Abstract
Purpose
The aim of the study was to investigate the expression of selected genes encoding enzymes involved in the antioxidant defense system (superoxide dismutase 2, SOD2; aldehyde dehydrogenase 1, ALDH1A1; microsomal glutathione S-transferase 1, MGST1) in fragments of anterior lens capsules of patients with pseudoexfoliation syndrome (PEX). The specificity and sensitivity of these molecular markers for PEX development were also assessed.
Methods
The study group consisted of 20 patients (9 women and 11 men) with diagnosed PEX and cataract. The control group included 23 patients (8 women and 15 men) who needed cataract surgery but did not have PEX. Quantification of SOD2, ALDH1A1, and MGST1 messenger ribonucleic acid (mRNA) was performed with quantitative real-time PCR.
Results
SOD2, ALDH1A1, and MGST1 mRNAs were detected in all studied samples. The examined genes had statistically significant higher expression in the group of patients with PEX than in the control group (SOD2, p=0.0015; ALDH1A1, p=0.0001; MGST1, p=0.0001, Mann–Whitney U test). The areas under the curve (AUC) of SOD2, MGST1, and ALDH1A1 were 0.766, 0.818, and 0.957, respectively.
Conclusions
Differential expression of SOD2, ALDH1A1, and MGST1 genes in the anterior lens capsules of patients with PEX suggest that diseased tissue appears to respond to the previously reported oxidative stress. A possible role of ALDH1A1 mRNA level as a risk factor or marker for PEX needs further confirmation.
Introduction
Pseudoexfoliation syndrome (PEX) is a serious ocular manifestation of a common age-related systemic disorder, and is frequently associated with severe chronic secondary open-angle glaucoma and cataract [1]. PEX involves pathologic excessive production, abnormal crosslinking [2,3], and progressive accumulation of elastic microfibrils in many organs [4,5].
Several mechanisms of the pseudoexfoliation material formation are possible, but a molecular background is more frequently suggested [6]. The imbalance between the antioxidant defense mechanism and reactive oxygen species (ROS) production results in oxidative stress leading to cellular damage. In addition, oxidative stress mechanisms (e.g., exposure to environmental chemicals, radiation, and atmospheric oxygen) in ocular tissues promotes various pathological conditions, including eye diseases such as cataract, glaucoma, uveitis, and age-related macular degeneration [7-9]. The cellular antioxidant defense system comprises enzymatic (i.e., superoxide dismutases [SODs], catalase, glutathione peroxidase) and non-enzymatic antioxidants (i.e., vitamins A, C, and E, selenium, zinc, carotenoids, and various metabolites) [10-13]. Moreover, Lobo et al. [14] and Sies [15] distinguished three levels of the antioxidant defense system. The first line contributes to preventing formation of free radicals and comprises enzymes such as superoxide dismutase, catalase, and glutathione peroxidase. The second line of defense contributes to suppressing chain initiation and/or breaking the chain propagation reactions and involves the low-molecular-weight antioxidants, i.e., vitamins and enzymes involved in its pathway such as aldehyde dehydrogenase 1 (ALDH1A1). The third line of defense is composed of a repair enzyme such as glutathione peroxidase and microsomal glutathione S-transferase 1 (MGST1) [14,15].
ROS are formed as normal metabolic products and are important in normal cellular functioning, but their production can be increased under pathological conditions and cause damage [4,5]. The generation of ROS can cause lipid peroxidation, protein modification and denaturation, and DNA damage [16]. Therefore, numerous antioxidant systems act as protective mechanisms. The type of antioxidant used in the free radical reaction depends on the site of free radical generation and its properties. The main ROS molecules that generate in the lens include superoxide anion, hydroxyl radical, and H2O2 [17]. Growing evidence supports the importance of oxidative stress in PEX [3-5,18]. Understanding the role of oxidative stress in the development of PEX may provide novel opportunities for improved therapeutic intervention. However, the exact pathogenesis of this disorder has not been well understood yet. Oxidative stress may be determined through markers of lipid peroxidation, the activity of antioxidant enzymes, and the levels of low-molecular-weight antioxidants [9]. We proposed single genes as components of three levels of the antioxidant defense system for the activity analysis of those lines. Therefore, the present study focused on quantitative relationships of SOD2, ALDH1A1, and MGST1 messenger ribonucleic acid (mRNA) levels between patients presenting with cataracts with and without PEX. Additionally, the specificity and sensitivity of these molecular markers for PEX development were assessed.
Methods
Subjects
The forty three patients with PEX and cataract or with cataract alone participated in this study (17 women and 26 men, mean age 73 years; range 63-80 years). The examined group comprised 20 patients (9 women and 11 men, mean age 74.5 years; range 63–80 years) with clinically diagnosed PEX and cataracts. The control group consisted of 23 cataract patients (8 women and 15 men, mean age 71 years; range 66–80 years) without PEX (Table 1). All the patients were treated at the Department of Ophthalmology, University Hospital No. 5, Medical University of Silesia, Katowice, Poland.
