSERT; and mazindol, MDPV, MDMA, and METH at hNET. All compounds tested had moderate to high potency at hDAT (lC50 values lower than 350 nM) and, except for butylone, moderate to high potency at hNET (lower than 250 nM). In contrast, there were five compounds with very low potencies at hSERT (greater than 1 µM).
Looking at specific compounds, MDPV had high affinity (Ki) for hDAT, moderate affinity for hNET, and low affinity for hSERT (Table 2). Naphyrone had high affinity for both hDAT and hSERT and slightly lower affinity for hNET. At hDAT, MDPV had similar potency at inhibition of uptake (IC50 value = 0.0126 nM) compared to the Ki value for radioligand binding (0.019 nM, Table 2). For naphyrone and MDPV, the ratio of Ki value to IC50 value at each of the transporters is less than six, indicating that binding affinity is predictive of uptake potency. This similarity of binding affinity and uptake potency at each transporter is also observed for cocaine and mazindol, which are both uptake blockers at the transporters. Thus, this pharmacological pattern suggests that MDPV and naphyrone may primarily interact with the transporters as uptake blockers.
In contrast, butylone, 4-FMC, mephedrone and methylone have low affinities for the [125I]RTI-55 binding site ( micromolar range) of hDAT, hSERT and hNET (Table 2). METH, MDMA and methcathinone also have low affinities for the [125I]RTI-55 binding site on the transporters, and these compounds have higher potency at inhibiting uptake at the transporters compared to their Ki values (Table 2), as compared to MDPV and naphyrone. For mephedrone, the ratios of binding Ki to uptake IC50 are 49, 41, and 220 and for methylone the ratios are 15, 50 and 71 at the hDAT, hSERT and hNET, respectively. For 4-FMC, these ratios are 38 and 276 at the hDAT and hNET, respectively, while the ratio cannot be determined at hSERT. The ratios of Ki to uptake IC50 are lower for butylone: 8.2, 5.3, and 7.5 at the hDAT, hSERT and hNET, respectively. Similar to 4-FMC, mephedrone and methylone, the ratios of binding Ki to uptake IC50 for METH are 152, 59, and 53, for MDMA the ratios are 109, 135, 1280, and for methcathinone the ratios are 23, >7.4, and 144 at hDAT, hSERT, and hNET, respectively. METH and MDMA are substrates at the transporters, and we and others have previously observed that substrates have higher potency at inhibition of uptake compared to affinity for radioligand binding sites [22;31]. Thus, the results suggest that mephedrone and methylone are substrates at hDAT, hNET and hSERT; 4-FMC is a substrate at least at hDAT and hNET; and butylone may be a substrate, but has less selectivity for inhibition of uptake.
Based on potencies to inhibit transport of [3H]neurotransmitters, butylone has relative selectivity for hDAT > hNET > hSERT. 4-FMC, mephedrone, methylone and methcathinone have similar relative selectivities for inhibition of uptake for hNET > hDAT > hSERT while METH has relative selectivity hDAT similar to hNET >> hSERT. MDMA is unique in having higher potency at hSERT compared to hDAT, but is most potent at hNET. Thus, the substituted methcathinones that are likely substrates (i.e. 4-FMC, mephedrone and methylone) have an effect on the transporters most similar to the parent compound methcathinone.
3.2. hDAT, hSERT and hNET: Substituted methcathinone efficacy and potency at stimulating release of preloaded [3H]neurotransmitter
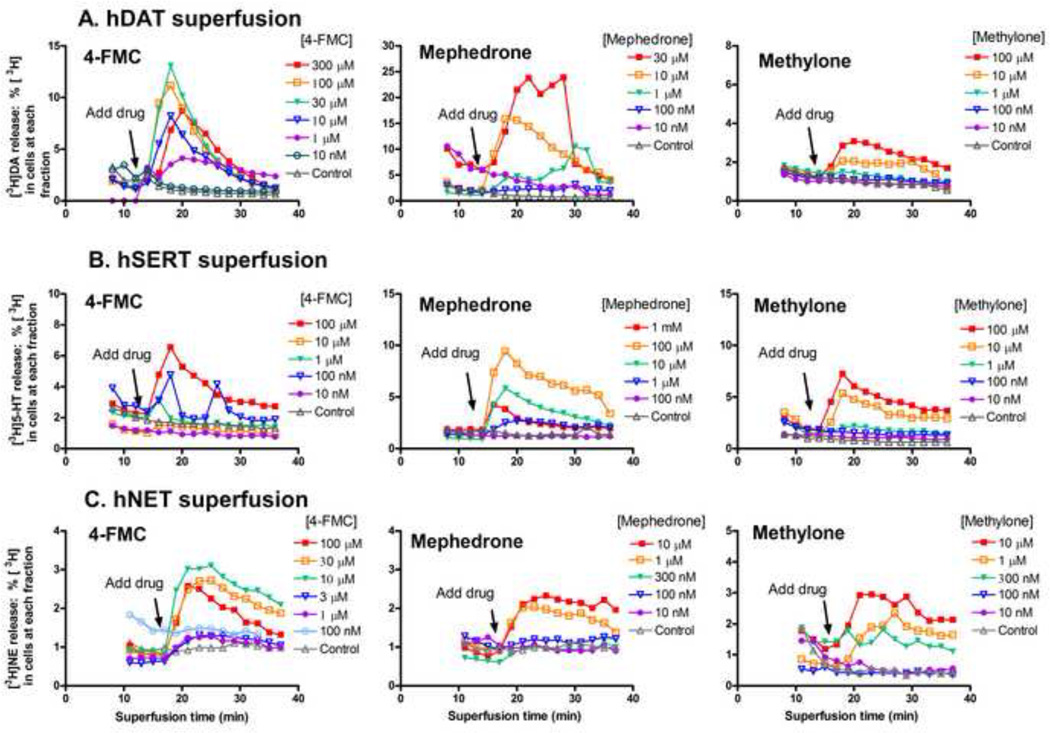
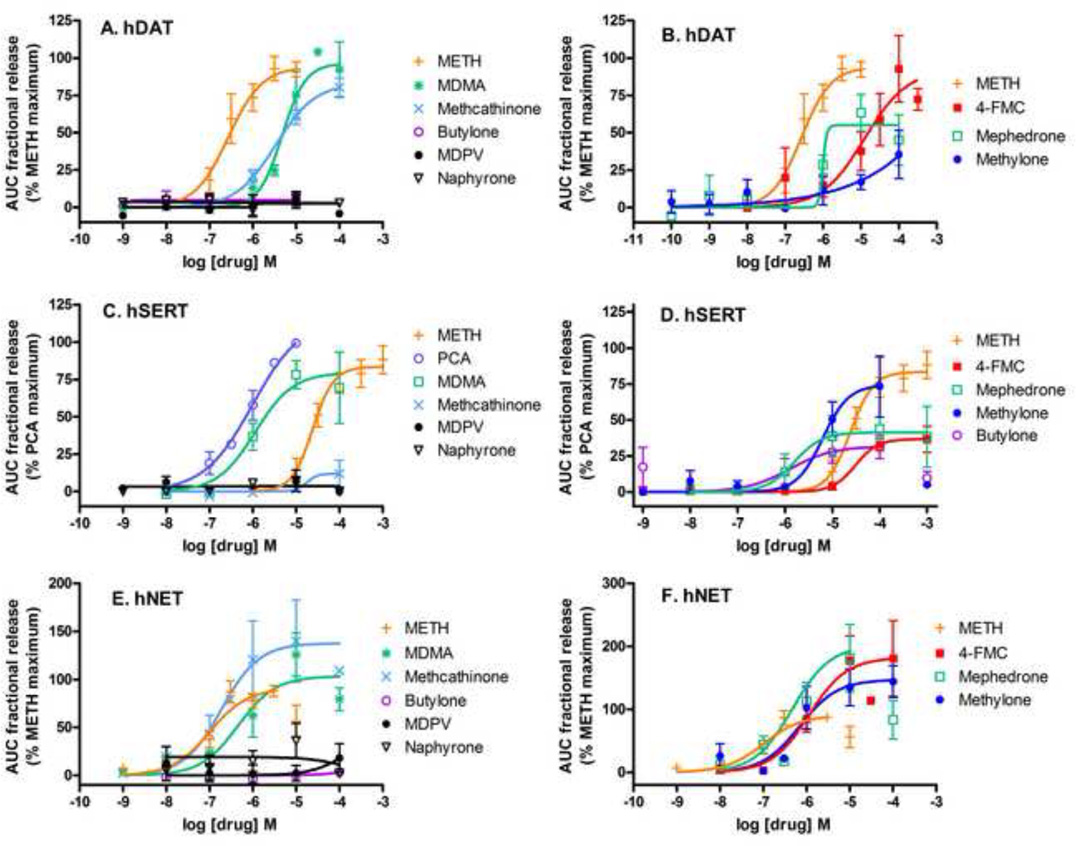
To determine if the substituted methcathinones stimulate release of neurotransmitter via the transporters, intact cells were preloaded with [3H]neurotransmitter and assays were conducted with a superfusion device, as described in the methods. One advantage of this system is that released [3H]neurotransmitter is removed continuously, minimizing reuptake of released [3H]neurotransmitter, which can be a confound [8]. Representative release time courses for effective compounds at the hDAT, hSERT and hNET are shown in Figure 2, the average concentration response curves for the substituted methcathinones and standards are shown in Figure 3, and the EC50 values and maximal efficacies are given in Table 3.
