Abstract
The speed of virus replication has typically been seen as an advantage for a virus in overcoming the ability of the immune system to control its population growth. Under some circumstances, the converse may also be true: more slowly replicating viruses may evoke weaker cellular immune responses and therefore enhance their likelihood of persistence. Using the model of lymphocytic choriomeningitis virus (LCMV) infection in mice, we provide evidence that slowly replicating strains induce weaker cytotoxic-T-lymphocyte (CTL) responses than a more rapidly replicating strain. Conceptually, we show a “bell-shaped” relationship between the LCMV growth rate and the peak CTL response. Quantitative analysis of human hepatitis C virus infections suggests that a reduction in virus growth rate between patients during the incubation period is associated with a spectrum of disease outcomes, from fulminant hepatitis at the highest rate of viral replication through acute resolving to chronic persistence at the lowest rate. A mathematical model for virus-CTL population dynamics (analogous to predator [CTL]-prey [virus] interactions) is applied in the clinical data-driven analysis of acute hepatitis B virus infection. The speed of viral replication, through its stimulus of host CTL responses, represents an important factor influencing the pathogenesis and duration of virus persistence within the human host. Viruses with lower growth rates may persist in the host because they “sneak through” immune surveillance.
Antigen localization, dose, and kinetics influence the magnitude and duration of an immune response in a nonlinear manner. Understanding the kinetic aspects of this fundamental area of immunology, using a combination of experimental studies and mathematical models of the key dynamic interactions, is the objective of the present study. A bell-shaped dose-response pattern reflects two basic kinetic modes of adaptive immune reaction. The first is amplification, increase in the rates of growth or burst sizes of specific lymphocyte clones with increasing antigen concentrations, and the second is exhaustion, the induction of tolerance by higher antigen concentrations through physical deletion of antigen-reactive cells (16, 20, 45). Studies of the exhaustion phenomenon with the lymphocytic choriomeningitis virus (LCMV) model have most clearly shown that quickly replicating strains, such as Docile, are associated with a down-regulation of the cytotoxic-T-lymphocyte (CTL) clonal burst and thus favor viral persistence (32). The mode of negative correlation between the primary CTL response and the extent of virus replication has been utilized to explain the low frequencies of virus-specific CTLs that characterize chronic infections in humans by noncytopathic viruses, including those of major medical importance, such as hepatitis B virus (HBV) and hepatitis C virus (HCV) (8, 9, 23, 29). In chronic infection with HCV and HBV, the frequencies of virus-specific CTLs are generally very low in the face of high virus loads compared to the frequencies in those patients who successfully resolve the infection. It is still not clear to what extent this reflects differences in the initial status of the antiviral response or subsequent exhaustion (24, 27, 28, 39). Under some circumstances, however, the ability of such viruses to persist correlates with acute CTL responses which appear to be weaker from the outset (10, 15, 39, 42).
A general theory for understanding the sensitivity of the immune response to the antigen growth-accumulation kinetics has been proposed by Grossman and colleague (17-19) based upon the notion of “perturbation.” According to this theory, the immune system tends to respond to strong perturbations caused by rapid increases in antigen appearance and by inflammation, which are characteristic of acute infections, but to adapt to and/or tolerate slow changes. In particular, although a stable or quasistable steady state may often be possible, reflecting equilibrium between low-level concentrations of the pathogen and small numbers of effector lymphocytes, establishment of such equilibrium is typically avoided in the response to acute infections. Inherent delays in the control of the pathogen's growth by an initially undeveloped specific immune response would result, in the case of rapidly growing pathogens, in a transiently overshooting level of pathogen concentration followed by an overshooting immune response that effectively clears the pathogen from the tissues. In contrast, effector cells may keep up with a slowly growing pathogen or immunogenic tumor, in which case a steady state or quasi-steady state can be smoothly approached with only modest fluctuations that do not necessarily lead to clearance but rather to chronicity. This provides an explanation, in particular, for the “sneaking-through” phenomenon known from tumor immunology (19, 25), namely, the ability of slowly growing immunogenic tumors to evade a potentially effective immune response. In addition, the “balance of lymphocyte growth and differentiation” models proposed by Grossman and Paul provided a theoretical explanation for exhaustion of the immune response under the pressure of exceedingly high levels of antigenic stimulation (18, 20) via anergy induction and apoptosis.
Comprehensive experimental analyses by Zinkernagel and Hengartner of model infections with the noncytopathic LCMV (45, 46) led to the formulation of a set of rules for the induction of immunity versus tolerance in relation to antigen localization, dose, and time of availability, which are in agreement with the above theory. In the present study, we first explored the effect of virus growth kinetics on primary CTL expansion using the well-characterized LCMV system. We provide evidence for a “bell-shaped” relationship of the peak immune response to the virus growth rate, suggesting sensitivity to both this rate and the antigen dose as such. Using published data on HCV infections, we demonstrate that fulminant, resolving, and progressing infection outcomes tend to correspond to the highest, intermediate, and lowest rates of virus doubling during the incubation period, suggesting a sneaking-through-like phenomenon. Since similar data sets are not available for HBV infection, we applied a mathematical model to analyze the impacts of various viral replication rates on acute HBV infection.
MATERIALS AND METHODS
Mice and viruses.
