In early studies, herpes simplex virus 1 (HSV-1) proteins were identified on the basis of two criteria. The first consisted of characterization of proteins contained in virions purified from cells whose proteins were labeled prior to infection. These proteins designated by the prefix VP were numbered in the order of decreasing apparent molecular weight or, conversely, ascending electrophoretic mobility in denaturing gels (147). The second criterion identified putative viral proteins accumulating in infected cells but absent from uninfected cells. These proteins, designated infected-cell proteins (ICPs), were also numbered in order of decreasing apparent molecular weight (73). One protein, however, while clearly apparent only in infected cells, varied with respect to electrophoretic mobility depending on the composition of the denaturing gel (74). This anomalously migrating protein was designated ICP0 (74). In subsequent studies, viral proteins were designated either by their known primary function (e.g., DNA polymerase, etc.) or the position of the gene along the unique long (UL) or short (US) components of the viral genome (105, 107). The original ICP designation, however, was retained primarily for five proteins recognized in early studies as being the products of α or immediate-early genes expressed after infection in the absence of de novo viral protein synthesis (74, 75). The five proteins, ICP0, ICP4, ICP22, ICP27, and ICP47, have been extensively studied, and for the most part, there is at least a semblance of concordance between the phenotype of cells infected with the mutant lacking the gene, the behavior of the protein in transduced cells, and the molecular functions expressed by the protein (reviewed in reference 132). ICP4 is an essential positive and negative regulator of gene expression (reviewed in reference 132). The protein blocks gene expression by binding to high-affinity DNA consensus sites located at transcription initiation sites of at least two genes. The mechanism of gene activation is less understood, although ICP4’s affinity for transcription factors and binding to highly degenerate or nonconsensus sites are suggestive of how it might act. ICP27 is also a multifunctional protein whose phenotype can be largely explained by its ability to block RNA splicing but not transport of unspliced RNA early in infection and by its activity as a chaperone of newly made viral mRNA across the nuclear membranes at late times after infection (reviewed in reference 137). Available data indicate that the carboxyl-terminal half of ICP22 enables full expression of a subset of late (γ2) viral genes by causing cdc2 cyclin-dependent kinase activity to survive the degradation of its physiologic partners cyclins A and B, by an aberrant partnership with the UL42 DNA polymerase processivity factor (2, 5, 6, 19, 141). Optimal transcription of late genes requires binding and posttranslational modification of topoisomerase IIα by the two proteins (7). The sole known mission of ICP47 is to bind to and preclude TAP1/TAP2 from enabling the transport of antigenic peptides into the endoplasmic reticulum for eventual presentation on the cell surface (50, 71, 161). Despite 3 decades of research and enormous interest, it is not known how the functions encoded in ICP0 account for its phenotype in either infected or transduced cells. However, available evidence suggests that ICP0 is a multifunctional protein and that its role in viral infection reflects the sum of its multiple and diverse functions (132).
Studies published nearly 20 years ago established that ICP0 activates genes introduced into cells by transfection or infection (29, 30, 54, 118, 129). Furthermore, ICP0 also activates a specific subset of cellular genes, including several p53-responsive genes that it activates independently of p53 (72). ICP0 is considered a promiscuous transactivator, inasmuch as it activates transcription from HSV (16, 22, 104) and heterologous (58, 114, 143) promoter elements independently of a single cis-acting element (40). ICP0 activates transcription of viral genes in synergy with or independent of ICP4 (29, 31, 55, 129). ICP0 has been shown to interact with ICP4, and this interaction is believed to mediate cooperative activation of gene expression, as it maps to a region of ICP0 (residues 617 to 775) that contains the domain involved in synergy with ICP4 (residues 680 to 767) (33, 159).
In most cell lines infected at low multiplicity with mutants lacking the gene encoding ICP0 (Δα0), viral yields are 10- to 100-fold lower than those from cells infected with wild-type virus (34, 136, 148). At higher multiplicities of infection, viral yields and protein expression are similar to those of wild-type virus (34, 136, 148). Exceptions are a few cell lines, exemplified by the line U20S, in which Δα0 mutants replicate as well as wild-type viruses (160). Because of the lethargy of Δα0 mutants, ICP0 has been held responsible for the establishment of latency and a myriad of other functions. Nevertheless, none of these phenotypic properties of ICP0 correlate directly with the emerging patterns of interaction of ICP0 with cellular proteins—the subject of this review.
α0 GENE STRUCTURE, SYNTHESIS, AND PROPERTIES OF THE GENE PRODUCT
α0, the gene encoding ICP0, maps in the inverted repeat sequences flanking the unique sequences of UL; therefore, the genome contains two copies of the gene. α0 consists of three exons encoding codons 1 to 19, 20 to 241, and 242 to 775. Intron 1 is by HSV standards large (767 bp) (123) and contains elements that may regulate the expression of the gene (61, 125). Intron 1 RNA accumulates in the cytoplasm and on the basis of its abundance appears to be more stable than ICP0 mRNA (18). Although RNA analyses identified four splice donors and one splice acceptor sequence for intron 1, alternatively spliced products have not been demonstrated (K. L. Carter, A. P. W. Poon, and B. Roizman, unpublished data). A product of an intron 2 unspliced RNA (ICP0R) has been identified and is present in infected cells at low levels in a cell-type-dependent manner (38). ICP0R functions as a promiscuous repressor of transcriptional activation by ICP0 and heterologous transcriptional activators (155). While the mechanism by which it represses transcription has not been determined, it has been suggested that ICP0R titrates a cellular factor(s) away from ICP0 (146). ICP0 encodes numerous functional domains, including a nuclear localization signal (residues 474 to 509) (33) and a high-affinity self-interaction domain in its C terminus that mediates the formation of stable dimers and higher-order oligomers (residues 617 to 711) (24, 40). α0 also encodes a C3HC4 RING finger zinc-binding motif between codons 116 and 156 of exon 2 (44, 48, 49).