Figure 2.
Time courses of [3H]neurotransmitter release induced by the substituted methcathinones 4-FMC, mephedrone and methylone from HEK-hDAT, -hSERT and -hNET cells. Data shown are representative experiments. Data are normalized to percent release of [3H]neurotransmitter remaining in cells at each time point. The last three buffer fractions prior to addition of drug and the 12 fractions in the presence of drug are shown.
Figure 3.
Substituted methcathinones, METH, and MDMA dose-response curves for release of [3H]neurotransmitter from HEK-hDAT (A,B), -hSERT (C,D) and -hNET (E,F) cells. The area under the curve (AUC) for each drug concentration was normalized to the maximal effect of METH for that experiment (hDAT and hNET) or the maximal effect of PCA (hSERT). A, C and E: Data show the lack of effect of minimal releasers compared to METH, methcathinone, MDMA and, for SERT, PCA. B, D, and F: Data show the effects of releasers at each or the transporters compared to METH.
Table 3.
Potency and efficacy of substituted methcathinones and standard compounds to release preloaded [3H]neurotransmitter from HEK-hDAT, HEK-hSERT and HEK-hNET cells.
| Drug | Drug-induced release of [3H]neurotransmitter EC50 ± sem (µM)(n) % maximum release ± sem* |
||
|---|---|---|---|
| hDAT [3H]DA | hSERT [3H]5-HT | hNET [3H]NE | |
| Butylone | >10 µM(5) 5.4 ± 3.8% |
3.1 ± 1.4 (6) 30.1 ± 6.5% |
>100 µM (3) 3.6 ± 2.2% |
| 4-FMC | 17.8 ± 7.6(5) 98 ± 14% |
39 ± 14 (6) 39.3 ± 9.7% |
1.53 ± 0.43 (5) 194 ± 58% |
| MDPV | >100 µM (3) 4.8 ± 5.3% |
>100 µM (3) 6.9 ± 2.1% |
>100 µM (5) 18 ± 15% |
| Mephedrone | 1.19 ± 0.34 (5) 68 ± 11% |
11.9 ± 4.9 (4) 53 ± 13% |
0.41 ± 0.13 (6) 152 ± 32% |
| Methylone | 11.8 ± 5.6 (8) 41 ± 12% |
6.7 ± 2.78 (8) 78 ± 18% |
0.426 ± 0.067 (4) 122 ± 32% |
| Naphyrone | >100 µM (2) <6% |
7.5 ± 3.0 (3) 20.1 ± 9.3% |
>100µM (3) 36 ± 18% |
| METH | 0.40 ± 0.12 (4) 101.9 ± 4.9% |
22.45 ± 0.24 (4) 97.6 ± 7.2% |
0.128 ± 0.036 (6) 92.7 ± 5.2% |
| MDMA | 4.8 ± 2.3 (5) 104 ± 14% |
1.04 ± 0.27 (4) 74 ±12% |
0.57 ± 0.19 (7) 116 ± 11% |
| Methcathinone | 3.57 ± 0.68 (3) 83.3 ± 4.9 |
>100 µM (3) 21.1 ± 9.8% |
0.228 ± 0.066 (4) 149 ± 42% |
| PCA | 0.80 ± 0.27 (4) 106.4 ± 2.0% |
||
(n) Number of independent experiments.
Maximum release is defined as the maximum release (maximal AUC) induced by METH (1 or 10 µM, hDAT; 0.3 or 1 µM, hNET) or p-chloroamphetamine (PCA, 10 µM, hSERT) for each experiment.
At the hDAT, 4-FMC (10 µM to 300 µM) elicited [3H]DA release that peaked about 6 min after addition of drug, with all concentrations above 10 nM having some effect (Fig 2A). Mephedrone was also an efficacious releaser of [3H]DA, with 30 µM eliciting maximal release 8 min after addition of drug. Methylone was less potent, with 100 µM eliciting the highest release by 6 min after addition of drug. In contrast, butylone, MDPV, and naphyrone had minimal releasing efficacy at concentrations ranging from 1 nM to 100 µM (Fig 3A). Comparing the maximal drug-induced releases (Fig 3A and 3B, Table 3, p<0.01, one-way ANOVA), MDMA, methcathinone, 4-FMC and mephedrone elicited maximal [3H]DA release similar to METH (ps>0.05, Dunnett’s multiple comparison test), while methylone was less efficacious (41%, p<0.01). Comparing the potencies of drugs to induce [3H]DA release (Table 3, p<0.01,one-way ANOVA), 4-FMC (45-fold), methylone (30 fold) and MDMA (18-fold) were less potent than METH (p<0.01, p<0.05, p<0.05, respectively, Dunnett’s test) while mephedrone and methcathinone had similar potencies (p>0.05).