C57BL/6 mice were obtained from the Institut für Labortierkunde (University of Zürich, Zürich, Switzerland). The following LCMV strains were used: Armstrong (ARM), WE, Docile (DOC), Traub, and a reassortant WE-ARM strain (35). LCMV was propagated on L929 cells at a low multiplicity of infection and plaqued as previously described (3).
Construction of tetrameric class I-peptide complexes and flow cytometry.
Major histocompatibility complex class I (H-2Db) tetramers complexed with the immunodominant LCMV glycoprotein epitope gp33 (KVYNFATC) were produced as previously described (1). Briefly, H2-Db and human β2 microglobulin molecules were recombinantly expressed in Escherichiacoli (the plasmids were kindly provided by John Altman, Emory University, Atlanta, Ga.). Biotinylated H2-Db peptide complexes were purified using an Aekta Explorer 10 chromatography system (Pharmacia, Uppsala, Sweden) and tetramerized by the addition of streptavidin-phycoerythrin (Molecular Probes, Eugene, Oreg.). At the appropriate times after immunization, animals were bled, and single-cell suspensions of spleen and lymph nodes were prepared. Aliquots of 5 × 105 cells or 3 drops of blood were stained using 50 μl of a solution containing tetrameric class I-peptide complexes at 37°C for 10 min, followed by staining with anti-CD8-fluorescein isothiocyanate (Pharmingen) at 4°C for 20 min. Erythrocytes in blood samples were lysed with fluorescence-activated cell sorter lysis solution (Becton Dickinson), and the cells were analyzed on a FACScalibur flow cytometer (Becton Dickinson) after being gated on viable leukocytes. For the determination of absolute cell counts, the total number of viable leukocytes was assessed in an improved Neubauer chamber. For blood, the total number of viable leukocytes was automatically determined in an Advia counter (Bayer, Leverkusen, Germany) in the Central Hematology Laboratory of the University Hospital Zürich.
Estimation of LCMV and hepatitis virus population doubling time.
The exponential growth rates of LCMV strains were estimated using a nonlinear regression procedure (Sigma Stat version 2.03). The logistic model of exponential LCMV growth with saturation, V(t) = K/[1 + (K/V(0) − 1)e−βt], where the parameters K, β, and V(0) stand for the carrying capacity, the exponential growth rate, and the initial size of infection for the spleen, was fitted to the log-scaled virus titer over days 1 to 3 postinfection to estimate the parameters.
The doubling times, τd, of hepatitis virus populations were quantitated from growth curves of published virus load data for the incubation periods in humans and chimpanzees. Using an exponential approximation for the initial growth of the virus, i.e., V(t) ∼ eβt, we calculated τd = ln2/β for monotonic growth patterns. Two estimates, τmin and τmax, characterizing the maximal and minimal growth phases of viral abundance, were used for curves which displayed a nonmonotonic growth pattern. Differences in the inoculum sizes, V(0), within the plausible range do not affect the slope but only the time when peak titer is reached.
Formal model for virus-CTL in HBV infection.
The mathematical model for acute CTL response to HBV infection is defined within a set of two delay differential equations for the virus (prey), V, and virus-specific CTLs (predator), E: dV(t)/dt = β · V(t) · [1 − V(t)/K] − γ · V(t) · E(t) and dE(t)/dt = b · V(t − τ) · E(t − τ)/[θSat + V(t)] − αE · E(t) + T*. (Symbols are defined in Table 1).
TABLE 1.
Model parameters and estimates for acute phase of HBV infection
| Parameter (units) | Notation | Estimate for primary acute infection | 95% confidence interval |
|---|---|---|---|
| HBV exponential growth rate (day−1) | β | 0.3 | 0.27-0.32 |
| Carrying capacity for virus (copies/ml) | K | 0.8 × 1010 | >0.4 × 1010 |
| Virus elimination rate (ml/copy/day) | γ | 1.75 × 10−3 | 10−3-0.34 × 10−2 |
| HBV-specific CTL stimulation rate (day−1) | b | 0.12 | 0.09-0.15 |
| CTL division time (days) | τ | 0.6 | Fixed ad hoc |
| Virus load for half-maximal CTL stimulation (copies/ml) | θSat | 0.12 × 104 | 0.34 × 102-106 |
| Death rate of CTL (day−1) | αE | 0.05 | 0.021-0.063 |
| Specific precursor CTL export from thymus (cell/ml/day) | T* | 0.1 | Fixed ad hoc |
The model differs from the classical Lotka-Volterra predator-prey system (41) in the following ways: (i) a Verhulst-Pearl logistic form for prey growth is used, (ii) we make use of the Holling type II response curve (22) for prey (virus)-dependent growth of the predators (CTLs), (iii) an immigration term for predators is included (reflecting the export of precursor CTLs from the thymus), and (iv) a time lag in the generation of predators (corresponding to CTL division time) is included.