Although ICP0 contains only 775 amino acids, its electrophoretic mobility in denaturing gels is that of a protein in excess of 1,000 amino acids in size (1, 100, 112, 123). The discordant migration of the protein may reflect the presence of a large number of prolines, especially in islands of highly conserved sequences in exon 3. The accumulation of ICP0 is cell type dependent and is also dependent on the accumulation of other α proteins (19, 60, 127).
ICP0 is extensively posttranslationally processed (1). It is phosphorylated by the viral protein kinase UL13 (117) and the cell cycle kinase cdc2 (3, 5) and nucleotidylylated by casein kinase II (9, 10, 110, 111). At least six major differentially modified isoforms of ICP0 are observed during the course of viral infection, and the pattern of isoforms observed varies as infection progresses (1, 3). Modifications of ICP0 are sequential and are associated with specific locations of the protein within cellular compartments (3, 110). Posttranslational modifications of ICP0 may determine the specific function performed by the protein at the specific time and/or in the cellular compartment in which it is localized (3). In this way, differential modification of ICP0 may contribute to its multifunctionality.
ICP0 in its pristine HSV-1 form is conserved in few herpesviruses. In HSV-2 the first intron is shorter and lacks the regulatory sequences (61, 106). The HSV-1 and HSV-2 proteins diverge particularly in the amino-terminal half of the sequences encoded by exon 3 (106). Nevertheless, several herpesviruses of the Alphaherpesvirinae subfamily contain genes at least in part homologous to HSV-1 ICP0 (23, 123, 150, 158).
DISTRIBUTION OF ICP0 IN INFECTED CELLS AND EFFECT OF THE PROTEIN ON THE STRUCTURE AND FUNCTION OF THE CELL
Newly synthesized ICP0 accumulates initially at or near nuclear structures knows as PODs, K bodies, or ND10 (39, 102, 103). The main organizing component of these structures is the promyelocytic leukemia (PML) protein (77, 163). PML recruits other constituents (Daxx, CBP, Sp100, etc.) in both native and sumoylated forms (reviewed in reference 116). The number and range of functions ascribed to PML are egregious (reviewed in refererences 12, 80, and 130). The prevailing evidence suggests that ND10 structures are repositories of transactivating factors and that SUMOylated proteins act as transcriptional repressors (reviewed in references 116 and 142). ND10 structures and accumulation of some ND10 components are regulated by cytokine signaling (e.g., interferons) (reviewed in reference 130).
As the amount of ICP0 increases, the protein spreads out and fills the nucleus. Late in infection, after the onset of viral DNA synthesis, ICP0 is found in the cytoplasm (88, 96, 151). ICP0 appears to shuttle and may linger rather than reside in the cytoplasm at late times after infection. In the presence of MG132, a proteasome inhibitor, ICP0 reappears in the nucleus and associates with proteasome subunits (96, 152). In cells abortively infected with a Δα4 mutant, d120, that generally does not express β or γ genes (26), ICP0 migrates from the nucleus to the cytoplasm at a much earlier time point, suggesting that retention of ICP0 in the nucleus until the onset of viral DNA synthesis requires at least expression of post-α genes (96). In cells infected with this mutant, ICP0 forms dense spherical or oval structures containing proteasome subunits (96).
The peregrinations of ICP0 appear to depend on both viral and cellular factors. ICP0 binds cyclin D3, and all available evidence suggests that this interaction promotes the relocalization of cyclin D3 to ND10 structures (84, 151). ICP0 in which aspartate 199 has been replaced with alanine does not bind cyclin D3 and is not translocated into the cytoplasm (151, 153). Conversely, overexpression of cyclin D3 accelerates the translocation (151). The mechanism by which cyclin D3 enables the export of ICP0 to the cytoplasm is unknown.
Concomitant with the spread of ICP0 throughout the nucleus, ICP0 promotes the disaggregation of ND10 structures (39, 102, 103) and degradation of ND10 components, including PML (45) and Sp100 (21, 120), especially SUMOylated isoforms. Unambiguous evidence implicates the RING finger domain in the disruption of ND10 structures and the proteasome-dependent degradation of its constituents (39, 45, 47, 102, 120).
In addition to ND10 components, ICP0 has been reported to cause the degradation of the catalytic subunit of the DNA-dependent protein kinase (90, 122), centromeric proteins C and A (46, 95), and as-yet-unidentified SUMOylated proteins (45) in a RING finger-dependent manner. With time, the number and diversity of cellular proteins known to be targeted for destruction by ICP0 are likely to increase as more information regarding its functions becomes available.
INTERACTION OF ICP0 WITH CELLULAR PROTEINS
The preceding section recited the apparent interaction of ICP0 with three cellular components, cyclin D3, ND10, and proteasome subunits. In cells infected with Δα0 or ICP0 D199A mutant virus, both cyclins D1 and D3 are rapidly degraded in a proteasome-dependent manner (63, 84, 151, 153). However, D-type cyclins are stabilized and cdk4 is activated at least through the time of nuclear localization of ICP0 in wild-type virus infection (63, 84, 151, 153). Nonetheless, cdk2 is not activated, and analysis of E2F proteins does not support the hypothesis that the stabilization of cyclins is designated to activate S-phase cellular protein synthesis (4). There is no evidence that either cyclin D1 or cyclin D3 is made de novo in infected cells, and various studies failed to show any genetic or physical interaction between cyclin D1 and ICP0 (151).