Table 1. Selected clinical features of patients with clinically diagnosed PEX syndrome and the control group.
| Characteristic | PEX syndrome group (n=20) | Control group (n=23) |
|---|---|---|
| Gender |
||
| Female |
9 |
8 |
| Male |
11 |
15 |
| Age (years) |
74.5 (63 - 80) * |
71 (66 - 80) |
| Eye |
||
| Right |
9 |
15 |
| Left |
11 |
8 |
| Visual acuity † |
0.1 (0.01 – 0.5) |
0.2 (0.01–0.7) |
| Axial length (mm) |
23.11 (22.34 – 24.98) |
23.52 (22 – 27) |
| IOP (mmHg) ‡ | 17 (16 – 20) | 17 (16 - 21) |
* Values of clinical parameters are expressed as means (minimum-maximum) † Snellen chart ‡ IOP - intraocular pressure
All subjects underwent a complete ophthalmic examination: best-corrected visual acuity (BCVA) using a 6-m Snellen chart, Goldmann applanation tonometry, gonioscopy with the use of three mirror Goldmann goniolens in the primary position of gaze, and direct and indirect slit-lamp biomicroscopy after pupil dilation with 1% Tropicamide (Haag-Streit, Köniz, Switzerland, +78D Oculus). The presence of PEX was confirmed clinically by PEX material deposits on anterior segment structures on the pupillary margin, anterior lens capsule (ALC), corneal endothelium, and various pigment-related signs—loss of pigment at the pupillary ruff, irregular pigmentation of trabecular meshwork, and the accumulation of pigment along Schwalbe’s line (Sampaolesi’s line).
The criteria for inclusion in the molecular analysis were as follows: aged ≥60 years, PEX exfoliation material on anterior segment structures, and scheduled for routine phacoemulsification cataract surgery. Patients with a history of previous intraocular surgery, glaucoma, or any other systemic or ocular conditions were excluded from the study. In addition, we excluded patients with no known history or symptoms of diabetes or other chronic disease, which could affect the measurements.
The study was approved by the Bioethics Committee of the Medical University in Katowice (KNW-6501–12/I/08) in accordance with the Declaration of Helsinki regarding medical research involving human subjects. All patients were informed about the research and signed an informed consent form.
Ribonucleic acid extraction from tissue specimens
Circular sections of ALCs from 20 eyes with PEX and cataract and 23 eyes with cataract alone were collected during phacoemulsification cataract surgery. The sections were stored for 48 h at −70 °C until RNA extraction. Total RNA was extracted from ALCs using a TRIzol reagent (Invitrogen, Carlsbad, CA). RNA extracts were treated with DNase I (MBI Fermentas, Vilnius, Lithuania) according to the manufacturer’s instructions. The quality of the extracts was checked electrophoretically using 0.8% agarose gel stained with ethidium bromide. The results were analyzed and recorded using the 1D Bas-Sys gel documentation system (Biotech-Fisher, Perth, Australia). Total RNA concentration was determined with spectrophotometric measurement in 5 μl capillary tubes using the Gene Quant II RNA/DNA Calculator (Pharmacia Biotech, Cambridge, UK).
Quantitative real-time polymerase chain reaction assay
Detection of the expression of SOD2, ALDH1A1, MGST1, and glyceraldehyde-3-phosphate (GAPDH) mRNAs was performed using a quantitative real-time (RT) PCR with SYBR Green chemistry (SYBR Green Quantitect RT–PCR Kit, Qiagen, Valencia, CA) according to the manufacturer’s instructions, and an Opticon DNA Engine Continuous Fluorescence detector (MJ Research, Waltham, MA) as described previously [19]. All samples were tested in triplicate. GAPDH for each sample was measured to exclude possible RT-PCR inhibitors. Oligonucleotide primers, specific for SOD2 [20], ALDH1A1 [21], MGST1 [18], and GAPDH [19], were chosen based on the published data (Table 2). The thermal profile for one-step RT-PCR was as follows: reverse transcription at 50 °C for 30 min, denaturation at 95 °C for 15 min, and 40 cycles consisting of the following temperatures and time intervals, 94 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. A cycle threshold (Ct), which is the point at which a PCR product is for the first time detected above a fixed threshold, was determined for each sample.
Table 2. Characteristics of primers used for real-time QRT-PCR.