We estimated the model parameters using data from a single patient (patient 1) from a study by Webster et al. (42) and following a nonlinear relative-deviation least-squares approach (Table 1). Five patients were identified in the study during the incubation phase of acute hepatitis B so that the dynamics of viral and immune events were defined (42). The virus load kinetics and the dynamics of cellular immune responses were most extensively documented for patient 1, permitting a good description of the virus-CTL dynamics data in the acute phase of hepatitis infection (see Fig. 5A). We assumed that the infecting inoculum established the initial virus load of 10 HBV DNA particles per ml of blood (in agreement with Whalley et al. [44]) and did not consider the virus data after the acute phase, i.e., for days 119 and 140, as they are not relevant for our study. The confidence intervals for each parameter characterizing the reliability of the single-patient data-based estimates were quantitated using the maximum-likelihood profile method (40). The sensitivity analysis of predictions regarding the effect of virus growth kinetics on the clonal expansion of virus-specific CTLs for the ad hoc fixed parameters (including the time lag) shows that they are robust. It should be emphasized that the ad hoc fixed parameters τ and T* could have been neglected without significantly affecting the analysis (T* also serves to initialize the variable E) and that the model is also not sensitive to changing the value θSat (Table 1). One can extend the simple model by incorporating additional processes and parameters in order to reproduce the biphasic elimination kinetics of the virus or a bell-shaped effect of the virus load on CTL activation. However, in view of the limited data sets available, this is not justified, as it will lead to a decrease in the model parsimony.
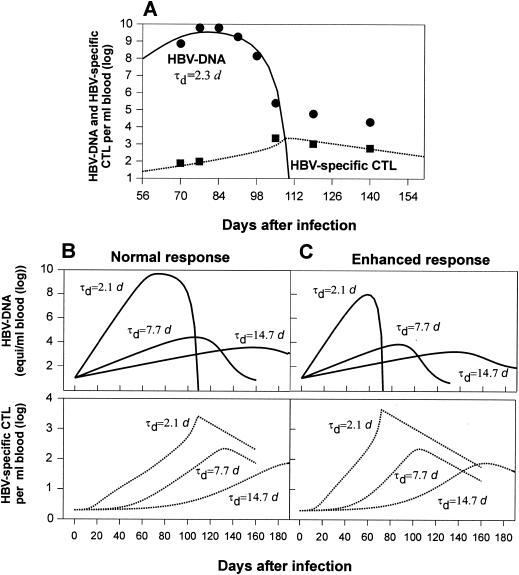
FIG. 5.
Model predictions of HBV-CTL dynamics for different virus population doubling times. (A) Clinical data for patient 1 (42) and the corresponding mathematical model simulation. d, days. (B and C) Viremia and CTL numbers in blood of a normal responder (B) and a high responder (C). A reduction in the HBV growth rate during the incubation period leads to a weaker CTL expansion and underwhelming of the host immune response.
RESULTS
Weaker CTL responses are induced by slowly replicating LCMV strains.
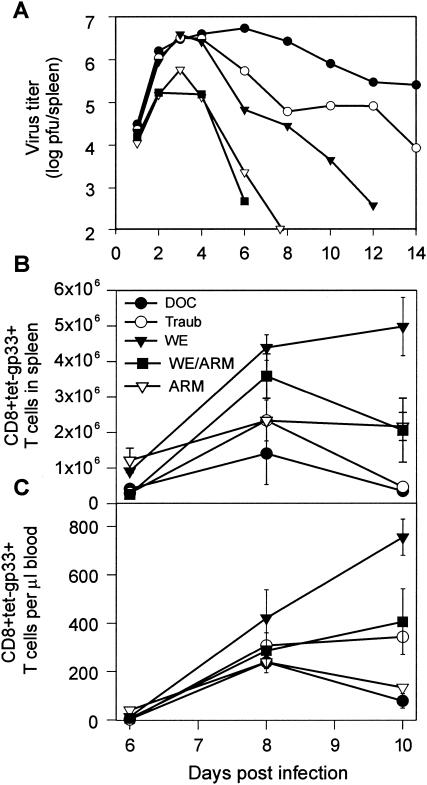
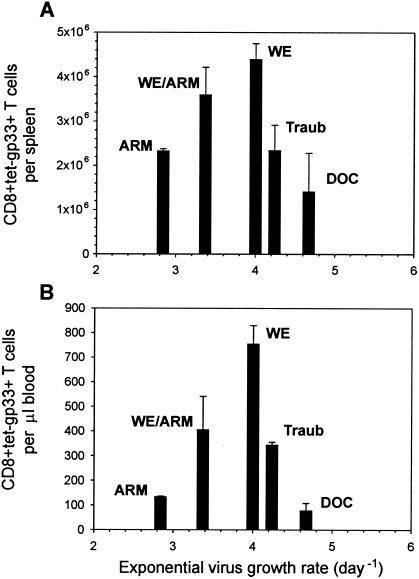
Available data on the growth kinetics of LCMV in mice (12, 31) suggest a range for τd of ∼1.4 to 8 h for the virus population doubling time, depending on the strain and whether conventional or specific-pathogen-free housing conditions are used to maintain the animals. In order to determine how the rate of virus growth affects the CTL response, we infected C57BL/6 mice intravenously with 200 PFU of five different LCMV strains (ARM, reassortant WE-ARM, WE, Traub, and DOC). All of the tested LCMV strains induce strong CTL responses against the gp33 epitope, and antibodies do not play a role in the early infection (7, 36). Viral titers in spleens were determined on days 1, 2, 3, 4, 6, 8, 10, and 12 postinfection (Fig. 1A), and the clonal expansion of CD8+ T cells specific for the gp33 epitope in spleens and blood was assessed using tetramer analysis (Fig. 1B and C). Mice infected with 200 PFU of the WE strain showed the strongest CTL response in the spleen and blood. Maximal expansion of gp33-specific CTLs was lower in mice infected with 200 PFU of the slowly replicating ARM or WE-ARM strains. Likewise, the rapidly replicating strains Traub and DOC elicited weaker gp33-specific CTL responses than WE (Fig. 1). The doubling times for the virus loads of all five LCMV strains were estimated from the growth phase data by fitting the logistic growth equation. According to their growth rates (doubling time; τd), the strains can be ranked from slowly to quickly replicating: ARM (5.86 h), WE-ARM (4.94 h), WE (4.16 h), Traub (3.92 h), and DOC (3.56 h). These doubling times correspond to a daily increase of the viral population by factors of 17, 29, 55, 69, and 107, respectively. Thus, the slowly replicating WE-ARM and ARM strains induced weaker CTL responses than the more quickly replicating WE strain, suggesting a positive correlation between the initial virus growth rate and the clonal burst size. In contrast, virus strains with high replication kinetics, such as Traub and DOC, are associated with a suppression of CTL responses, indicating a negative effect of virus growth on clonal CTL expansion. The peak numbers of gp33-specific CTL responses in the spleen (day 8) and blood (day 10) thus exhibit a nonmonotone (bell-shaped) dependence on the virus growth rate (Fig. 2).