ND10 structures are a common target of viruses belonging to many diverse families (reviewed in references 37 and 130). Although ICP0 has been linked to the disruption of ND10 structures and degradation of their constituents (39, 45, 102, 103, 120), there is no evidence that ICP0 interacts directly with any components of ND10 domains or that ND10 structures directly affect viral replication. Overexpression of PML in uninfected cells causes the ND10 structures to increase in size and sequester components present also in a dispersed form (97, 145). Cells containing these enlarged ND10 structures replicate HSV-1 in a manner indistinguishable from those of untreated cells (97). Furthermore, the ND10 structures remain intact throughout infection (97). An explanation for the targeting of PML for degradation by the virus emerged from comparisons of viral replication in murine PML−/− and PML+/+ cells. In the absence of exogenous alpha or gamma interferon, wild-type virus replicated equally well in both cell lines (20). In the presence of interferon, viral gene expression and yields were significantly reduced in PML+/+ cells but not in PML−/− cells (20). Furthermore, replication of Δα0 viruses in cultured cells is exquisitely sensitive to interferon (69, 113). Finally, a virus in which the RING finger has been inactivated by two point mutations is hypersensitive to interferons in PML+/+ cells but demonstrates interferon sensitivity comparable to that of wild-type virus in PML−/− cells (R. Hagglund and Roizman, unpublished results). The degradation of PML appears to be a preemptive function designed to preclude exogenous interferon from interfering with viral replication.
In cells in which ND10 structures are not disrupted (e.g., infection with a Δα0 mutant or under conditions where PML is overexpressed), viral DNA localizes adjacent to ND10 structures prior to replication and replication occurs in close association with ND10 (101, 145). This association of viral genomes with ND10 structures requires ICP4 and ICP27 and is mediated by the interaction of these proteins with the ND10 component Daxx (149). ICP4 is recruited into foci adjacent to ND10 structures (41). In wild-type virus infection in the absence of PML overexpression, viral DNA associates with ND10 structures prior to their disruption by ICP0 (101), but replication of viral DNA in association with ND10 structures cannot be detected because of the destruction of ND10. As ND10 structural integrity is clearly not required for viral DNA replication, the implications of the association of viral DNA with ND10 structures are not clear.
ICP0 has been linked to other proteins. Among the earliest reported is the herpesvirus ubiquitin-specific protease now known as USP7 (43, 108, 109). This high-affinity interaction is abolished by the substitution of ICP0 residue 620 or substitution of both residues 623 and 624 (42). Another cellular protein interacting with ICP0 is BMAL1, a transcription factor that forms a heterodimer with CLOCK and regulates circadian oscillation (87). p60, a protein of unknown function identified in yeast-two hybrid assays, interacts with both ICP0 and an unprocessed form of ICP22 (15). ICP0 also interacts with the translation elongation factor EF-1δ (88). A potential significance of this interaction is signaled by the observation that members of all three herpesvirus subfamilies phosphorylate EF-1δ through homologs of the highly conserved HSV-1 UL13 protein kinase (82, 85, 86, 89). Thus, hyperphosphorylation of EF-1δ may enhance the ability of the cell to sustain a high level of protein synthesis.
ICP0 IS A TWO-HEADED UBIQUITIN LIGASE
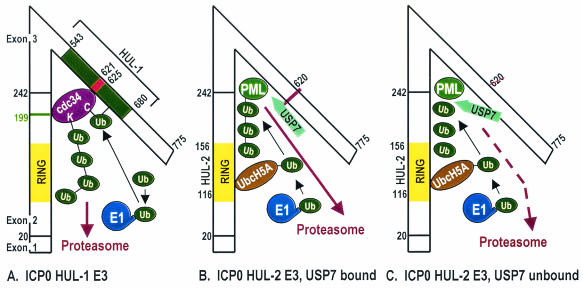
Multiple lines of evidence led to the hypothesis that ICP0 is a component of the ubiquitin proteasome pathway and may act as an ubiquitin ligase (Fig. 1). (i) The C-terminal region of ICP0 (residues 594 to 646) interacts with USP7 (42, 43, 108, 109). (ii) ICP0 promotes the degradation of several cellular proteins (45, 46, 90, 95, 120, 122) and induces the accumulation of and colocalizes with conjugated ubiquitin (36, 121) in a RING finger-dependent manner. (iii) ICP0 dynamically associates with 26S proteasomes in infected cells and remains bound to proteasomes in the presence of the proteasome inhibitor MG132 (96, 152).
FIG. 1.
Models showing interactions of ICP0 with the ubiquitin-proteasome system. (A) HUL-1 E3 domain. ICP0 HUL-1 E3 activity, mapping in sequences encoded in exon 3, promotes the autoubiquitylation and degradation of the E2 enzyme cdc34 (62-64, 152). The model depicted is described in the text. The figure is adapted from reference 62. (B) RING finger (HUL-2) E3 domain mapping to sequences encoded by exon 2. ICP0 RING finger E3 activity promotes the ubiquitylation and degradation of PML and other cellular proteins (13, 45, 46, 95, 120, 122). While the ICP0 RING finger has E3 ligase activity in in vitro reactions containing either UbcH5a or UbcH6 E2 ubiquitin-conjugating enzymes (14, 64), the degradation of PML and Sp100 appears to be mediated by UbcH5a (60). The E2 enzyme(s) involved in the degradation of the other known substrates of the ICP0 RING finger has not been identified. Available data indicate that ICP0 binds USP7 and sequesters it from deubiquitylating substrates ubiquitylated by ICP0. (C) Model of HUL-2 E3 in cells infected with a mutant incapable of binding USP7. The model shows that unbound USP7 would be available to deubiquitylate newly ubiquitylated ICP0 substrates, decreasing the efficiency of their degradation. This model is consistent with data showing that mutants unable to bind USP7 have a reduced capacity to degrade PML and Sp100 (42; Hagglund and Roizman, unpublished).