| Gene | Sequence of primers | Length of amplicon (bp) * | Tm (°C) † |
|---|---|---|---|
| SOD2 |
Forward: 5′-CTGATTTGGACAAGCAGCAA-3′ |
199 |
81.6 |
| Reverse: 5′-CTGGACAAACCTCAGCCCTA-3′ |
|||
| ALDH1A1 |
Forward: 5′-TACTCACCGATTTGAAGATT-3′ |
151 |
77.2 |
| Reverse: 5′-TTGTCAACATCCTCCTTATC-3′ |
|||
| MGST1 |
Forward: 5′- ATTGGCCTCCTGTATTCCTTG-3′ |
311 |
80.2 |
| Reverse: 5′-TAATCCCTCTGCTCCCCTCC-3′ |
|||
| GAPDH |
Forward: 5′-GAAGGTGAAGGTCGGAGTC-3′ |
226 |
80.0 |
| Reverse: 5′-GAAGATGGTGATGGGATTC-3′ |
* bp – base pairs † Tm – melting temperature
To quantify the results obtained with RT-PCR for SOD2, ALDH1A1, MGST1, and GAPDH, a standard curve method was used [22,23]. Commercially available standards of β-actin cDNA (TaqMan DNA Template Reagent Kit, PE Applied Biosystems, Foster, CA) were used at five different concentrations (0.6, 1.2, 3.0, 6.0, and 12.0 ng/µl), to simultaneously detect the expression profile of each investigated gene. For standards, the calculation of copy number values was based on the following relationship: 1 ng of DNA=333 genome equivalents (PE Applied Biosystems). Amplification plots for each dilution of a commercially available standard template were used to determine the Ct values. A standard curve was generated by plotting the Ct values against the log of the known amount of β-actin cDNA copy numbers. Correlation coefficients for standard curves ranged from 0.988 to 0.995 indicating a high degree of confidence for measurement of the copy number of molecules in each sample.
Each run was completed using melting curve analysis to confirm the specificity of amplification and the absence of primer dimers. RT-PCR products were separated on 6% polyacrylamide gels and visualized with silver salts.
Statistical analyses
Statistical analyses were performed using Statistica 8.0 software (StatSoft, Tulsa, OK), and the level of significance was set at p<0.05. Values were expressed as median with the 25th and 75th quartiles, and minimum and maximum. The Mann–Whitney U test was applied to assess differences in the expression of SOD2, ALDH1A1, and MGST1.
Respective cutoff values for gene expression were assessed with the receiver operating characteristic (ROC) curve analysis. With the use of ROC analysis, sensitivities and specificities were calculated by varying the criterion of positivity from the least (cut at probability of 0) to the most stringent (cut at probability of 1). Optimal sensitivity and specificity were determined for the SOD2, MGST1, and ALDH1A1 genes, and a corresponding cutoff value of each gene was identified. The area under the curve (AUC) of each gene and a relative risk of PEX development were also calculated. The AUC has a value from 0.5 to 1.0, where 1.0 represents perfect ability to discriminate, and 0.5 represents the discrimination resulting from pure chance An AUC greater than 0.9 is considered excellent, greater than 0.8 to 0.9 very good, 0.7 to 0.8 good, 0.6 to 0.7 average, and <0.6 poor [24,25].
Results
Specificity of the real-time polymerase chain reaction assay
RT-PCR specificity for the target genes was confirmed experimentally based on amplimers melting temperatures. For each RT-PCR product, a single peak at the expected temperatures was observed: SOD2, 81.6 °C; ALDH1A1, 77.2 °C; MGST1, 80.2 °C; and GAPDH, 80.0 °C (data not shown). Gel electrophoresis also revealed the presence of a single product of predicted length (data not shown).
The messenger ribonucleic acid level of superoxide dismutase 2, aldehyde dehydrogenase 1, and microsomal glutathione S-transferase 1 in anterior lens capsules of patients with pseudoexfoliation syndrome
SOD2, ALDH1A1, and MGST1 mRNAs were detected in all tested samples obtained from the control and study groups. In the ALCs, the SOD2 mRNA copies/µg of total RNA was about ninefold higher in the patients with PEX and cataract (median=23,288 mRNA copies/µg of total RNA) compared to the control cataract group (median=2,600 mRNA copies/µg of total RNA), and statistical significance was found (p=0.0015, Mann–Whitney U test; Figure 1A). In the case of ALDH1A1 mRNA, there was a statistically significant 13-fold increase in the study group (median=153,001 mRNA copies/µg of total RNA) compared to the control group (median=11,598 mRNA copies/µg of total RNA; p=0.0001, Mann–Whitney U test; Figure 1B). The mRNA level of MGST1 was about threefold higher in the PEX and cataract samples (median=4,439 mRNA copies/µg of total RNA) than in the control cataract group samples (median=1,243 mRNA copies/µg of total RNA). A comparative analysis of MGST1 expression also revealed statistically significant differences between the patients with PEX and cataract and the control cataract groups (p=0.0001, Mann–Whitney U test; Figure 1C).
Figure 1.
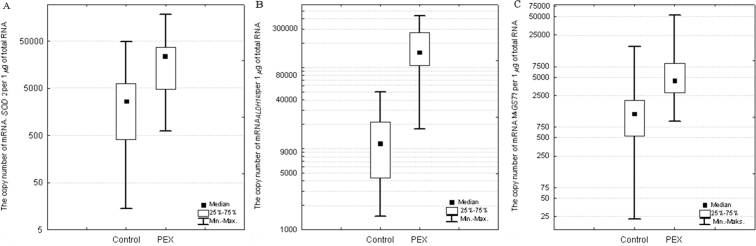
The messenger ribonucleic acid (mRNA) level of superoxide dismutase 2 (SOD2; A), aldehyde dehydrogenase 1 (ALDH1A1; B), and microsomal glutathione S-transferase 1 (MGST1; C) in the ALCs of patients with clinically diagnosed pseudoexfoliation syndrome (PEX) and in the control group. (Box and whisker plots present medians±quartiles and extreme values of copy numbers per 1 μg of total RNA; p<0.05, Mann–Whitney U test.)