At the hSERT, 4-FMC, mephedrone and methylone elicited release of [3H]5-HT that peaked about 6 min after addition of drug (Fig 2B). The highest concentration tested sometimes elicited less release, as observed with 1 mM methylone (Fig 3D), which may be due to the high concentrations of methylone that are transported inward competing with intracellular [3H]5-HT for reverse transport. Butylone elicited release only at the three highest concentrations tested, 1 µM, 10 µM and 100 µM. In contrast, MDPV and naphyrone had minimal releasing efficacy at concentrations ranging from 1 nM to 100 µM (Fig 3C). Compared to the maximal release elicited by the standard compound p-chloroamphetamine (PCA, Fig 3C, Table 3, p<0.0001, one-way ANOVA), METH and MDMA elicited similar amounts of [3H]5-HT release (p>0.05, Dunnett’s test, Table 3), while methcathinone was significantly less efficacious (p<0.01). Compared to the potency of PCA (p<0.0001, one-way ANOVA), MDMA had similar potency as PCA (p>0.05, Dunnett’s test, Table 3), while METH was 28 fold less potent (p<0.01). Comparing the substituted methcathinone potencies to that of METH (Fig 3D, Table 3, p<0.01, one-way ANOVA), butylone had higher potency (p<0.05, Dunnett’s test), while 4-FMC, mephedrone, methylone, and naphyrone had similar potencies to METH (p>0.05, Table 3). Comparing the substituted methcathinone efficacies to those of METH (p<0.01, one-way ANOVA), mephedrone and methylone had similar efficacies compared to METH (p>0.05, Dunnett’s test) while butylone, 4-FMC and naphyrone were less efficacious (p<0.05).
At the hNET, 4-FMC, mephedrone and methylone elicited release of [3H]NE that peaked 8–16 min after addition of drug, with concentrations above 10 nM having some effect (Fig 2C). The highest concentration tested sometimes elicited less release, as observed with 100 µM mephedrone (Fig 3F). Butylone, MDPV and naphyrone had minimal releasing efficacy at concentrations ranging from 10 nM to 100 µM (Fig 3E). Compared to the maximal release elicited by METH (Fig 3F, Table 3), 4-FMC released more [3H]NE than METH (p<0.05, Dunnett’s test, Table 3), while mephedrone, methylone, MDMA and methcathinone elicited similar amounts of [3H]NE release (p>0.05). Compared to the potency of METH, 4-FMC, mephedrone and methylone had lower potencies (p<0.05, Dunnett’s test, Table 3).
For compounds that stimulated release via the transporters, 4-FMC, mephedrone, METH and methcathinone had relative potencies of hNET > hDAT > hSERT, while methylone and MDMA had relative potencies of hNET > hSERT > hDAT.
3.3. hVMAT2: Inhibition of [3H]DHTB binding and [3H]5-HT uptake
At hVMAT2, the compounds used as standards, RO4–1284 and reserpine, had moderate affinity displacing specific [3H]DHTB binding (Table 4). Naphyrone had the highest affinity of the tested compounds, (Ki = 119 µM). The other substituted methcathinones had very low to no measurable affinity, up to the maximal concentration tested (1 mM). METH and MDMA had high micromolar affinity for VMAT2, while methcathinone had no measurable affinity at concentrations up to 1 mM (Table 4). In the [3H]5-HT uptake assay, reserpine had low nanomolar potency and RO4–1284 had mid nanomolar potency to block uptake. All other compounds tested had IC50 values in the micromolar range or higher. Compared to the potency of METH to inhibit uptake (p<0.0001, one-way ANOVA), butylone, 4-FMC, mephedrone and methcathinone had lower potency (p<0.01, p<0.01, p<0.01, p<0.05, respectively, Dunnett’s test) while MDMA and naphyrone had similar potency (p>0.05). There was no measurable potency for MDPV and methylone.
Table 4.
Substituted methcathinones and standard compounds: Affinity for the [3H]DHTB binding site and potency to inhibit [3H]5-HT uptake and stimulate release of [3H]NE at hVMAT2.
| Drug | Inhibition of [3H]DHTB binding Ki ± sem (µM)(n) |
Inhibition of [3H]5-HT uptake IC50 ± sem (µM)(n) |
Drug-induced release of [3H]NE EC50 ± sem (µM) (n) |
Release of [3H]NE % maximum release* ± sem or range** |
|---|---|---|---|---|
| Butylone | 730 ± 110(4) | 162 ± 55 (4) | 4.40 ± 0.52 (2) | 17.4 ± 1.1% |
| 4-FMC | >1 mM (2) | 178 ± 36 (6) | >100 µM (2) | 24.9 ± 5.7% |
| MDPV | 990 ± 270 (5) | >100 µM(3) | 148 ± 84 (5) | 35.8 ± 9.6% |
| Mephedrone | >1 mM (2) | 115.7 ± 8.8(5) | 66 ± 23 (3) | 33.1 ± 1.9% |
| Methylone | >1 mM (2) | >100 µM(3) | >100 µM (3) | 21.8 ± 8.5% |
| Naphyrone | 119 ± 20(5) | 21.4 ± 7.1 (3) | >100 µM (4) | 34 ± 14% |
| METH | 920 ± 110(11) | 4.72 ± 0.61 (5) | 79 ± 29(11) | 95.5 ± 2.1% |
| MDMA | 661 ± 58(3) | 5.8 ± 1.4 (3) | 114 ± 40(3) | 63 ± 13% |
| Methcathinone | >1 mM (3) | 117 ± 57(3) | 11.6 ± 7.3(3) | 42 ± 10% |
| Reserpine | 0.417 ± 0.061 (7) | 0.0066 ± 0.0013 (5) | ||
| RO4-1284 | 0.150 ± 0.015(8) | 0.098 ± 0.032 (5) |
(n) Number of independent experiments.
Hill slopes for [3H]DHTB binding ranged from −0.89 to −1.38.
Maximum release is defined as the maximum release (maximal AUC) induced by METH (100 µM or 1 mM).
The range is given when n=2.
3.4. hVMAT2: Efficacy and potency to stimulate release of preloaded [3H]NE
To determine if the substituted methcathinones stimulate release of neurotransmitter via the VMAT2, permeabilized cells were preloaded with [3H]NE and assays were conducted with a superfusion device, as described in the methods. When compared to the maximal efficacy of METH to elicit [3H]NE release from HEK-hVMAT2 cells (p<0.001, one-way ANOVA), the six substituted methcathinones, MDMA and methcathinone were less efficacious (p<0.01, Dunnett’s test, Table 4). Only MDMA released more than 50% of the maximal release induced by METH (Table 4). For all drugs that elicited release in a dose-dependent manner, EC50 values were in the micromolar range. The EC50 values for release were greater than 100 µM for 4-FMC, methylone and naphyrone, and each elicited less than 35% of the release elicited by 1 mM METH.