The enzymatic cascade leading to the ubiquitylation and degradation of substrate proteins has been reviewed in detail elsewhere (70, 78). Briefly, activated ubiquitin is transesterified from the E1 ubiquitin-activating enzyme to a conserved cysteine of an E2 ubiquitin-conjugating enzyme. E3 ubiquitin ligases represent the most diverse components of the system and bind both the E2 and substrate while functioning catalytically to promote the transfer of ubiquitin from the E2 to the substrate. Since RING finger domains are characteristic of a class of E3 ubiquitin ligase enzymes (78, 81, 98) and since ICP0 is not degraded by the 26S proteasome in infected cells (152), ICP0 became a prime candidate as a virus-encoded component of the cellular ubiquitylation machinery.
Investigation of cdc34 as a possible target of degradation by ICP0 was initially based on the observation that ICP0 caused the stabilization of both cyclins D3 and D1, even though there was no evidence that cyclin D1 interacts with ICP0 (151). One hypothesis that was investigated was that ICP0 targets the E2 enzyme responsible for the turnover of the D cyclins in uninfected cells. cdc34 is the major E2 enzyme integrating into the Skp1-cdc34-F-box E3 complex (78), which promotes the degradation of cyclin D1 and cyclin D3 in conjunction with the F-box protein Skp2 (52, 133, 134, 162). Supporting evidence that ICP0 targets cdc34 for degradation is as follows. (i) Ubiquitylated cdc34 isoforms are bound to proteasomal subunits in infected cells in the presence of MG132 (152). (ii) cdc34 is degraded in a proteasome-dependent manner in infected cells (64). (iii) cdc34 strongly interacts with ICP0 in the region encoded by exon 2 (residues 20 to 241), and the D199A mutation attenuates but does not destroy this interaction (64, 152). Concordant with these observations, both the targeting of ubiquitylated cdc34 to proteasomes and cdc34 degradation do not occur in cells infected with a recombinant virus carrying the ICP0 D199A mutation (64, 152). (iv) Contrary to expectations, the ICP0 ligase activity associated with cdc34 mapped to exon 3 (residues 543 to 680) rather than to the RING finger domain in exon 2 (62, 64, 152). In in vitro substrate-independent ubiquitylation reactions, ICP0 exon 3 polypeptides encoding this E3 function promoted the autoubiquitylation of cdc34 (62, 152). Moreover, this domain of exon 3 interacts with cdc34, albeit not as strongly as the exon 2 domain described above (62). Further studies demonstrated that residues 621 to 625 are essential for both polyubiquitylation and binding of cdc34 by the amino acids encoded in exon 3 (62). (v) As would be predicted, a viral mutant lacking ICP0 residues 621 to 625 does not stabilize D-type cyclins or cause the degradation of cdc34 (63). (vi) The ubiquitin ligase activity mapping in exon 3 was not affected in cells infected with a viral mutant in which the RING finger was disrupted by mutagenesis (63).
The E3 activity mapping in exon 3 (Fig. 1A), designated herpesvirus ubiquitin ligase 1 (HUL-1), is noteworthy in two respects. First, the HUL-1 activity specifically promotes the ubiquitylation and degradation of cdc34 (62, 63, 152) and no other target has been identified to date. The ability of an E3 enzyme to catalyze the autoubiquitylation of its cognate E2 is not unusual inasmuch as yeast cdc34 is autoubiquitylated in vitro and in vivo (8, 59, 144). As illustrated in Fig. 1A, the interaction of ICP0 with cdc34 at residue D199 may serve to tether cdc34 to ICP0 and promote its autoubiquitylation by bringing it into proximity to the HUL-1 domain (62, 64). cdc34 also weakly binds to exon 3 (62), as could be expected for the interaction of E2 and E3 enzymes (78). These activities are independent of the exon 2 RING finger domain both in the infected cell and in vitro (62-64, 152). Second, the HUL-1 E3 represents a novel class of ubiquitin ligases in that it functions independently of the ICP0 RING finger domain and shares no homology with other E3 enzymes lacking a RING finger (62).
The RING finger domain of ICP0 acts as a second, independent ubiquitin ligase site designated HUL-2 (Fig. 1B) (14, 64). Of the many E2 enzymes tested independently in two laboratories, only two, UbcH5a and UbcH6, promoted ubiquitin-protein ligation in in vitro substrate-independent assays (14, 64). As noted earlier, the RING finger is associated with degradation of PML and Sp100, constituents of ND10 structures (45, 120). Evidence linking the in vitro E3 ligase activities with degradation of PML and Sp100 in infected cells emerged from studies in which cells transduced with dominant-negative E2 enzymes were infected with wild-type virus. In these cells dominant-negative UbcH5a but not dominant-negative UbcH6 or UbcH7 blocked PML and Sp100 degradation and delayed ND10 disruption by at least several hours (60).