Assessment of superoxide dismutase 2, aldehyde dehydrogenase 1, and microsomal glutathione S-transferase 1 messenger ribonucleic acid level as molecular markers of pseudoexfoliation syndrome
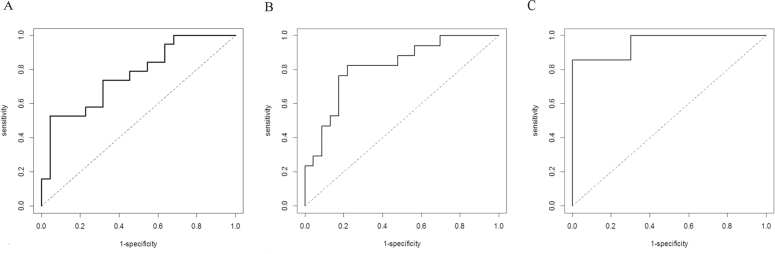
To evaluate the predictive power of SOD2, MGST1, and ALDH1A1 mRNA levels as potential molecular markers of PEX development, the AUC, sensitivity, specificity, and cutoff value were shown with use of the ROC curve analysis (Table 3). The SOD2 mRNA level above 23,288 copies/µg of total RNA and the MGST1 mRNA level above 2,155 copies/µg of total RNA were associated with a 1.7-fold and 1.8-fold increase in the relative risk of PEX development, respectively. The cutoff value for the ALDH1A1 gene (mRNA level above 67,000 copies/µg RNA) corresponded to a 2.4-fold increase in a relative risk of PEX development. The AUCs of SOD2, MGST1, and ALDH1A1 were 0.766, 0.818, and 0.957, respectively, and all were higher than 0.7 (p<0.001; Figure 2A–C).
Table 3. The ROC analysis and the diagnostic values of the SOD2, MGST1 and ALDH1A1 mRNA levels.
| Variable | AUC * | cutoff value |
specificity [%] |
sensitivity [%] |
|---|---|---|---|---|
| (mRNA copies/µg RNA) | ||||
| SOD2 |
0.766 |
23,288 |
95.5% |
52.6% |
| MGST1 |
0.818 |
2155 |
78.3% |
82.4% |
| ALDH1A1 | 0.957 | 67,000 | 100.0% | 85.7% |
* AUC - area under the curve
Figure 2.
The receiver operating characteristic (ROC) curves for superoxide dismutase 2 (SOD2; A), aldehyde dehydrogenase 1 (ALDH1A1; B), and microsomal glutathione S-transferase 1 (MGST1; C) mRNA levels display molecular markers of pseudoexfoliation syndrome (PEX).
Discussion
The pathogenesis of PEX seems to be multifactorial but remains unexplained [26]. PEX can be associated with a disturbed extracellular matrix synthesis, uncontrolled basement membrane metabolism, and the action of free radicals [27]. Moreover, the oxidant-antioxidant balance may be altered in various ocular pathologies such as cataract, glaucoma, uveitis, and age-related macular degeneration [12,28-31]. Oxidative stress leads to damage to lipids and DNA and the inhibition and deactivation of proteins such as collagen and elastin resulting in disruption of overall biologic function [27,32,33]. At the cell membrane, lipid hydroperoxides induce changes in permeability and cause an uncoupling of the membrane-bound enzyme sodium, potassium-adenosine triphosphatase (Na,K-ATPase) and oxidative inhibition of calcium-adenosine triphosphatase (Ca2+-ATPase) in several tissues, including the lens. The decreased activity of these enzymes is thought to be connected to oxidative damage to the sulfhydryl groups of the molecules. In turn, within the cell, lipid peroxides can damage DNA [34]. Gartaganis et al. [27] confirmed an increase in lipid peroxidation in PEX lens epithelial cells.
The antioxidants in the defense systems act at different levels such as preventive, radical scavenging, and repair. In the present study, qRT-PCR was applied to investigate the SOD2, ALDH1A1, and MGST1 mRNA levels encoding selected enzymes, which are antioxidants acting in the defense systems at all three levels.
In previous research, antioxidant activity changed due to eye diseases in various ocular tissues such as the aqueous humor, the conjunctiva, the cornea, and the lens [27,35-39]. Many attempts have also been made to identify a total antioxidant status and concentrations of individual antioxidant enzymes in serum, which can be easily assessed [28,29,40-42]. However, the systemic alterations in antioxidants might not exactly reflect the situation in the structures of eyes [28]. In addition, the change in the protein level is preceded by alteration of gene transcriptional activity encoding this protein. In turn, analysis of ocular samples has been notoriously difficult because of the technical obstacles. This is especially true for the lens and cornea [43]. Unfortunately, in our study we did not obtain sufficient quantities of the specimens for further analyses. Therefore, we could not measure the activity and level of other oxidative enzymes or metabolites, such as catalase or malondialdehyde, in this study. Although there are published data regarding differences between mRNA levels of antioxidants in the ocular tissues of patients with PEX [18,44,45], the identification of candidate genes as molecular markers of PEX in previous studies has not been performed.