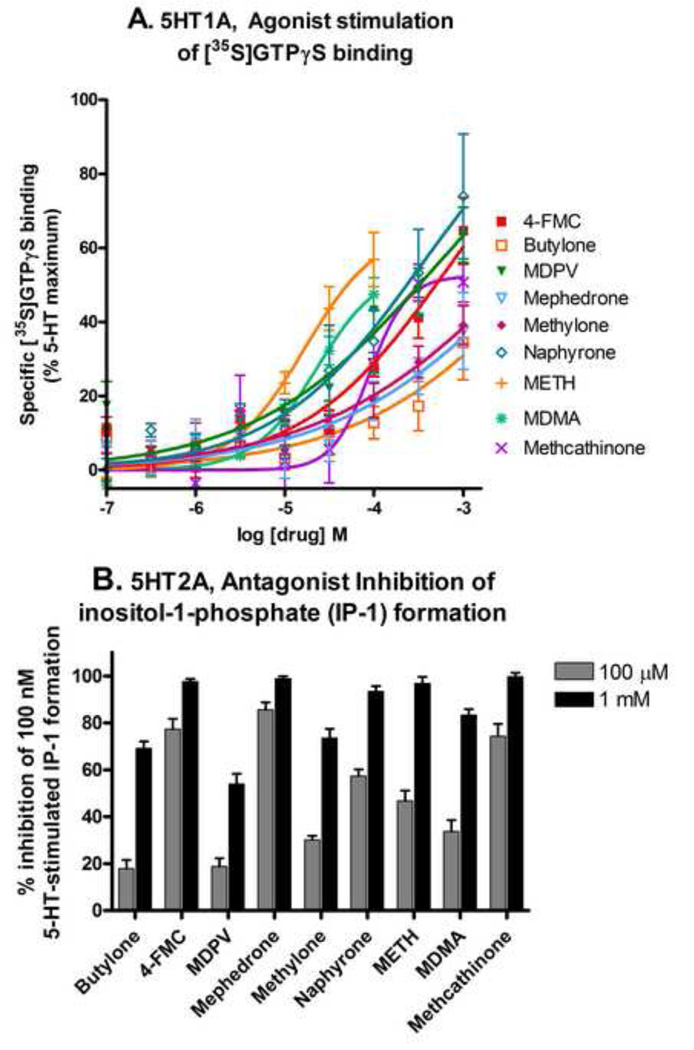
3.5. h5-HT1A: Affinity for [3H]8-OH-DPAT binding site and potency to stimulate [35S]GTPγS binding
In the [3H]8-OH-DPAT binding assay, .METH, MDMA, methcathinone and the substituted methcathinones all had binding affinities in the micromolar range for the h5-HT1A receptor (Table 5). The rank order of affinities (Ki) was WAY 100,635 > LSD > 5-HT >> naphyrone, METH > MDMA, MDPV > methcathinone > methylone > 4-FMC > mephedrone, butylone. In the [35S]GTPγS functional assay, 5-HT and LSD, the standard compounds, were full agonists with potencies in the low nanomolar range (Fig 4A, Table 6). METH, MDMA, methcathinone and the six substituted methcathinones were partial agonists with very low potencies ranging from 21.7 to 440 µM. The rank order of potency at stimulating [35S]GTPγS binding was LSD > 5-HT >> METH > MDMA > methcathinone > MDPV > naphyrone, mephedrone > methylone, 4-FMC > butylone.
Table 5.
Affinity of substituted methcathinones and standard compounds at h5-HT1A, h5-HT2A and h5-HT2C receptors.
| Inhibition of radioligand binding Ki ± sem (µM) (n) | |||
|---|---|---|---|
| Drug | h5-HT1A [3H]8OH-DPAT |
h5-HT2A [125I]DOI |
h5-HT2C [125I]DOI |
| Butylone | >96# (3) | 118 ± 17(3) | 41 ± 12(3) |
| 4-FMC | 71.6 ± 3.4(3) | 10.0 ± 1.0(3) | 12.6 ± 2.4(4) |
| MDPV | 14.8 ± 3.8(3) | 207 ± 29 (3) | 107 ± 30(3) |
| Mephedrone | >91# (3) | 9.0 ± 1.5(4) | 9.1 ± 2.1(4) |
| Methylone | 63.28 ± 0.98 (3) | 48.5 ± 8.0 (3) | 10.6 ± 1.8(3) |
| Naphyrone | 4.64 ± 0.60 (4) | 17.7 ± 2.6(4) | 23.2 ± 6.4 (3) |
| METH | 5.90 ± 0.85 (3) | 43.5 ± 9.1 (4) | 10.2 ± 2.9(9) |
| MDMA | 14.1 ± 2.4(5) | 8.3 ± 1.2 (4) | 1.36 ± 0.44(6) |
| Methcathinone | 29.1 ± 3.6(4) | 29.2 ± 3.3 (4) | 16.0 ± 4.2 (4) |
| 5-HT | 0.00304 ± 0.00053(6) | 0.0076 ± 0.0013(10) | 0.00170 ± 0.00054(7) |
| LSD | 0.00132 ± 0.00032(10) | 0.000148 ± 0.000030(4) | 0.00129 ± 0.00016(3) |
| WAY 100,635 | 0.000161 ± 0.000040(3) | ||
| Ketanserin | 0.0156 ± 0.0048(4) | ||
| SB242084 | 0.00247 ± 0.00050(8) | ||
(n) Number of independent experiments conducted in duplicate.
If some experiments yielded Ki values less than 100 µM and other experiments yielded Ki values greater than 100 µM, the latter experiments were assigned a value of 100 µM and averages calculated. The actual value is greater than that average and no standard error is reported.
Binding Hill slopes for [3H]8-OH-DPAT ranged from −0.59 to −1.04, for h5-HT2A [125I]DOI ranged from −0.85 to −1.17, and for h5-HT2C [125I]DOI ranged from −0.65 to −1.46.
Figure 4.
Effects of substituted methcathinones, METH and MDMA on h5-HT1A and h5-HT2A function. A. The h5-HT1A agonist assay, stimulation of [35S]GTPγS binding. The methcathinones were full to partial agonists, tested at concentrations ranging from 100 nM to 1 mM. B. The h5-HT2A antagonist assay, inhibition of 100 nM-5-HT-stimulated inositol-1-phosphate formation. The compounds had no agonist activity (data not shown). The substituted methcathinones were weak to full antagonists and were tested at concentrations ranging from 100 nM to 1 mM. The percent inhibition by each compound at 100 µM and 1 mM concentrations is shown.
Table 6.
Potency and efficacy of substituted methcathinones and standard compounds at h5-HT1A, h5-HT2A and h5-HT2C receptors.