Efficient degradation of PML and translocation of ICP0 from the nucleus to the cytoplasm require the presence of the UL13 and US3 protein kinases. In cells infected with mutants lacking either one of the kinases, both the degradation of PML and the translocation of ICP0 to the cytoplasm are delayed by several hours. The degradation of cdc34 is also dependent on the UL13 kinase (A. Esclatine, Hagglund, Poon, and Roizman, unpublished). These observations are consistent with the evidence that degradation of PML is not essential for viral gene expression, as evident from studies on the effects of overexpression of PML (97). The data also indicate that in infected cells efficient PML degradation involves post-α gene expression.
ICP0 also encodes a novel mechanism to augment the efficiency of the degradation of RING finger substrates (Fig. 1C). Examination of the data published by Parkinson and Everett (120) suggests that ICP0 binds USP7 to sequester it from newly ubiquitylated HUL-2 substrates (Hagglund and Roizman, unpublished). It is of note that the USP7 binding site shares several critical determinants with the HUL-1 E3 domain (42, 62, 63). However, separation of these activities by the ICP0 K620I point mutation has allowed their respective functions to be dissected (42, 64; Hagglund and Roizman, unpublished).
A recent report demonstrated that the tumor suppressor p53 (reviewed in reference 93) binds to ICP0 in the region encoded by residues 241 to 594 and that ICP0 RING finger E3 function promotes low levels of p53 ubiquitylation in infected cells (13). Unlike those of other ICP0 RING finger E3 substrates, p53 levels are not affected by HSV-1 infection (72), and ICP0-mediated p53 ubiquitylation is much less efficient than p53 ubiquitylation by Mdm2 (13). Thus, the relevance of p53 ubiquitylation by ICP0 is unclear. As HSV-1 infection prevents induction of apoptosis by UV irradiation in an ICP0-dependent manner (13), it is possible that the effects of ICP0 on p53 block its ability to initiate apoptotic responses detrimental to the virus.
HSV-1 is not unique among viruses in encoding components of the ubiquitin-proteasome pathway to rid the infected cell of protein inimical to its replication (25, 51, 68, 76, 99, 128, 140), nor is it the only herpesvirus to encode an ubiquitin ligase (25, 51). To date, however, ICP0 is the only known protein exhibiting two independent E3 sites. The finding exemplifies both the range of cellular proteins that it deems threatening to its replication and the density of genetic information that is the hallmark of viral genomes.
Several unresolved issues are likely to be the focus of further work. The first centers on observations that both cdc34 and cyclin D3 bind ICP0 at or near D199. It is not clear whether cdc34 and cyclin D3 bind to ICP0 exon 2 cooperatively or competitively or if the binding of cyclin D3 is related to its role in mediating the translocation of ICP0 in the initial stages of HSV-1 infection. A second unresolved issue stems from the data showing that cyclin D1 is stabilized, even though there is no evidence that it interacts with ICP0 (63, 151). Whether stabilization of cyclin D1 reflects a need for this protein or unintended collateral damage in the quest to salvage cyclin D3 has not been discerned. While ICP0 exon 3 E3 activity might act on additional substrates, the degradation of cdc34, its cognate E2, mediated by ICP0 would render such activity inefficient. Another pending issue is whether Sp100 and PML are the only ND10 components targeted for degradation by the ICP0 RING finger. Finally, it has been proposed that SUMOylated PML functions as the central organizer of ND10 structures because SUMOylation of PML is required for proper formation of ND10 bodies (77, 163). Thus, an obvious question is whether degradation of Sp100 is collateral damage resulting from the degradation of PML.
ICP0, GENOME ORGANIZATION, AND INITIATION OF VIRAL GENE EXPRESSION
Three lines of investigation, some more tenuous than others, connect ICP0 to the structure of the viral genome in relation to its expression. The most senior in age is the view that ICP0 is a determinant of latency. In essence, the viral genome has been reported to be circular (reviewed in references 131 and 132) and encased in a nucleosomal structure (28) in the latent state. With the exception of the latency-associated transcripts, which are in part antisense to ICP0, the HSV genome is silent during its latent state in dorsal root neurons (reviewed in reference 132). The rate of spontaneous reactivation appears to be species specific in experimental animal systems and is probably at the low end in mice (133). The common procedure to measure reactivation is to explant the dorsal root ganglia in vitro (132). Δα0 virus is inefficient in establishing latency (66, 91, 157), but then to establish latency efficiently, the virus must replicate at or near the nerve endings that transport the virus from the site of entry into the body to the neuronal nucleus (83, 131, 138, 139). Δα0 virus does not readily reactivate (17, 67, 91, 135), but given its phenotype in cultured cells, this is hardly surprising. Many deletion mutants (e.g., ΔUL23, the gene encoding thymidine kinase) that replicate well in culture fail to reactivate efficiently from the latent state on explantation of ganglia (reviewed in reference 132).
The most recent association of ICP0 with latency stems from the observation that the recovery of circular virus from infected cells by electrophoresis in Gardella gels is far lower than would be expected if viral DNA were replicated by the rolling circle model (79). More important, the relative amount of circular forms recovered in this system, while small, is greater in cells infected with Δα0 mutant. The leap that anchors ICP0 to latency in this argument is that the establishment of circular forms in latently infected neurons is the consequence of absence of ICP0. This aspect of the argument has no opposition, since it has been generally accepted that expression of regulatory proteins ICP4, ICP0 and VP16, the virion transcription factor which activates α gene promoters, would be antithetical to the establishment of latency (65).