Our results revealed statistically significant higher mRNA levels of SOD2, ALDH1A1, and MGST1 in the ALCs of patients with PEX and cataract compared to the control cataract subjects suggesting that the pathways for regulating and responding to oxidative stress were present and transcriptionally responsive. Upregulation of SOD2 may be a compensatory mechanism against free radicals, and an adaptive cellular response to increased production of ROS [46]. Previous studies indicated that SOD2 in the ocular tissues can be induced by mediators of oxidative stress, including radiation, lipopolysaccharide, tumor necrosis factor, and interleukin 1 [47,48]. Zenkel et al. [18,45] determined the set of differentially expressed genes mainly related to extracellular matrix metabolism and cellular stress in anterior segment tissues obtained from patients with PEX-associated open-angle or closed-angle glaucoma patients, at the mRNA and protein levels. Corresponding to our results, the expression of SOD increased in their studies. Furthermore, Uçakhan et al. [49] demonstrated an increase in SOD activity in the lens capsules of patients with PEX, but in that study, qRT-PCR was not used. This may suggest the compensatory mechanism in response to the increased oxidative stress in eyes with PEX and cataract compared to those with cataract alone [49]. Lin et al. [50] suggested that overexpression of SOD could prevent cataract formation induced by oxidative stress. However, they studied rat intact lenses. All these results suggested that SOD activity may play a role in eliminating oxidative stress.
MGST1 may be a protective factor against oxidative damage from lipid peroxidation [51]. In the current studies, a statistically significant increase in the MGST1 mRNA level was revealed. This finding suggests that oxidative stress plays a role in the pathogenesis of PEX. However, the report presented by Zenkel et al. [45] contradicts our findings. These authors found up to threefold downregulation for MGST1 in PEX-associated open-angle or closed-angle glaucoma specimens. In addition, Yağci et al. [42] observed a decrease in SOD activity. The discrepancy could be due to the patient pool; e.g., the patients in the Zenkel et al. [45] study had glaucoma comorbidity rather than cataract comorbidity. Reduced expression of antioxidative enzymes can indicate defective protection against oxidative stress in patients with PEX [42,45]. Yildirim et al. [52] and Yilmaz et al. [53] also suggested that decreased trace element levels in the lenses of patients with PEX could reflect defective antioxidative defense systems. Additionally, high concentrations of lipid peroxidation products may promote oxidation, aggregation, and abnormal extracellular matrix metabolism in the anterior segment structures of eyes and cause disease progression [54].
ALDH1A1 plays a role in oxidation of a wide range of aldehydes, e.g., all-trans- and 9-cis-retinal, and exhibits a high affinity for metabolism of highly reactive products of lipid peroxidation of cellular membranes, including malondialdehyde (MDA) and 4-hydroxy-2-nonenal [55]. Thus, ALDH1A1 plays a role in preventing oxidative damage by free-radical species [26,56,57]. In ocular tissues, ALDH1A1 is found mainly in the lens and to a lesser extent in the cornea. ALDH1A1 can play a role in detoxifying 4-hydroxy-2-nonenal and MDA, protecting ocular tissues from protein crosslinking and aggregation, which can lead to proteasome inhibition and eventually cataract formation [58]. Yilmaz et al. indicated that MDA concentrations were much higher in patients with PEX [38]. However, data are lacking regarding ALDH1A1 expression in PEX. For the first time, our results showed increased expression of ALDH1A1 gene in the ALCs of patients with this disease, which may be explained through the involvement of ALDH1A1 in the metabolism of vitamin A [59] and participation in the second and third lines of antioxidant defense [60].
The second part of this study focused on assessing SOD2, MGST1, and ALDH1A1 mRNA levels as molecular markers of PEX development. While we were launching our study, we could not find any published reports regarding the identification of correlations between PEX and selected markers. Our findings suggest the clinical usefulness of these molecular markers for PEX. Moreover, the ALDH1A1 holds great potential for developing diagnostics for PEX because ROC analysis revealed sensitivity and specificity of 85.7% and 100%, respectively, with an AUC of 0.957 as an index of diagnostic accuracy. However, the suggestion that the level of ALDH1A1 mRNA could be a good predictor of risk for PEX seems speculative. ALDH1A1 appears to be a good discriminator of controls and patients with PEX in a population with sufficiently advanced disease to allow for clinical diagnosis, but there is no evidence that ALDH1A1 mRNA levels measured earlier in the progression of the disease will be as sensitive or accurate. A possible role of ALDH1A1 mRNA level as a risk factor or marker for PEX requires further investigation. Moreover, our data are limited in that not the entire PEX population but only samples from patients with PEX and cataract were evaluated.
In conclusion, SOD2, ALDH1A1, and MGST1 were overexpressed in the ALCs of patients with PEX suggesting that diseased tissue appears to respond to oxidative stress. Knowledge of the molecular pathophysiology of PEX may provide insight into the development of possible strategies for early diagnosis of PEX. Moreover, a detailed examination of the antioxidant defense system might help to identify a new therapeutic agent for PEX treatment.