FIG. 1.
Kinetics of early LCMV replication and expansion of gp33-specific CD8+ T cells after infection with different LCMV strains. C57BL/6 mice were infected intravenously with 200 PFU of LCMV-ARM, WE-ARM, WE, Traub, or DOC. (A) LCMV titers in spleens were determined at the indicated times postinfection. (B and C) Total numbers of CD8+ tet-gp33+ CTLs were assessed in the spleen (B) and blood (C) at the indicated times by fluorescence-activated cell sorter analysis. The values represent means ± standard errors for two or three mice per group.
FIG. 2.
Bell-shaped relationship between initial virus growth rate and peak CTL responses in spleen (A) and blood (B) established using data on CTL expansion in C57BL/6 mice infected with distinct LCMV strains exhibiting different growth rates. The error bars indicate standard errors. Variation in the LCMV growth rate can have either an enhancing or a down-regulating effect on the clonal burst size of virus-specific CTLs. Virus replication above a threshold is required for strong CTL response. LCMV-WE appears to be an “optimally” replicating strain in C57BL/6 mice, as it induces the strongest CTL response.
Growth kinetics of hepatitis virus infections.
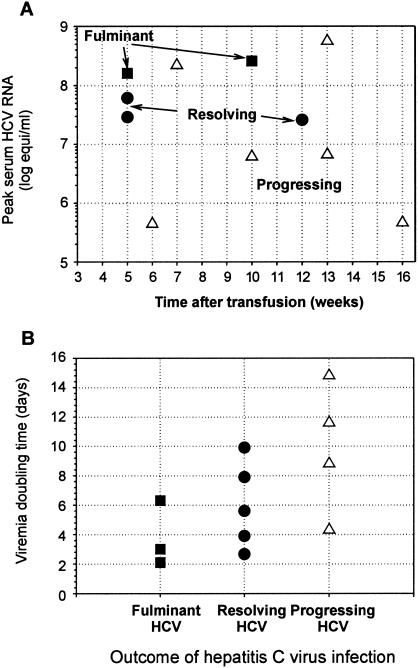
The overall dynamics of hepatitis virus infection is much slower than that of LCMV. A regular association between the extent of virus growth and the clinical outcome of HCV infection (see Table 1 in reference 14 for details) is observed for a cohort of prospectively studied patients, as summarized in Fig. 3A. Despite interpatient variability, the resolving and fulminant infections tend to reach higher virus concentrations earlier than progressing and chronic infections, reflecting faster initial growth. Data on the growth of HCV (5, 13, 14, 43) in sera of humans and chimpanzees during the incubation period suggest virus population doubling times (τd) in the range of ∼2.1 to 15 days for the whole spectrum of HCV infections. Expressed in terms of the virus load doubling time (estimated as described in Materials and Methods), the hierarchy is as follows (Fig. 3B): chronic progressing hepatitis, 4 days < τd < 15 days; acute resolving hepatitis, 2.3 days < τd < 10 days; fulminant hepatitis, 2.1 < τd < 6.2 days. Although the ranges overlap, slower replication is evident in HCV patients with longer viral persistence.
FIG. 3.
Associations among the characteristics of HCV growth and the clinical outcomes resulting from hepatitis infection. (A) Peak serum HCV RNA level versus time posttransfusion in a well-defined cohort of prospectively followed patients (14). (B) Summary of virus doubling times and infection outcomes from data on HCV in humans and chimpanzees (5, 13, 14, 43). The hierarchy suggests that the kinetics of virus growth can affect the outcome of HCV infection, and slower replication is evident in patients with longer HCV persistence.
It is noteworthy that similar comprehensive data sets that permit the correlation of virus growth kinetics with the phenotype of infection are not available for HBV infection. The only HBV study known to us that allows HBV growth rate estimation was performed by Whalley et al. (44), who analyzed a cohort of seven patients. The analysis of these data suggests a range for τd of ∼2.2 to 5.8 days for acute HBV infection (which corresponds to an expansion factor of ∼1.13 to 1.37 per day).