| Drug | h5-HT1A Stimulation of [35S]GTPγS binding EC50 ± sem (µM)(n) % maximum stimulation ± sem* |
h5-HT2A Inhibition of 5- HT- stimulated IP-1 formation IC50 ± sem (µM)(n) % maximum inhibition ± sem** |
h5-HT2C Inhibition of 5-HT- stimulated IP-1 formation IC50 ± sem (µM) (n) % maximum inhibition ± sem** |
|---|---|---|---|
| Butylone | 440 ± 110(3) 52 ± 13% |
410 ± 110 (3) 95.9 ± 4.1% |
>1 mM (3) 11 ± 12% |
| 4-FMC | 156.0 ± 3.4(3) 70.7 ± 8.3% |
23.6 ± 3.0 (3) 97.0 ± 1.6% |
>1 mM (3) 15 ± 20% |
| MDPV | 60.8 ± 8.4 (3) 69.0 ± 6.7% |
270 ± 86 (3) 67.7 ± 5.4% |
>1 mM (3) 24.5 ± 8.4% |
| Mephedrone | 126 ± 29(3) 44 ± 12% |
14.8 ± 1.8 (3) 98.7 ± 1.3% |
>440# (3) 66.3 ± 8.4% |
| Methylone | 143 ± 32(3) 47 ± 10% |
159 ± 11 (3) 84.7 ± 5.2% |
>1 mM (3) 28.0 ± 7.5% |
| Naphyrone | 116 ± 30(3) 80 ± 15% |
65 ± 11 (3) 98.18 ± 0.94% |
>1 mM (3) 32 ± 14% |
| METH | 18.1 ± 4.2(3) 66.0 ± 8.0% |
112 ± 12 (4) 100.0 ± 0.0% |
>1 mM (2) <3% |
| MDMA | 35 ± 14(10) 65.8 ± 8.5% |
189 ± 52 (4) 96.3 ± 3.8% |
>1 mM (2) 9 ± 12% |
| Methcathinone | 49 ± 21 (4) 52.8 ± 6.2% |
40.0 ± 9.5 (5) 99.63 ± 0.37% |
>1 mM (2) 17.5 ± 4.0% |
| 5-HT | 0.0110 ± 0.0024(6) 100.3 ± 2.2% |
||
| LSD | 0.0058 ± 0.0017(8) 107.8 ± 4.3% |
||
| Ketanserin | 0.00298 ± 0.00097 (11) 95.9 ± 1.5% |
||
| SB242084 | 0.00028 ± 0.00012 (4) 86.5 ± 5.3% |
(n) Number of independent experiments conducted in duplicate.
Drug-induced stimulation is normalized to the maximal stimulation by 5-HT.
Inhibition of 100 nM 5-HT-induced IP-1 is normalized to maximal inhibition by ketanserin (10 µM, h5-HT2A) or by SB242084 (1 µM, h5-HT2C). On average, 5-HT-(100 nM) stimulated 640 ± 140 nM IP-1 in h5-HT2A cells and 1920 ± 130 nM IP-1 in h5-HT2C cells
If some experiments yielded IC50 values less than 1 mM and other experiments yielded IC50 values greater than 1 mM, the latter experiments were assigned a value of 1 mM and averages calculated. The actual value is greater than that average and no standard error is reported.
3.6. h-5-HT2A: Affinity for [125I]DOI binding site and potency to stimulate inositol monophosphate accumulation
In the [125I]DOI binding assay, METH, MDMA, methcathinone and the substituted methcathinones all had affinities for the h5-HT2A receptor in the micromolar range (Table 5). The rank order of affinities (Ki) was LSD > 5-HT > ketanserin >> MDMA, mephedrone, 4-FMC > naphyrone > methcathinone > METH, methylone > butylone > MDPV. In the assay for inositol monophosphate, a measure of receptor function, 5-HT and LSD, the standard compounds, were full agonists with potencies in the low nanomolar range, METH had partial agonist activity at millimolar concentrations, but all other compounds had no agonist activity (data not shown). In the antagonist assay, ketanserin, the standard compound, had potency in the low nanomolar range (the 100 nM concentration of 5-HT used in this assay is approximately an EC65). METH, MDMA, methcathinone and the six substituted methcathinones were antagonists with very low potency ranging from 14.8 to 410 µM (Fig 4B, Table 6). The rank order of potency for inhibition of 100 nM 5-HT-stimulated IP-1 formation was ketanserin >>> mephedrone > 4-FMC > methcathinone > naphyrone > METH > Methylone > MDMA > MDPV > butylone.
3.7. h5-HT2C: Affinity for [125I]DOI binding site and potency to stimulate inositol monophosphate accumulation
In the [125I]DOI binding assay, LSD, 5-HT and SB242084 had low nanomolar affinities. METH, MDMA, methcathinone and the substituted methcathinones all had micromolar affinities in the h5-HT2C receptor binding assay, ranging from 1.36 µM to 107 µM (Table 5). The rank order of affinity (Ki) was LSD, 5-HT > SB242084 >> MDMA > mephedrone, METH, methylone, 4-FMC > methcathinone > naphyrone > butylone > MDPV (Table 5). In the inositol monophosphate assay of receptor function, the standard compounds 5-HT and LSD were full to partial agonists with potencies in the low nanomolar range, MDMA was a full agonist with low micromolar potency, and METH and methcathinone were partial agonists with midmicromolar potencies while the remainder of the drugs had no agonist activity (data not shown). In the antagonist assay, SB242084, the internal standard, had potency in the subnanomolar range. Mephedrone was a weak antagonist, with maximal efficacy of 66.3% and potency in the high micromolar range. The data for all other compounds tested could not be fit to a sigmoidal curve, and maximal efficacy ranged between <3% (METH) to 32% (naphyrone, Table 6).
3.8. DA receptors: Affinity for radioligand binding sites
In the DA receptor binding assays, the standards SCH23390 and SKF38393 (D1 receptor) had high affinity for the [3H]SCH23390 binding site. The standards butaclamol and quinpirole (D2 receptor) had subnanomolar and low micromolar affinity, butaclamol and quinpirole (D3 receptor) had low and mid nanomolar affinity, and haloperidol and quinpirole (D4.4 receptor) had low and high nanomolar affinity, for the [3H]YM-09151-2 binding sites on these receptors. METH, MDMA, methcathinone and the substituted methcathinones had no measurable affinity for the DA D1, DA D2, DA D3 and DA D4.4 receptor up to the maximal concentration tested (10 µM).
3.9. hSigma1 receptors: Affinity for radioligand binding site
At the hSigma1 receptor, the standards haloperidol and spiperone had subnanomolar and low micromolar affinity, respectively, for the [3H]pentazocine binding site (Table 7), in agreement with previously published pharmacology of the receptor (Lee et al., 2008). In addition, METH had a Ki = 3.18 µM, similar to the published value (5.2 µM, Lee et al., 2008). There were significant differences in affinity among the compounds tested. Among the substituted methcathinones, naphyrone had the highest affinity, which was significantly higher than METH (p<0.05, one way ANOVA followed by Dunnett’s multiple comparison test). The other substituted methcathinones had micromolar affinities, ranging from 4.4 µM for MDPV to ~25 µM for 4-FMC and methylone; the latter two had significantly lower affinity than METH (p<0.01). The rank order of affinity for the sigma1 receptor was haloperidol >> naphyrone > spiperone > METH, MDPV, mephedrone, butylone > MDMA, 4-FMC, methylone > methcathinone.
Table 7.
Affinity of substituted methcathinones and standard compounds at hSigma1 receptors.
| Drug | Inhibition of [3H]pentazocine binding Ki ± sem (µM) (n) |
|---|---|
| Butylone | 7.9 ± 1.8 (7) |
| 4-FMC | 24.2 ± 9.0 (5) |
| MDPV | 4.4 ± 1.7 (6) |
| Mephedrone | 7.8 ± 2.0 (6) |
| Methylone | 25.5 ± 7.7 (6) |
| Naphyrone | 0.509 ± 0.052 (6) |
| METH | 3.18 ± 0.74 (9) |
| MDMA | 19.4 ± 6.3 (3) |
| Methcathinone | 117 ± 40 (3) |
| Haloperidol | 0.00094 ± 0.00039 (6) |
| Spiperone | 1.31 ± 0.24 (5) |
(n) Number of independent experiments conducted in duplicate.