References
- 1.Schlötzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141:921–37. doi: 10.1016/j.ajo.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 2.Ritch R. Exfoliation syndrome. Curr Opin Ophthalmol. 2001;12:124–30. doi: 10.1097/00055735-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Schlötzer-Schrehardt U. Genetics and genomics of pseudoexfoliation syndrome/glaucoma. Middle East Afr J Ophthalmol. 2011;18:30–6. doi: 10.4103/0974-9233.75882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlötzer-Schrehardt UM, Koca MR, Naumann GO, Volkholz H. Pseudoexfoliation syndrome. Ocular manifestation of a systemic disorder? Arch Ophthalmol. 1992;110:1752–6. doi: 10.1001/archopht.1992.01080240092038. [DOI] [PubMed] [Google Scholar]
- 5.Streeten BW, Li ZY, Wallace RN, Eagle RC, Jr, Keshgegian AA. Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol. 1992;110:1757–62. doi: 10.1001/archopht.1992.01080240097039. [DOI] [PubMed] [Google Scholar]
- 6.Schlötzer-Schrehardt U. Molecular pathology of pseudoexfoliation syndrome/glaucoma–new insights from LOXL1 gene associations. Exp Eye Res. 2009;88:776–85. doi: 10.1016/j.exer.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2010;12:93–124. doi: 10.1089/ars.2008.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasiliou V, Nebert DW. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genomics. 2005;2:138–43. doi: 10.1186/1479-7364-2-2-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yildirim Z, Ucgun NI, Yildirim F. The role of oxidative stress and antioxidants in the pathogenesis of age-related macular degeneration. Clinics (Sao Paulo) 2011;66:743–6. doi: 10.1590/S1807-59322011000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manda G, Nechifor MT, Neagu TM. Reactive Oxygen Species, Cancer and Anti-Cancer Therapies. Curr Chem Biol. 2009;3:22–46. [Google Scholar]
- 11.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219–36. [PMC free article] [PubMed] [Google Scholar]
- 12.Cabrera MP, Chihuailaf RH. Antioxidants and the integrity of ocular tissues. Vet Med Int. 2011;2011:905153. doi: 10.4061/2011/905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Mehta G, Vasiliou V. Antioxidant defenses in the ocular surface. Ocul Surf. 2009;7:176–85. doi: 10.1016/s1542-0124(12)70185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sies H. Total antioxidant capacity: appraisal of a concept. J Nutr. 2007;137:1493–5. doi: 10.1093/jn/137.6.1493. [DOI] [PubMed] [Google Scholar]
- 16.Spector A. Review: Oxidative stress and disease. J Ocul Pharmacol Ther. 2000;16:193–201. doi: 10.1089/jop.2000.16.193. [DOI] [PubMed] [Google Scholar]
- 17.Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;22:657–82. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 18.Zenkel M, Pöschl E, von der Mark K, Hofmann-Rummelt C, Naumann GO, Kruse FE, Schlötzer-Schrehardt U. Differential gene expression in pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci. 2005;46:3742–52. doi: 10.1167/iovs.05-0249. [DOI] [PubMed] [Google Scholar]
- 19.Strzalka-Mrozik B, Stanik-Walentek A, Kapral M, Kowalczyk M, Adamska J, Gola J, Mazurek U. Differential expression of transforming growth factor-β isoforms in bullous keratopathy corneas. Mol Vis. 2010;16:161–6. [PMC free article] [PubMed] [Google Scholar]
- 20.Gottipati S, Cammarata PR. Mitochondrial superoxide dismutase activation with 17 beta-estradiol-treated human lens epithelial cells. Mol Vis. 2008;14:898–905. [PMC free article] [PubMed] [Google Scholar]
- 21.Nezzar H, Chiambaretta F, Marceau G, Blanchon L, Faye B, Dechelotte P, Rigal D, Sapin V. Molecular and metabolic retinoid pathways in the human ocular surface. Mol Vis. 2007;13:1641–50. [PubMed] [Google Scholar]
- 22.Berezowski P, Strzalka-Mrozik B, Forminska-Kapuscik M, Mazurek U, Filipek E, Nawrocka L, Pieczara E, Banasiak P, Kimsa M. Posttraumatic temporal TGF-β mRNA expression in lens epithelial cells of paediatric patients. Folia Biol (Praha) 2012;58:24–9. [PubMed] [Google Scholar]
- 23.Kapral M, Wawszczyk J, Jurzak M, Hollek A, Węglarz L. The effect of inositol hexaphosphate on the expression of selected metalloproteinases and their tissue inhibitors in IL-1β-stimulated colon cancer cells. Int J Colorectal Dis. 2012;27:1419–28. doi: 10.1007/s00384-012-1445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi BC. Slopes of a receiver operating characteristic curve and likelihood ratios for a diagnostic test. Am J Epidemiol. 1998;148:1127–32. doi: 10.1093/oxfordjournals.aje.a009592. [DOI] [PubMed] [Google Scholar]