More contentious is the conclusion that viral DNA synthesis occurs on linear rather than circular templates as a consequence of the presence of ICP0 (79). The data supporting this model are based entirely on the physical separation of linear DNA and circular DNA (79). A central question is whether the technique employed in this study effectively discriminates between linear DNA and circular forms of molecules containing nicks and gaps—a common feature of viral DNAs packaged in virions. The circular model of viral replication (reviewed in reference 11) is founded on separation by different techniques as well as direct detection of the formation of head-to-tail junctions and pulsed-field gel electrophoresis (27, 53, 124). In particular, the circular model is supported by evidence that, within 30 min after entry, a large fraction of virus competent to express ICP0 had fused ends, that the ratio of linked to unlinked ends remained stable for several hours after infection, and that linkage did not require viral protein or DNA synthesis (53, 124). While Jackson and DeLuca correctly assert that an increase in joint regions could be indicative of circularization or concatemerization (79), reconciliation of the divergent results obtained by these methods is of paramount importance.
The third line of evidence that ICP0 is a determinant for the initiation of viral gene expression emerged from studies designed initially to determine whether the cDNA of ICP0 could replace the genomic variant. Viruses carrying the cDNA copy were made by two laboratories, and both reported that the cDNA viruses were capable of replication, establishment of latency, and reactivation (35, 115, 119). A closer examination of one of these mutants revealed that they resembled the wild-type virus in all but one peculiar characteristic. In some cell lines, notably HEp-2 and rabbit skin cells, but not Vero cells, the initiation of viral gene expression could be delayed by several hours in a multiplicity-dependent manner—the higher the multiplicity of infection, the shorter the delay. However, even at very low multiplicities of infections that resulted in a delay of several hours, the final yield of virus could not be differentiated from that of the wild-type virus (125). Two additional observations are of interest. First, inhibitors of histone-deacetylating enzymes shortened the delay in viral gene expression at low multiplicities by several hours. Second, expression of viral genes by the cDNA virus but not that of wild-type virus was blocked by MG132 added to the medium 3 h after infection (125, 126).
The significance of these studies stems from a more general issue concerning the state of the viral DNA released into the nuclei. In virions, the DNA is known to be bound by polyamines (56, 57), leading to the question of whether viral DNA remains unencumbered from cellular proteins until it and its progeny are repackaged in virions. One scenario that incorporates a role for ICP0 is that viral DNA becomes loosely bound to proteins and that, in the absence of transcriptional activity, the rate of conversion into closed nucleosomal structures is cell type dependent. In the absence of ICP0 but at a high multiplicity of infection, the low initial level of transcription becomes a virtual torrent as viral proteins accumulate, interact with viral DNA, and shift transcription into a higher gear. At low multiplicities, the genome may well be silenced before the accumulation of sufficient viral protein necessary to reverse the silencing of the viral genome. The virus carrying the cDNA copies may well represent an intermediate between a wild-type virus and Δα0 mutant. The report that the intron contains transcription factor binding sites (61) suggests the possibility that, in its absence, the accumulation of ICP0 proceeds at a lower rate. This in turn delays the divestiture of viral genome from cellular proteins for transcription and, ultimately, DNA synthesis. Questions arising in the wake of these studies center on the role of ICP0 in the initiation of viral gene expression and whether this function accounts for the ability of ICP0 to activate genes introduced into cells by transfection or infection.
CONCLUSIONS
ICP0 does not bind DNA and, with the exception of BMAL1, does not bind known transcriptional factors. An all-too-obvious explanation for the ability of ICP0 to transactivate gene expression is that this function reflects the sum of all of the functions performed by the protein. The breadth of this assumption can be narrowed on the basis of the observation that mutations that disrupt the RING finger are ineffective in transactivating gene expression (22, 32, 33, 47, 94). The possibility exists, however, that a functional RING finger is necessary in order to enable another, more direct action by ICP0.
Another approach to this question is to examine the impact of each of the known functions in detail. Again, it is apparent that the functions dependent on the RING finger domain have the highest impact. For example, mutations in exon 3 and sequences distal to the RING finger decrease viral yields, especially in experimental animal systems, but the overall effects are not nearly as dramatic as those resulting from the disruption of the RING finger that are similar to those observed for ICP0 null viruses (22, 34, 42, 63, 94, 153). However, some mutations in exon 3 have a material effect on transactivation by ICP0 comparable to that of RING finger disruption, but these mutations have a less pronounced effect on viral growth than those inactivating the RING finger (34, 42). These observations suggest that, while transactivation is a major function of ICP0, the ICP0 RING finger domain makes additional contributions to the function of ICP0 in viral replication.
The most apparent consequence of the disruption of the RING finger is the structural maintenance of the ND10 bodies and the stabilization of several cellular proteins cited earlier in the text. A readily identifiable advantage resulting from the degradation of PML is the lessened susceptibility to interferons produced by distal, abortively infected cells. The evidence that ICP0 indeed plays a role in blocking the antiviral effects of interferon emerged from studies showing that a Δα0 mutant was virulent in mice defective for interferon receptors (92). Nevertheless, it is difficult to see a connection between the anti-interferon functions of ICP0 and transactivation of cellular genes in the context of infected cells in culture.
Another hypothesis is that the transactivating properties of ICP0 are the consequence of transcriptional factors released from the disrupted ND10 structures. This hypothesis suffers from two defects. First, as discussed above, preclusion of ND10 disruption by overexpression of PML has no effect on viral replication in cultured cells (97). Second, this hypothesis is inconsistent with the observation that the transactivation function of ICP0 is restricted to genes introduced into the cells by transfection or infection and a specific subset of cellular genes rather than affecting a diversity of viral and cellular genes contained in the infected cell (30, 72).