Both LCMV and hepatitis viruses are noncytopathic, but the kinetics of acute infections differ: (i) LCMV replicates ∼10 times faster, and (ii) the elimination time of LCMV is also almost 10 times shorter than that of HBV or HCV. The latter difference reflects the fact that the population of hepatitis virus-infected cells probably exceeds the peak CTL number by several orders of magnitude (21). Furthermore, there is an upper limit of the capacity of the immune system to mount a CTL response, even in a strongly reactive patient. Unlike the experimental LCMV model, human hepatitis virus infection is not amenable to a similar level of quantitative experimental analysis of the effect of virus growth on CTL response and virus dynamics. This is where mathematical models can be helpful. We used recent data relating HBV and CTL dynamics in acute hepatitis infection (42) as a reference set similar to the LCMV data to estimate key parameter values for a simple predator (CTL)-prey (virus) mathematical model.
Modeling the kinetics of virus and CTL in acute HBV infection.
Although protection against infection is a multifactorial phenomenon depending on both the innate and adaptive immune mechanisms, CTLs play a vital role in the control of human HBV infection via both cytolytic and noncytolytic mechanisms (8, 21). The dynamics of virus infections can be biologically characterized in terms of the interacting populations of viruses and effector CTLs (11). Well-established concepts from population biology, such as the predator-prey system (22, 30, 41), facilitate a quantitative analysis of virus-host interactions within a mathematical framework (4, 34). In the amplification mode, the virus acts as a positive regulator of the CTL population, whereas CTLs function to eliminate the virus population, so their mutual interaction dynamics can be viewed and formally described as a predator (CTL)-prey (virus)-type system (Fig. 4). The classical approach to modeling the dynamics of a deterministic one-predator- one-prey system is the model of Lotka and Volterra and its modifications (22, 41). Our analysis of the limited set of HBV data are based on a simple predator-prey-type model, which differs from the classical model in that the rate of growth of the predator population (CTLs) saturates at a defined predator density. This allows us to more accurately mimic the kinetic patterns of virus and CTLs observed in a patient during acute HBV infection (42), as shown in Fig. 5A. The formal framework of the model is defined in Materials and Methods, whereas the parameter estimates and the confidence intervals are listed in Table 1. The major point of the modeling process was to examine the sensitivity of the immune response to a decrease in the HBV growth rate compared to the acute infection. Since the high virus load suppression aspect was not in the scope of the HBV data analysis, the bell-shaped dependence of the lymphocyte growth rate on the virus load was not considered; therefore, the fulminant phenotype of infection was not modeled. The effects of variability in factors such as inoculum size, HLA haplotype, and gender can (in principle) be examined with the model by changing the parameters listed in Table 1.
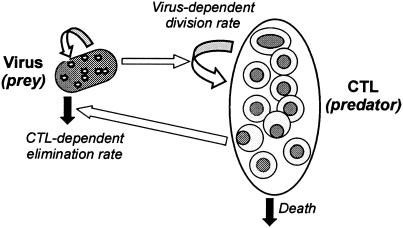
FIG. 4.
Schematic view of the predator-prey framework for the dynamic analysis of the CTL-virus interaction representing the amplification mode.
Decrease in human hepatitis virus doubling time leads to weaker CTL response.
To investigate the virus growth effect on CTL expansion, we conducted mathematical analyses based on the model simulating CTL responses of patients to infection with HBV mutants with different doubling times. The replication rate estimates (τd, ∼2.2 to 5.8 days) for acute HBV infection are derived from a study of a cohort of seven patients performed by Whalley et al. (44). The results summarized in Fig. 5B and C show that a decrease in the virus population doubling time proportionally increases the peak virus number, the magnitude of the CTL response, and the overall efficacy of virus elimination. An HBV infection with a slowly replicating strain, with the doubling time, τd, set at ∼14.7 days, induces only a weak response, and the virus tends to persist.
Patients may have different major histocompatibility complex backgrounds, resulting in various patterns of responsiveness to a given virus strain. Variations in the clonal burst size and dynamics of virus-specific CTLs might reflect differences in the initial CTL precursor frequency and/or in the activation thresholds of lymphocytes. The latter are also determined by the genetic makeup of the host. To simulate the difference in the HBV dynamics between a “high” responder and a “normal” responder, we increased the CTL population division rate by 50%. It has been recorded (16) that minor quantitative differences in the lymphocyte responsiveness parameter may result in large changes in the magnitude of the response due to nonlinear amplification, akin to the growth of cells. The overall dynamics of infection shift toward a lower viremia, a stronger CTL response, and earlier virus elimination (Fig. 5C). However, the slowly replicating strain (τd, ∼14.7 days) still has moderate growth but elicits a weak and transient CTL response that decreases after day 200 (not shown). We noticed that the mathematical model allows viruses with extremely low replication rates (below γT*/αE, i.e., a doubling time longer than 300 days) to be eradicated by the preexisting CTLs. However, we consider here only the realistic range of significantly higher HBV growth rates.
Our modeling analyses thus suggest that transition from acute to chronic HBV infection might be explained (at least in part) by a decrease in the replication rate of the evolving virus population. In patients, ranges for virus growth rates corresponding to different clinical outcomes of HBV infection might overlap, as seen in HCV infection. The observed variations between patients could reflect the responder status of the host or genetic features of the virus.