- 25.Ying GS, Maguire M, Quinn G, Kulp MT, Cyert L. ROC analysis of the accuracy of Noncycloplegic retinoscopy, Retinomax Autorefractor, and SureSight Vision Screener for preschool vision screening. Invest Ophthalmol Vis Sci. 2011;52:9658–64. doi: 10.1167/iovs.11-8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stafiej J, Malukiewicz G, Lesiewska-Junk H, Rość D, Kaźmierczak K. Endothelial cell markers in patients with pseudoexfoliation syndrome. ScientificWorldJournal. 2012;2012:863949. doi: 10.1100/2012/863949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gartaganis SP, Patsoukis NE, Nikolopoulos DK, Georgiou CD. Evidence for oxidative stress in lens epithelial cells in pseudoexfoliation syndrome. Eye (Lond) 2007;21:1406–11. doi: 10.1038/sj.eye.6702596. [DOI] [PubMed] [Google Scholar]
- 28.Abu-Amero KK, Kondkar AA, Mousa A, Osman EA, Al-Obeidan SA. Decreased total antioxidants status in the plasma of patients with pseudoexfoliation glaucoma. Mol Vis. 2011;17:2769–75. [PMC free article] [PubMed] [Google Scholar]
- 29.Cumurcu T, Gunduz A, Ozyurt H, Nurcin H, Atis O, Egri M. Increased oxidative stress in patients with pseudoexfoliation syndrome. Ophthalmic Res. 2010;43:169–72. doi: 10.1159/000272019. [DOI] [PubMed] [Google Scholar]
- 30.Engin KN, Yemişci B, Yiğit U, Ağaçhan A, Coşkun C. Variability of serum oxidative stress biomarkers relative to biochemical data and clinical parameters of glaucoma patients. Mol Vis. 2010;16:1260–71. [PMC free article] [PubMed] [Google Scholar]
- 31.Gherghel D, Griffiths HR, Hilton EJ, Cunliffe IA, Hosking SL. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:877–83. doi: 10.1167/iovs.04-0777. [DOI] [PubMed] [Google Scholar]
- 32.Marchitti SA, Chen Y, Thompson DC, Vasiliou V. Ultraviolet radiation: cellular antioxidant response and the role of ocular aldehyde dehydrogenase enzymes. Eye Contact Lens. 2011;37:206–13. doi: 10.1097/ICL.0b013e3182212642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lassen N, Black WJ, Estey T, Vasiliou V. The role of corneal crystallins in the cellular defense mechanisms against oxidative stress. Semin Cell Dev Biol. 2008;19:100–12. doi: 10.1016/j.semcdb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Babizhayev MA. Biomarkers and special features of oxidative stress in the anterior segment of the eye linked to lens cataract and the trabecular meshwork injury in primary open-angle glaucoma: challenges of dual combination therapy with N-acetylcarnosine lubricant eye drops and oral formulation of nonhydrolyzed carnosine. Fundam Clin Pharmacol. 2012;26:86–117. doi: 10.1111/j.1472-8206.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- 35.Koliakos GG, Befani CD, Mikropoulos D, Ziakas NG, Konstas AG. Prooxidant-antioxidant balance, peroxide and catalase activity in the aqueous humour and serum of patients with exfoliation syndrome or exfoliative glaucoma. Graefes Arch Clin Exp Ophthalmol. 2008;246:1477–83. doi: 10.1007/s00417-008-0871-y. [DOI] [PubMed] [Google Scholar]
- 36.Koliakos GG, Konstas AG, Schlötzer-Schrehardt U, Hollo G, Katsimbris IE, Georgiadis N, Ritch R. 8-Isoprostaglandin F2a and ascorbic acid concentration in the aqueous humour of patients with exfoliation syndrome. Br J Ophthalmol. 2003;87:353–6. doi: 10.1136/bjo.87.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorkhabi R, Ghorbanihaghjo A, Javadzadeh A, Rashtchizadeh N, Moharrery M. Oxidative DNA damage and total antioxidant status in glaucoma patients. Mol Vis. 2011;17:41–6. [PMC free article] [PubMed] [Google Scholar]
- 38.Yimaz A, Adigüzel U, Tamer L, Yildirim O, Oz O, Vatansever H, Ercan B, Değirmenci US, Atik U. Serum oxidant/antioxidant balance in exfoliation syndrome. Clin Experiment Ophthalmol. 2005;33:63–6. doi: 10.1111/j.1442-9071.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- 39.Zoric L, Miric D, Milenkovic S, Jovanovic P, Trajkovic G. Pseudoexfoliation syndrome and its antioxidative protection deficiency as risk factors for age-related cataract. Eur J Ophthalmol. 2006;16:268–73. doi: 10.1177/112067210601600212. [DOI] [PubMed] [Google Scholar]
- 40.Koliakos GG, Konstas AG, Schlötzer-Schrehardt U, Bufidis T, Georgiadis N, Ringvold A. Ascorbic acid concentration is reduced in the aqueous humor of patients with exfoliation syndrome. Am J Ophthalmol. 2002;134:879–83. doi: 10.1016/s0002-9394(02)01797-x. [DOI] [PubMed] [Google Scholar]