Hill slopes for [3H]pentazocine binding ranged from −0.68 to −1.23.
4. Discussion
Herein, we characterized the binding affinities and functional activities of six substituted methcathinones and structurally related compounds at a wide array of biogenic amine transporters and receptors. Our data suggest that butylone, MDPV and naphyrone are primarily transporter blockers, while 4-FMC, mephedrone and methylone are transporter substrates that can profoundly alter neurotransmitter levels, while functional effects on other receptors may play a negligible role in the psychotomimetic and physical effects of methcathinone analogues. This data corroborates and adds to the growing body of research on these abused compounds [6;8;14;20]. Consistent with previous reports, we find that the compounds have high affinity for, and potency at, biogenic amine transporters, with differing efficacies, as some are substrates and others blockers. Additionally, the substrates have higher potency to induce release via the NET compared to the DAT and SERT. This is the first report that all tested methcathinone analogs are low potency partial agonists at the 5-HT1A receptors, and are antagonists with very low potency at the 5-HT2A receptor.
It is important to determine if the concentrations necessary to interact with the neuronal targets will be reached during voluntary human consumption. In postmortem cases, methylone, MDPV and mephedrone concentrations ranged from 0.06–3.3 mg/L (~0.25–14 µM) in blood from heart, periphery, and femoral veins [32;33];[34]. Although brain concentrations weren’t included, 10 µM blood concentrations are possible during drug overdose. The substituted methcathinones generally inhibited .hDAT, hSERT and hNET uptake in vitro at concentrations below 10 µM (Table 1); in vivo this would increase extracellular neurotransmitters in the central nervous system following exocytosis. Additionally, substrates would cause non-exocytotic release of neurotransmitters.
While both transporter blockers and substrates increase extraneuronal dopamine concentrations, these compounds can have differential effects on transporter function and on longer lasting sequelae. For example, high in vivo doses of substrates including METH and methcathinone decrease the activity of DAT in well-washed rat striatal synaptosomes, while transporter blockers including cocaine and methylphenidate have little residual effect (reviewed in [35]), suggesting that there could be differential effects of substituted methcathinone blockers and substrates on transporter trafficking. Substrates enter the presynaptic neuron via transporters with possible subsequent intracellular actions, while blockers do not have access to the intracellular space via transporters. Some substrates are neurotoxic, as suggested by reductions in DAT and dopamine D2 receptors and increased development of Parkinson’s disease in human METH abusers, and depletion of DA and striatal axonal degeneration in rats treated with methcathinone [36,37–39]. Acute, high dose mephedrone alone does not damage dopaminergic neurons 2 or 7 days after administration to rats, but exacerbates the dopaminergic neuron toxicity elicited by METH, MDMA and amphetamine [20;40–42], suggesting that polysubstance ingestion is of additional concern (reviewed in [43]). Similar to MDMA, mephedrone has neurotoxic effects on serotonergic neurons [42] . One striking difference among the biochemical pharmacologies of METH, MDMA and the methcathinones is the much lower affinity and potency of the latter at VMAT2, which suggests that the methcathinones may not alter vesicular storage of neurotransmitters.
“Bath salt” overdose can cause psychosis [2;44]; a possible mechanism is direct interaction as h5-HT2A receptor agonists, like LSD and some substituted phenethylamines [23]. The substituted methcathinones had no h5-HT2A agonist activity; they completely blocked (partial blockade by MDPV) 5-HT-induced h5-HT2A activity with mid-to-high micromolar antagonist potency. Thus, very low binding affinity and lack of agonist activity eliminate a direct h5-HT2A interaction for this symptom, although uptake blockade or substrate-induced release would increase synaptic 5-HT concentrations and indirectly stimulate h5-HT2A receptors.
Among other receptors tested, substituted methcathinones had weak, inhibitory effects on h5-HT2C function while MDMA and METH were agonists with low potency, which may help explain differences in neurotoxicity between methcathinones and amphetamines [45]. The substituted methcathinones, METH, MDMA and methcathinone were partial agonists with very low potency at h5-HT1A receptors and had no measurable affinity for DA receptors. Naphyrone had mid-nanomolar, and the other substituted methcathinones had low-to-mid micromolar, affinity for the hSigma1 receptor, an endoplasmic reticulum chaperone protein. The hSigma1 receptor, expressed by both neurons and glial cells in brain areas including the prefrontal cortex and hippocampus, modulates the function of some voltage-gated and ligand-gated ion channels, and is implicated in the effects of abused drugs (reviewed in [46]).
MDPV and naphyrone were very potent uptake blockers at biogenic amine transporters, with high affinity for binding sites, high potency for uptake inhibition and no drug-induced substrate release. Interactions at other target proteins were at least 60–350-fold less potent, in agreement with others [8]. MDPV, along with naphyrone, had highest potency at hDAT and hNET uptake blockade of all drugs tested, with lower potency at hSERT, consistent with its inhibition of DAT leak currents [47]. MDPV is a stimulant as it elicits rat wheel running [13]. MDPV and naphyrone are substituted pyrovalerones and share structural similarities; they are highly lipophilic, which may result in effective crossing of the blood-brain barrier and long exposure time in vivo [7;7;8;47]. Naphyrone (100 mg) has had adverse effects 48 hours after ingestion [5]. These compounds have similar action to cocaine at transporters, but at much lower concentrations and with potentially longer effect times. Butylone had very low affinities for, and low to very low potencies for uptake inhibition at, the hDAT, hNET, and hSERT, in agreement with other reports [8;14]; it was primarily a transporter inhibitor with low releasing capabilities at hSERT. Although a partial agonist at h5-HT1A receptors and a complete antagonist at h5-HT2A receptors, butylone will probably not interact with these targets during recreational drug taking because the high concentrations required for these effects will not be achieved.
There are reoccurring discrepancies between rank orders of affinity (Ki) in binding assays and of potency (EC50 or IC50) in functional assays, two measures of drug interactions with transporters or receptors. The differences are primarily driven by the much lower affinity compared to potency of substrates at the transporters (see section 3.1, above). Thus for substrates, there is less recognition of the RTI-55 (cocaine analog) binding site as compared to the binding pocket for the neurotransmitter. For example, the highest affinities measured for 4-FMC were at h5-HT2A, hDAT, h5-HT2C and hNET in binding assays, while in functional assays 4-FMC had highest potencies at hNET and hDAT uptake, in agreement with [8], and at hNET and hDAT release as measured with superfusion assays, and h5-HT2A inhibition. Thus 4-FMC was primarily a hNET and hDAT substrate, as evidenced by stimulation of non-exocytotic release and differential potency at uptake compared to binding. 4-FMC stimulated more release via hNET compared to METH. Increased NE release peripherally could impact cardiac events, and centrally could affect forebrain areas via NE neurons projecting from the locus coeruleus and the stress response via NE neurons terminating in the paraventricular nucleus, and elicit acute panic attacks [48]. Overdose with a bath salt product containing both 4-FMC and MDPV elicited hallucinations, agitation and tachycardia; the 4-FMC serum concentration was 346 ng/mL (~1.6 uM, [44], which would block DA and NE uptake and elicit release of NE (Tables 2 and 3). Herein, minimal effect on the 5-HT system was observed, so this drug may act similarly to METH, consistent with other observations [8].