DISCUSSION
There is growing interest in immunology in understanding rate effects in pathogen-host interactions. We present evidence from the experimental murine LCMV model that infection of genetically identical mice with a slowly replicating virus strain results in weaker CTL responses. A similar relationship between LCMV growth and the extent of specific CD8+-T-cell response was recently observed in C57BL/6 versus gamma interferon-deficient mice (2). The observation of this positive correlation adds another dimension to the interpretation of virus-host interactions and further extends a widely accepted view of a negative correlation observed between noncytopathic-virus growth kinetics and the magnitude of the CTL response, commonly known as exhaustion (32). Conceptually, we showed a bell-shaped relationship between the LCMV growth rate and the peak CTL response. Therefore, variations in the LCMV doubling time during the incubation period can have either an enhancing or a down-regulating effect on the clonal burst size of virus-specific CTLs, depending on whether the CTL population responds to the presence of viral antigen in the amplification-enhancement or exhaustion mode. Earlier studies suggested that by increasing the dose of infection or initial CTL numbers it is possible to shift the system from one mode to another (12, 32, 45).
According to the general theory (17, 18, 20) (see the introduction), slower replication of the virus may not only be quantitatively associated with a weaker response but may result in qualitatively different outcomes, reminiscent of the classical sneaking-through phenomenon (19, 25). Indeed, it has been postulated that the generation of a sizable effector response in the lymphoid tissues depends critically on the rapid kinetics of antigen growth (20, 45). Accordingly, lymphocytes preferentially respond, individually and collectively, to a rapid change in the level of stimulation rather than to stimulation per se. In particular, slowly progressing viral infections might present a greater challenge for control by the immune system on purely kinetic grounds, because of the reduction in the CTL overshoot that is necessary to facilitate efficient clearance of the foreign antigen (20).
Indeed, reported clinical and experimental evidence (although somewhat limited at present) indicates that more slowly replicating mutants of HBV and HCV result, or are observed, in patients with chronic hepatitis (6, 15). The majority of HCV-infected patients (85%) fail to clear the virus. Many reasons have been put forward to explain why viruses such as HCV and HBV may persist (immune escape, exhaustion, and tolerogenic effects in the liver). We suggest that, in addition, the kinetic aspects of virus growth need to be considered, as indicated by the hierarchy depicted in Fig. 3. A direct relationship between the initial viral replication kinetics and cellular responses in humans infected with HCV has also been observed by Thimme and associates (39). One patient (subject 1 in the study) with faster virus growth kinetics produced a stronger CTL response (percentage of HCV tet+ CD8 T cells) and cleared the virus, while a second patient (subject 2) with an HCV population doubling time about twice as long (four-times-lower peak CTL) developed a chronic infection. The latest studies of HCV infection in chimpanzees (37, 38) indicated that in the two animals who cleared the virus, peak viremias of 105 and 106.5 genome equivalents/ml were reached in weeks 6 and 8 postinfection, whereas the virus load grew slowly, reaching the first peak of 103.5 after 14 weeks, in the animal with a persistent outcome.
We have applied a mathematical predator-prey-like model to mimic relevant aspects of the dynamics of the CTL-virus interaction. The model reproduces the postulated overshooting-versus-adaptation behavior of the immune response to quickly growing and slowly growing viruses, respectively. Mathematically, overshooting reflects a fluctuation approach to the steady state following perturbation of the system. Such fluctuations have been studied in animal systems and in many other nonlinear dynamic systems (22, 30, 33, 41). By calibrating the model's parameters by fitting detailed kinetic data from a single HBV-infected patient, we predict the potential implications of variation in the viral growth rate for the outcome of the infection (Fig. 5).
The mathematical model presented (or its modifications) can be used as a platform for in silico-based predictions for other virus infections, such as human immunodeficiency virus or cytomegalovirus. It particular, it should assist other investigators with formulating rational conceptual programs of data collection and analysis. There are many efficient and reliable software packages available, such as MATLAB (http://www.mathworks.com) or Berkeley Madonna (http://www.berkeleymadonna.com/), that help to automate the process of programming model equations, running computer simulations, and solving data-fitting problems.
In applying such a theory to immune responses to virus infections, several caveats must be borne in mind. First, mathematical-model construction represents a reductionist approach to the analysis of biological systems that in reality are complex in structure. Our study focuses on the kinetic aspects of regulation of the immune response at the cell population level and therefore does not consider the intimate molecular processes underlying the observed correlation. This would require, for example, detailed experimental analysis of antigen distribution, presentation, and gene activation. Furthermore, although CTLs are considered to play a critical role, other effector mechanisms (cytokines or neutralizing antibodies) are likely to contribute to viral dynamics and the eventual outcome of infection. Indeed, interferon gamma-deficient mice fail to clear the slowly replicating LCMV-ARM, despite an elevated and otherwise functional CTL response (2). Second, although CTL temporal dynamics and viral growth dynamics can under many circumstances be successfully captured using simple models (such as predator-prey systems), there are other phenomena, such as CTL exhaustion, CTL competition, or CTL dysfunction, which do not fit in this simple predator-prey interpretation and demand more complex models (e.g., the “balance of growth and differentiation” models) to capture reality.