- 41.Yağci R, Ersöz I, Erdurmuş M, Gürel A, Duman S. Protein carbonyl levels in the aqueous humour and serum of patients with pseudoexfoliation syndrome. Eye (Lond) 2008;22:128–31. doi: 10.1038/sj.eye.6702751. [DOI] [PubMed] [Google Scholar]
- 42.Yağci R, Gürel A, Ersöz I, Keskin UC, Hepşen IF, Duman S, Yiğitoğlu R. Oxidative stress and protein oxidation in pseudoexfoliation syndrome. Curr Eye Res. 2006;31:1029–32. doi: 10.1080/02713680601001319. [DOI] [PubMed] [Google Scholar]
- 43.Diehn JJ, Diehn M, Marmor MF, Brown PO. Differential gene expression in anatomical compartments of the human eye. Genome Biol. 2005;6:R74. doi: 10.1186/gb-2005-6-9-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee RK. The molecular pathophysiology of pseudoexfoliation glaucoma. Curr Opin Ophthalmol. 2008;19:95–101. doi: 10.1097/ICU.0b013e3282f49cda. [DOI] [PubMed] [Google Scholar]
- 45.Zenkel M, Kruse FE, Naumann GO, Schlötzer-Schrehardt U. Impaired cytoprotective mechanisms in eyes with pseudoexfoliation syndrome/glaucoma. Invest Ophthalmol Vis Sci. 2007;48:5558–66. doi: 10.1167/iovs.07-0750. [DOI] [PubMed] [Google Scholar]
- 46.Reddy VN, Kasahara E, Hiraoka M, Lin LR, Ho YS. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp Eye Res. 2004;79:859–68. doi: 10.1016/j.exer.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Hirose K, Longo DL, Oppenheim JJ, Matsushima K. Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anticancer drugs, and ionizing radiation. FASEB J. 1993;7:361–8. doi: 10.1096/fasebj.7.2.8440412. [DOI] [PubMed] [Google Scholar]
- 48.Visner GA, Dougall WC, Wilson JM, Burr IA, Nick HS. Regulation of manganese superoxide dismutase by lipopolysaccharide, interleukin-1, and tumor necrosis factor. Role in the acute inflammatory response. J Biol Chem. 1990;265:2856–64. [PubMed] [Google Scholar]
- 49.Uçakhan OO, Karel F, Kanpolat A, Devrim E, Durak I. Superoxide dismutase activity in the lens capsule of patients with pseudoexfoliation syndrome and cataract. J Cataract Refract Surg. 2006;32:618–22. doi: 10.1016/j.jcrs.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 50.Lin D, Barnett M, Grauer L, Robben J, Jewell A, Takemoto L, Takemoto DJ. Expression of superoxide dismutase in whole lens prevents cataract formation. Mol Vis. 2005;11:853–8. [PubMed] [Google Scholar]
- 51.Johansson K, Järvliden J, Gogvadze V, Morgenstern R. Multiple roles of microsomal glutathione transferase 1 in cellular protection: a mechanistic study. Free Radic Biol Med. 2010;49:1638–45. doi: 10.1016/j.freeradbiomed.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Yildirim Z, Uçgun NI, Kiliç N, Gürsel E, Sepici-Dinçel A. Pseudoexfoliation syndrome and trace elements. Ann N Y Acad Sci. 2007;1100:207–12. doi: 10.1196/annals.1395.020. [DOI] [PubMed] [Google Scholar]
- 53.Yilmaz A, Ayaz L, Tamer L. Selenium and pseudoexfoliation syndrome. Am J Ophthalmol. 2011;151:272–6. doi: 10.1016/j.ajo.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 54.Gartaganis SP, Georgakopoulos CD, Patsoukis NE, Gotsis SS, Gartaganis VS, Georgiou CD. Glutathione and lipid peroxide changes in pseudoexfoliation syndrome. Curr Eye Res. 2005;30:647–51. doi: 10.1080/02713680590968367. [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, Koppaka V, Thompson DC, Vasiliou V. Focus on molecules: ALDH1A1: from lens and corneal crystallin to stem cell marker. Exp Eye Res. 2012;102:105–6. doi: 10.1016/j.exer.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choudhary S, Xiao T, Vergara LA, Srivastava S, Nees D, Piatigorsky J, Ansari NH. Role of aldehyde dehydrogenase isozymes in the defense of rat lens and human lens epithelial cells against oxidative stress. Invest Ophthalmol Vis Sci. 2005;46:259–67. doi: 10.1167/iovs.04-0120. [DOI] [PubMed] [Google Scholar]
- 57.King G, Holmes R. Human ocular aldehyde dehydrogenase isozymes: distribution and properties as major soluble proteins in cornea and lens. J Exp Zool. 1998;282:12–7. [PubMed] [Google Scholar]
- 58.Lassen N, Bateman JB, Estey T, Kuszak JR, Nees DW, Piatigorsky J, Duester G, Day BJ, Huang J, Hines LM, Vasiliou V. Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(−/−)/Aldh1a1(−/−) knock-out mice. J Biol Chem. 2007;282:25668–76. doi: 10.1074/jbc.M702076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duester G. Families of retinoid dehydrogenases regulating vitamin A function: production of visual pigment and retinoic acid. Eur J Biochem. 2000;267:4315–24. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 60.Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4:697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]