Mephedrone had highest binding affinities for hDAT, h5-HT2A, h5-HT2C and hNET, while it had highest functional potencies at hNET and hDAT uptake, hNET release, hSERT uptake and hDAT release. Mephedrone may initially bind to other protein targets, but at lowest concentrations is a hNET substrate, followed by substrate effects at hDAT and hSERT. Our results are generally similar to other reports of release, although relative potencies differ [8;20] possibly due to differences between superfusion versus other more “static” release assays. Mephedrone is unlikely to cause neurotransmitter release from synaptic vesicles, since hVMAT2 effects occurred at high micromolar concentrations that are unlikely to be reached in vivo unless mephedrone is concentrated by transport into the presynaptic neuron. Mephedrone’s Ki and IC50 values at hDAT were similar to METH’s, while its Ki and IC50 values at hSERT were similar to MDMA’s, consistent with observations of others [42] and survey results by mephedrone users that the subjective effects were more similar to MDMA than cocaine [49]. Other evidence of mephedrone being a transporter substrate include efficacy midway between METH and MDMA at DAT release measured with rotating disk voltammetry [42] and induction of an inward current in Xenopus oocytes expressing DAT, similar to METH [47]. Mephedrone is self-administered by rats with increasing intake over time [42], decreases rat wheel running similar to MDMA [13], and elicits conditioned place preference [50], suggesting a potential for abuse.
Similar to mephedrone, the most potent effects of methylone were as hNET, hDAT and hSERT substrates. Normalized to METH’s releasing efficacy at each transporter, methylone had higher efficacy at hNET compared to hDAT and hSERT. In rat brain synaptosomes, methylone has generally higher potencies for release via DAT and SERT and similar potency at NET compared to values reported herein [20]. Methylone’s potency for hDAT, hNET and hSERT uptake (Table 2) were higher than in C6 cells expressing rat DAT and hNET, and platelets (hSERT); there is similar very low potency at VMAT2 in bovine chromaffin granules [17]. Binge-like dosing of rats, but not mice, with methylone decreases 5-HT concentrations in frontal cortex, striatum and hippocampus, suggesting possible neurotoxic effects [45].
Substituted methcathinones may have other effects on presynaptic neurotransmitter disposition. Amphetamine inhibits monoamine oxidase, decreasing DA, NE and 5-HT metabolism [51]. METH, in addition to VMAT2 interactions, is highly lipophilic and induces neurotransmitter release by altering vesicular pH (the weak base hypothesis, [52]). Amphetamine and other substrates facilitate ion currents through transporters [53;54], which may be requisite for eliciting substrate release. At DAT, mephedrone increases, while MDPV blocks, this current [47]. The effect of other substituted methcathinones on these targets and processes is unknown. In addition, effects of metabolites could modulate the in vivo pharmacology. Further experimentation is required to determine if behavioral or neurotoxicological differences exist between the substituted methcathinones that are transporter substrates as compared to transporter blockers.
Acknowledgments
We thank the Research Triangle Institute (RTI) and the NIDA drug supply program for providing the drugs used in the studies. We thank David White, Jane Acri, David McCann, and Michael Baumann for thoughtful discussions, and David Buck for the figure of substituted methcathinone structures.
This work was supported by the National Institutes of Health National Institute on Drug Abuse Interagency agreement ADA12013 (AJ, AJE), Grant P50 DA018165 (A.J), and Veterans Affairs Merit Review and Career Scientist programs (AJ)
Abbreviations
- LSD
lysergic acid diethylamide
- METH
(+)methamphetamine
- MDMA
3,4-methylenedioxymethylamphetamine
- MDPV
3,4-methylenedioxypyrovalerone
- mephedrone
4-methyl-N-methylcathinone
- methylone
3,4-methylenedioxy-N-methylcathinone
- 4-FMC
4-fluoromethcathinone
- naphyrone
naphthylpyrovalerone
- HEK
human embryonic kidney
- IP-1
inositol-monophosphate
- GTPγS
guanosine 5'-O-[gamma-thio]triphosphate
- DA
dopamine
- 5-HT
5-hydroxy-tryptamine, serotonin
- NE
norepinephrine
- hDAT
human dopamine transporter
- hSERT
human serotonin transporter
- hNET
human norepinephrine transporter
- VMAT2
vesicular monoamine transporter2
- DMEM
Dulbecco’s Modified Eagle's Medium
- BCS
bovine calf serum;
- DHTB
dihydrotetrabenezine
- RO4-1284
(2R,2S,11bs)-2-ethyl-3-isobutyl-9,10-dimethoxy-2,2,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-ol
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- WAY
100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]-ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide
- DOI
(±)-1-(2,5,-dimethoxy-4-iodophenyl)-2-aminopropane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this work were presented at the College on Problems of Drug Dependence (2012) annual meeting.
The authors have no actual or potential conflicts of interest.
Reference List
- 1.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 2.Striebel JM, Pierre JM. Acute psychotic sequelae of “bath salts”. Schizophr Res. 2011;133:259–260. doi: 10.1016/j.schres.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA. Psychoactive “bath salts” intoxication with methylenedioxypyrovalerone. Am J Med. 2012;125:854–858. doi: 10.1016/j.amjmed.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3:439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- 5.Derungs A, Schietzel S, Meyer MR, Maurer HH, Krahenbuhl S, Liechti ME. Sympathomimetic toxicity in a case of analytically confirmed recreational use of naphyrone (naphthylpyrovalerone) Clin Toxicol (Phila) 2011;49:691–693. doi: 10.3109/15563650.2011.592838. [DOI] [PubMed] [Google Scholar]
- 6.Iversen L, Gibbons S, Treble R, Setola V, Huang XP, Roth BL. Neurochemical profiles of some novel psychoactive substances. Eur J Pharmacol. 2012 doi: 10.1016/j.ejphar.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener M, Liechti M. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glennon RA, Yousif M, Naiman N, Kalix P. Methcathinone: a new and potent amphetamine-like agent. Pharmacol Biochem Behav. 1987;26:547–551. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- 10.Young R, Glennon RA. Cocaine-stimulus generalization to two new designer drugs: methcathinone and 4-methylaminorex. Pharmacol Biochem Behav. 1993;45:229–231. doi: 10.1016/0091-3057(93)90110-f. [DOI] [PubMed] [Google Scholar]
- 11.Gygi MP, Fleckenstein AE, Gibb JW, Hanson GR. Role of endogenous dopamine in the neurochemical deficits induced by methcathinone. J Pharmacol Exp Ther. 1997;283:1350–1355. [PubMed] [Google Scholar]
- 12.Dal Cason TA, Young R, Glennon RA. Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol Biochem Behav. 1997;58:1109–1116. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- 13.Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone mephedrone and methylone. Br J Pharmacol. 2012;167:407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–125. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 16.Camp DM, Browman KE, Robinson TE. The effects of methamphetamine and cocaine on motor behavior and extracellular dopamine in the ventral striatum of Lewis versus Fischer 344 rats. Brain Res. 1994;668:180–193. doi: 10.1016/0006-8993(94)90523-1. [DOI] [PubMed] [Google Scholar]