Arising from data indicating that ICP0 has an effect similar to that of a histone deacetylase inhibitor (72), the hypothesis that remains to be explored in greater depth is that DNA introduced into cells by infection or transfection becomes bound to cellular proteins immediately upon entry but that the organization of the DNA into stable chromatin structures may take many hours and varies from cell to cell. ICP0 may affect these prechromatin interactions and enable the transcription of viral genomes at a stage before histones are bound and modified to silence the DNA. As noted earlier, silencing of the genome is overcome in the absence of ICP0 if sufficient amounts of viral proteins are present to enable transcription and replication to take place. Consistent with this hypothesis, U20S cells complement ΔVP16 mutant viruses as well as Δα0 viruses (160). Thus, at high multiplicities, high levels of virion VP16 might be able to drive sufficient viral gene expression in the absence of ICP0. A viral protein suitable for this function is ICP8, a DNA-binding protein made very early in infection that cooperatively binds to viral DNA (reviewed in references 11 and 132). The test of this hypothesis remains a major challenge in the elucidation of the role of ICP0 in the biology of HSV.
A key unresolved question arising from the ICP0 studies conducted hitherto is whether ICP0 RING finger E3 ubiquitin ligase function mediates the RING finger-dependent transactivation function of ICP0. No mutations in the RING finger domain tested to date have been able to separate E3 and transactivation functions (14, 94; Hagglund and Roizman, unpublished). Furthermore, mutations in ICP0 exon 3 that disrupt the USP7 interaction site, resulting in less efficient degradation of RING finger substrates, impair the transactivation function of ICP0 (42, 120; Hagglund and Roizman, unpublished). If one takes into account the hypothesis described above, an attractive possibility is that ICP0 RING finger E3 activity promotes the degradation of a global transcriptional repressor(s) functioning in the formation of chromatin structure, which is adverse to transcription from viral DNA and transfected plasmids. While several possible candidates have arisen, the identity of such a putative substrate remains unknown. This hypothesis would also explain the mechanism by which ICP0R and truncations missing exon 3 act as dominant-negative promiscuous transrepressors (154, 155). While ICP0 ubiquitylation in infected cells has not been detected, ICP0R is stably ubiquitylated in infected cells (156). Thus, ICP0R may titrate the E2 that mediates the degradation of the substrate(s) responsible for the transactivation function away from ICP0. Considering that the integrity of regions of ICP0 exon 3 not involved in USP7 binding, nuclear localization, or multimerization is required for transactivation activity (33), exon 3 might function to localize such a substrate(s) to ICP0.
A key feature of ICP0 that emerges from a review of all of its activities and interactions is that the protein exhibits an enormous conformational diversity. To interact with so many diverse cellular and viral proteins, it must have a structure that enables it to change conformations as a consequence of posttranslational modifications or event-induced fit. These are properties of proteins that have arisen early in evolution. Analyses of the structure of ICP0 may provide valuable lessons in protein design.
Acknowledgments
We thank Sunil Advani, Haidong Gu, and Alice P. W. Poon for careful reading of the manuscript and thank them and Roger Everett for useful discussions.
The studies performed in this laboratory were aided by National Cancer Institute Grants CA87661, CA83939, CA71933, CA78766, and CA88860 and by the U.S. Public Health Service. R.H. is funded in part by the Howard Hughes Medical Institute.
REFERENCES
- 1.Ackermann, M., D. K. Braun, L. Pereira, and B. Roizman. 1984. Characterization of herpes simplex virus 1 α proteins 0, 4, and 27 with monoclonal antibodies. J. Virol. 52:108-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advani, S. J., R. Brandimarti, R. R. Weichselbaum, and B. Roizman. 2000. The disappearance of cyclins A and B and the increase in activity of the G2/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the α22/US1.5 and UL13 viral genes. J. Virol. 74:8-15. [PMC free article] [PubMed] [Google Scholar]
- 3.Advani, S. J., R. Hagglund, R. R. Weichselbaum, and B. Roizman. 2001. Posttranslational processing of infected cell proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize. J. Virol. 75:7904-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2000. E2F proteins are posttranslationally modified concomitantly with a reduction in nuclear binding activity in cells infected with herpes simplex virus 1. J. Virol. 74:7842-7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2000. The role of cdc2 in the expression of herpes simplex virus genes. Proc. Natl. Acad. Sci. USA 97:10996-11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2001. cdc2 cyclin-dependent kinase binds and phosphorylates herpes simplex virus 1 UL42 DNA synthesis processivity factor. J. Virol. 75:10326-10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2003. Herpes simplex virus 1 activates cdc2 to recruit topoisomerase II alpha for post-DNA synthesis expression of late genes. Proc. Natl. Acad. Sci. USA 100:4825-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee, A., L. Gregori, Y. Xu, and V. Chau. 1993. The bacterially expressed yeast CDC34 gene product can undergo autoubiquitination to form a multiubiquitin chain-linked protein. J. Biol. Chem. 268:5668-5675. [PubMed] [Google Scholar]
- 9.Blaho, J. A., C. Mitchell, and B. Roizman. 1993. Guanylylation and adenylylation of the α regulatory proteins of herpes simplex virus require a viral β or γ function. J. Virol. 67:3891-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaho, J. A., C. Mitchell, and B. Roizman. 1994. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J. Biol. Chem. 269:401-410. [PubMed] [Google Scholar]
- 11.Boehmer, P. E., and I. R. Lehman. 1997. Herpes simplex virus DNA replication. Annu. Rev. Biochem. 66:347-384. [DOI] [PubMed] [Google Scholar]