The general implication of the above analysis is that more slowly replicating viruses appear to be more difficult for the immune system to control and may therefore sneak through immunosurveillance. Our ability to demonstrate this effect has been limited by the scarcity of quantitative data and by confounding factors that contribute to variability in the observed patterns. Still, the present observations should help to draw more attention to this issue in future studies. This specific form of virus-host interaction may also be important during antiviral drug therapy, because long-lasting suppression of viral replication may lead to the loss of sufficiently stimulated cellular immune responses and, eventually, to treatment failure (26). Down-regulation of the virus growth kinetics (via a whole range of potential mechanisms), resulting in weaker CTL responses, might represent an effective strategy, in evolutionary terms, used by noncytopathic viruses to survive in immunocompetent hosts. Thus, such viruses may sneak through immune surveillance by “underwhelming” the immune response of the host, rather than overwhelming it, in order to establish a persistent infection.
Acknowledgments
We thank Hans Hengartner and Rolf Zinkernagel for critical reviews of the manuscript and continued support of this project.
This work was supported by the Swiss National Science Foundation, the Wellcome Trust, the Alexander von Humboldt Foundation, the Leverhulme Trust, and the Russian Foundation for Basic Research.
REFERENCES
- 1.Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 2.Bartholdy, C., J. P. Christensen, D. Wodarz, and A. R. Thomsen. 2000. Persistent virus infection despite chronic cytotoxic-T-lymphocyte activation in gamma interferon-deficient mice infected with lymphocytic choriomeningitis virus. J. Virol. 74:10304-10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battegay, M., S. Cooper, A. Althage, J. Banziger, H. Hengartner, and R. M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33:191-198. [DOI] [PubMed] [Google Scholar]
- 4.Bell, G. I. 1973. Predator-prey equations simulating an immune response. Math. Biosci. 16:291-314. [Google Scholar]
- 5.Bigger, C. B., K. M. Brasky, and R. E. Lanford. 2001. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J. Virol. 75:7059-7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brechot, C., V. Thiers, D. Kremsdorf, B. Nalpas, S. Pol, and P. Paterlini-Brechot. 2001. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology 34:194-203. [DOI] [PubMed] [Google Scholar]
- 7.Brundler, M. A., P. Aichele, M. Bachmann, D. Kitamura, K. Rajewsky, and R. M. Zinkernagel. 1996. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur. J. Immunol. 26:2257-2262. [DOI] [PubMed] [Google Scholar]
- 8.Chisari, F. V. 1997. Cytotoxic T cells and viral hepatitis. J. Clin. Investig. 99:1472-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13:29-60. [DOI] [PubMed] [Google Scholar]
- 10.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10:439-449. [DOI] [PubMed] [Google Scholar]
- 11.Doherty, P. C., and J. P. Christensen. 2000. Accessing complexity: the dynamics of virus-specific T cell responses. Annu. Rev. Immunol. 18:561-592. [DOI] [PubMed] [Google Scholar]
- 12.Ehl, S., P. Klenerman, R. M. Zinkernagel, and G. Bocharov. 1998. The impact of variation in the number of CD8(+) T-cell precursors on the outcome of virus infection. Cell Immunol. 189:67-73. [DOI] [PubMed] [Google Scholar]
- 13.Farci, P., S. J. Munoz, A. Shimoda, S. Govindarajan, D. C. Wong, A. Coiana, G. Peddis, R. Rubin, and R. H. Purcell. 1999. Experimental transmission of hepatitis C virus-associated fulminant hepatitis to a chimpanzee. J. Infect. Dis. 179:1007-1011. [DOI] [PubMed] [Google Scholar]
- 14.Farci, P., A. Shimoda, A. Coiana, G. Diaz, G. Peddis, J. C. Melpolder, A. Strazzera, D. Y. Chien, S. J. Munoz, A. Balestrieri, R. H. Purcell, and H. J. Alter. 2000. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288:339-344. [DOI] [PubMed] [Google Scholar]
- 15.Forns, X., R. Thimme, S. Govindarajan, S. U. Emerson, R. H. Purcell, F. V. Chisari, and J. Bukh. 2000. Hepatitis C virus lacking the hypervariable region 1 of the second envelope protein is infectious and causes acute resolving or persistent infection in chimpanzees. Proc. Natl. Acad. Sci. USA 97:13318-13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Germain, R. N. 2001. The art of the probable: system control in the adaptive immune system. Science 293:240-245. [DOI] [PubMed] [Google Scholar]
- 17.Grossman, Z. 1984. Recognition of self and regulation of specificity at the level of cell populations. Immunol. Rev. 79:119-138. [DOI] [PubMed] [Google Scholar]
- 18.Grossman, Z. 1993. Cellular tolerance as a dynamic state of the adaptable lymphocyte. Immunol. Rev. 133:45-73. [DOI] [PubMed] [Google Scholar]
- 19.Grossman, Z., and G. Berke. 1980. Tumor escape from immune elimination. J. Theor. Biol. 83:267-296. [DOI] [PubMed] [Google Scholar]
- 20.Grossman, Z., and W. E. Paul. 2000. Self-tolerance: context dependent tuning of T cell antigen recognition. Semin. Immunol. 12:197-203. [DOI] [PubMed] [Google Scholar]
- 21.Guidotti, L. G., and F. V. Chisari. 1996. To kill or to cure: options in host defense against viral infection. Curr. Opin. Immunol. 8:478-483. [DOI] [PubMed] [Google Scholar]
- 22.Hassell, M. P. 2000. The spatial and temporal dynamics of host-parasitoid interactions. Oxford University Press, Oxford, United Kingdom.