- 17.Cozzi NV, Sievert MK, Shulgin AT, Jacob P, III, Ruoho AE. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur J Pharmacol. 1999;381:63–69. doi: 10.1016/s0014-2999(99)00538-5. [DOI] [PubMed] [Google Scholar]
- 18.Nagai F, Nonaka R, Satoh Hisashi KK. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 19.Yubero-Lahoz S, Ayestas MA, Jr., Blough BE, Partilla JS, Rothman RB, de la Torre R, Baumann MH. Effects of MDMA and related analogs on plasma 5-HT: relevance to 5-HT transporters in blood and brain. Eur J Pharmacol. 2012;674:337–344. doi: 10.1016/j.ejphar.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumann MH, Ayestas MA, Jr., Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee IT, Chen S, Schetz JA. An unambiguous assay for the cloned human sigma1 receptor reveals high affinity interactions with dopamine D4 receptor selective compounds and a distinct structure-affinity relationship for butyrophenones. Eur J Pharmacol. 2008;578:123–136. doi: 10.1016/j.ejphar.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eshleman AJ, Carmolli M, Cumbay M, Martens CR, Neve KA, Janowsky A. Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J Pharmacol Exp Ther. 1999;289:877–885. [PubMed] [Google Scholar]
- 23.Gatch MB, Forster MJ, Janowsky A, Eshleman AJ. Abuse liability profile of three substituted tryptamines. J Pharmacol Exp Ther. 2011;338:280–289. doi: 10.1124/jpet.111.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilhelm CJ, Johnson RA, Lysko PG, Eshleman AJ, Janowsky A. Effects of methamphetamine and lobeline on vesicular monoamine and dopamine transporter-mediated dopamine release in a cotransfected model system. J Pharmacol Exp Ther. 2004;310:1142–1151. doi: 10.1124/jpet.104.067314. [DOI] [PubMed] [Google Scholar]
- 25.Staal RG, Hogan KA, Liang CL, German DC, Sonsalla PK. In vitro studies of striatal vesicles containing the vesicular monoamine transporter (VMAT2): rat versus mouse differences in sequestration of 1-methyl-4-phenylpyridinium. J Pharmacol Exp Ther. 2000;293:329–335. [PubMed] [Google Scholar]
- 26.Varoqui H, Erickson JD. Functional identification of vesicular monoamine and acetylcholine transporters. Methods Enzymol. 1998;296:84–99. doi: 10.1016/s0076-6879(98)96008-6. [DOI] [PubMed] [Google Scholar]
- 27.Newman-Tancredi A, Gavaudan S, Conte C, Chaput C, Touzard M, Verriele L, Audinot V, Millan MJ. Agonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: a [35S]GTPgammaS binding study. Eur J Pharmacol. 1998;355:245–256. doi: 10.1016/s0014-2999(98)00483-x. [DOI] [PubMed] [Google Scholar]
- 28.Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A) 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:114–123. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- 29.Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O'Brien A, White A, Kennedy JM, Craymer K, Farrington L, Auh JS. Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr. 1998;178:440–466. [PubMed] [Google Scholar]
- 30.Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 31.Rothman RB, Partilla JS, Baumann MH, Dersch CM, Carroll FI, Rice KC. Neurochemical neutralization of methamphetamine with high-affinity nonselective inhibitors of biogenic amine transporters: a pharmacological strategy for treating stimulant abuse. Synapse. 2000;35:222–227. doi: 10.1002/(SICI)1098-2396(20000301)35:3<222::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Cawrse BM, Levine B, Jufer RA, Fowler DR, Vorce SP, Dickson AJ, Holler JM. Distribution of methylone in four postmortem cases. J Anal Toxicol. 2012;36:434–439. doi: 10.1093/jat/bks046. [DOI] [PubMed] [Google Scholar]
- 33.Maskell PD, De PG, Seneviratne C, Pounder DJ. Mephedrone (4-methylmethcathinone)-related deaths. J Anal Toxicol. 2011;35:188–191. doi: 10.1093/anatox/35.3.188. [DOI] [PubMed] [Google Scholar]
- 34.Pearson JM, Hargraves TL, Hair LS, Massucci CJ, Frazee CC, III, Garg U, Pietak BR. Three fatal intoxications due to methylone. J Anal Toxicol. 2012;36:444–451. doi: 10.1093/jat/bks043. [DOI] [PubMed] [Google Scholar]
- 35.Fleckenstein AE, Gibb JW, Hanson GR. Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur J Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- 36.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 37.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 38.Granado N, Ares-Santos S, Moratalla R. Methamphetamine and Parkinson's disease. Parkinsons Dis. 2013;2013:308052. doi: 10.1155/2013/308052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sparago M, Wlos J, Yuan J, Hatzidimitriou G, Tolliver J, Dal Cason TA, Katz J, Ricaurte G. Neurotoxic and pharmacologic studies on enantiomers of the N-methylated analog of cathinone (methcathinone): a new drug of abuse. J Pharmacol Exp Ther. 1996;279:1043–1052. [PubMed] [Google Scholar]
- 40.Angoa-Perez M, Kane MJ, Briggs DI, Francescutti DM, Sykes CE, Shah MM, Thomas DM, Kuhn DM. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. J Neurochem. 2012 doi: 10.1111/jnc.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angoa-Perez M, Kane MJ, Francescutti DM, Sykes KE, Shah MM, Mohammed AM, Thomas DM, Kuhn DM. Mephedrone, an abused psychoactive component of 'bath salts' and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2012;120:1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol. 2012;8:33–42. doi: 10.1007/s13181-011-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thornton SL, Gerona RR, Tomaszewski CA. Psychosis from a bath salt product containing flephedrone and MDPV with serum, urine, and product quantification. J Med Toxicol. 2012;8:310–313. doi: 10.1007/s13181-012-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.den Hollander B, Rozov S, Linden AM, Uusi-Oukari M, Ojanpera I, Korpi ER. Long-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedrone. Pharmacol Biochem Behav. 2013;103:501–509. doi: 10.1016/j.pbb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124:195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cameron KN, Kolanos R, Solis E, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2012 doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM, Shekhar A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety. 2010;27:339–350. doi: 10.1002/da.20642. [DOI] [PubMed] [Google Scholar]
- 49.Carhart-Harris RL, King LA, Nutt DJ. A web-based survey on mephedrone. Drug Alcohol Depend. 2011;118:19–22. doi: 10.1016/j.drugalcdep.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Lisek R, Xu W, Yuvasheva E, Chiu YT, Reitz AB, Liu-Chen LY, Rawls SM. Mephedrone ('bath salt') elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012;126:257–262. doi: 10.1016/j.drugalcdep.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson JB. Stereoselectivity and isoenzyme selectivity of monoamine oxidase inhibitors. Enantiomers of amphetamine N-methylamphetamine and deprenyl. Biochem Pharmacol. 1985;34:4105–4108. doi: 10.1016/0006-2952(85)90201-1. [DOI] [PubMed] [Google Scholar]
- 52.Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: the actions of dopamine and psychostimulants. J Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C. Carrier-mediated, release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J Neurochem. 1998;71:1289–1297. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]