- 23.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA 96:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantzanou, M., M. Lucas, E. Barnes, H. Komatsu, G. Dusheiko, S. Ward, G. Harcourt, and P. Klenerman. 2003. Viral escape and T cell exhaustion in hepatitis C virus infection analysed using Class I peptide tetramers. Immunol. Lett. 85:165-171. [DOI] [PubMed] [Google Scholar]
- 25.Klein, G. 1976. Mechanisms of escape from immune surveillance. Natl. Cancer Inst. Monogr. 44:135-136. [PubMed] [Google Scholar]
- 26.Komarova, N. L., E. Barnes, P. Klenerman, and D. Wodarz. 2003. Boosting immunity by antiviral drug therapy: a simple relationship among timing, efficacy, and success. Proc. Natl. Acad. Sci. USA 100:1855-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lechner, F., N. H. Gruener, S. Urbani, J. Uggeri, T. Santantonio, A. R. Kammer, A. Cerny, R. Phillips, C. Ferrari, G. R. Pape, and P. Klenerman. 2000. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 30:2479-2487. [DOI] [PubMed] [Google Scholar]
- 28.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maini, M. K., C. Boni, C. K. Lee, J. R. Larrubia, S. Reignat, G. S. Ogg, A. S. King, J. Herberg, R. Gilson, A. Alisa, R. Williams, D. Vergani, N. V. Naoumov, C. Ferrari, and A. Bertoletti. 2000. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May, R. M. 2001. Stability and complexity in model ecosystems. Princeton University Press, Princeton, N.J.
- 31.Moskophidis, D., M. Battegay, M. A. Bruendler, E. Laine, I. Gresser, and R. M. Zinkernagel. 1994. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J. Virol. 68:1951-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moskophidis, D., F. Lechner, H. Pircher, and R. M. Zinkernagel. 1993. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 362:758-761. [DOI] [PubMed] [Google Scholar]
- 33.Nisbet, R. M., and W. S. C. Gurney. 1982. Modelling fluctuating populations. John Wiley & Sons, New York, N.Y.
- 34.Nowak, M. A., and R. M. May. 2000. Virus dynamics. Oxford University Press, Oxford, United Kingdom.
- 35.Riviere, Y., R. Ahmed, P. J. Southern, M. J. Buchmeier, and M. B. Oldstone. 1985. Genetic mapping of lymphocytic choriomeningitis virus pathogenicity: virulence in guinea pigs is associated with the L RNA segment. J. Virol. 55:704-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seiler, P., M. A. Brundler, C. Zimmermann, D. Weibel, M. Bruns, H. Hengartner, and R. M. Zinkernagel. 1998. Induction of protective cytotoxic T cell responses in the presence of high titers of virus-neutralizing antibodies: implications for passive and active immunization. J. Exp. Med. 187:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su, A. I., J. P. Pezacki, L. Wodicka, A. D. Brideau, L. Supekova, R. Thimme, S. Wieland, J. Bukh, R. H. Purcell, P. G. Schultz, and F. V. Chisari. 2002. Genomic analysis of the host response to hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 99:15669-15674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thimme, R., J. Bukh, H. C. Spangenberg, S. Wieland, J. Pemberton, C. Steiger, S. Govindarajan, R. H. Purcell, and F. V. Chisari. 2002. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc. Natl. Acad. Sci. USA 99:15661-15668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venzon, D. J., and S. H. Moogavkor. 1988. A method for computing profile-likelihood-based confidence intervals. Appl. Statistician 37:87-94. [Google Scholar]
- 41.Volterra, V. 1979. Variations and fluctuations in the numbers of co-existing animal species, p. 65-236. In F. M. Scudo and J. R. Ziegler (ed.), The golden age of theoretical biology 1923-1940. Springer, Berlin, Germany.
- 42.Webster, G. J., S. Reignat, M. K. Maini, S. A. Whalley, G. S. Ogg, A. King, D. Brown, P. L. Amlot, R. Williams, D. Vergani, G. M. Dusheiko, and A. Bertoletti. 2000. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology 32:1117-1124. [DOI] [PubMed] [Google Scholar]
- 43.Weiner, A. J., X. Paliard, M. J. Selby, A. Medina-Selby, D. Coit, S. Nguyen, J. Kansopon, C. L. Arian, P. Ng, J. Tucker, C. T. Lee, N. K. Polakos, J. Han, S. Wong, H. H. Lu, S. Rosenberg, K. M. Brasky, D. Chien, G. Kuo, and M. Houghton. 2001. Intrahepatic genetic inoculation of hepatitis C virus RNA confers cross-protective immunity. J. Virol. 75:7142-7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whalley, S. A., J. M. Murray, D. Brown, G. J. Webster, V. C. Emery, G. M. Dusheiko, and A. S. Perelson. 2001. Kinetics of acute hepatitis B virus infection in humans. J. Exp. Med. 193:847-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zinkernagel, R. M. 2000. Localization dose and time of antigens determine immune reactivity. Semin. Immunol. 12:163-171. [DOI] [PubMed] [Google Scholar]
- 46.Zinkernagel, R. M., and H. Hengartner. 2001. Regulation of the immune response by antigen. Science 293:251-253. [DOI] [PubMed] [Google Scholar]