- 12.Borden, K. L. B. 2002. Pondering the promyelocytic leukemia protein (PML) puzzle: possible functions for PML nuclear bodies. Mol. Cell. Biol. 22:5259-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutell, C., and R. Everett. 2003. The HSV-1 regulatory protein interacts with and ubiquitinates p53. J. Biol. Chem. 278:36596-36602. [DOI] [PubMed] [Google Scholar]
- 14.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruni, R., B. Fineschi, W. O. Ogle, and B. Roizman. 1999. A novel cellular protein, p60, interacting with both herpes simplex virus 1 regulatory proteins ICP22 and ICP0 is modified in a cell-type-specific manner and is recruited to the nucleus after infection. J. Virol. 73:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai, W., and P. A. Schaffer. 1992. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol. 66:2904-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai, W., T. L. Astor, L. M. Liptak, C. Cho, D. M. Coen, and P. A. Schaffer. 1993. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J. Virol. 67:7501-7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter, K. L., and B. Roizman. 1996. Alternatively spliced mRNAs predicted to yield frame-shift proteins and stable intron 1 RNAs of the herpes simplex virus 1 regulatory gene α0 accumulate in the cytoplasm of infected cells. Proc. Natl. Acad. Sci. USA 93:12535-12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter, K. L., and B. Roizman. 1996. The promoter and transcriptional unit of a novel herpes simplex virus 1 α gene are contained in, and encode a protein in frame with, the open reading frame of the α22 gene. J. Virol. 70:172-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chee, A. V., P. Lopez, P. P. Pandolfi, and B. Roizman. 2003. Promyleocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J. Virol. 77:7101-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chelbi-Alix, M. K., and H. de Thé. 1999. Herpes virus induced proteasome-dependent degradation of the nuclear bodies-associated PML and Sp100 proteins. Oncogene 18:935-941. [DOI] [PubMed] [Google Scholar]
- 22.Chen, J., and S. Silverstein. 1992. Herpes simplex viruses with mutations in the gene encoding ICP0 are defective in gene expression. J. Virol. 66:2916-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung, A. K. 1989. DNA nucleotide sequence analysis of the immediate-early gene of pseudorabies virus. Nucleic Acids Res. 17:4637-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciufo, D. M., M. A. Mullen, and G. S. Hayward. 1994. Identification of a dimerization domain in the C-terminal segment of the IE110 transactivator protein from herpes simplex virus. J. Virol. 68:3267-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coscoy, L., D. J. Sanchez, and D. Ganem. 2001. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J. Cell Biol. 155:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLuca, N. A., A. M. McCarthy, and P. A. Schaffer. 1985. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 56:558-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deshmane, S. L., B. Raengsakulrach, J. F. Berson, and N. W. Fraser. 1995. The replicating intermediates of herpes simplex virus type 1 DNA are relatively short. J. Neurovirol. 1:165-176. [DOI] [PubMed] [Google Scholar]
- 28.Deshmane, S. L., and N. W. Fraser. 1989. During latency, herpes simplex virus type 1 DNA is associated with nucleosomes in a chromatin structure. J. Virol. 63:943-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Everett, R. D. 1984. Trans activation of transcription by herpes virus products: requirements for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 3:3135-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everett, R. D. 1985. Activation of cellular promoters during herpes virus infection of biochemically transformed cells. EMBO J. 4:1973-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Everett, R. D. 1986. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J. Gen. Virol. 67:2507-2513. [DOI] [PubMed] [Google Scholar]
- 32.Everett, R. D. 1987. A detailed mutational analysis of Vmw110, a trans-acting transcriptional activator encoded by herpes simplex virus type 1. EMBO J. 6:2069-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Everett, R. D. 1988. Analysis of the functional domains of herpes simplex virus type 1 immediate-early polypeptide Vmw110. J. Mol. Biol. 202:87-96. [DOI] [PubMed] [Google Scholar]
- 34.Everett, R. D. 1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol. 70:1185-1202. [DOI] [PubMed] [Google Scholar]
- 35.Everett, R. D. 1991. Construction and characterization of herpes simplex type 1 viruses without introns in immediate early gene 1. J. Gen. Virol. 72:651-659. [DOI] [PubMed] [Google Scholar]
- 36.Everett, R. D. 2000. ICP0 induces the accumulation of colocalizing conjugated ubiquitin. J. Virol. 74:9994-10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everett, R. D. 2001. DNA viruses and viral proteins that interact with PML nuclear bodies. Oncogene 20:7266-7273. [DOI] [PubMed] [Google Scholar]
- 38.Everett, R. D., A. Cross, and A. Orr. 1993. A truncated form of herpes simplex virus type 1 immediate-early protein Vmw110 is expressed in a cell type dependent manner. Virology 197:751-756. [DOI] [PubMed] [Google Scholar]
- 39.Everett, R. D., and G. G. Maul. 1994. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 13:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everett, R. D., A. Orr, and M. Elliott. 1991. High level expression and purification of herpes simplex virus type 1 immediate early polypeptide Vmw110. Nucleic Acids Res. 19:6155-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Everett, R. D., G. Sourvinos, and A. Orr. 2003. Recruitment of herpes simplex virus type 1 transcriptional regulatory protein ICP4 into foci juxtaposed to ND10 in live, infected cells. J. Virol. 77:3680-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Everett, R. D., M. Meredith, and A. Orr. 1999. The ability of herpes simplex virus type 1 immediate-early protein Vmw110 to bind to a ubiquitin-specific protease contributes to its roles in the activation of gene expression and stimulation of virus replication. J. Virol. 73:417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Everett, R. D., M. Meredith, A. Orr, A. Cross, M. Kathoria, and J. Parkinson. 1997. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 16:1519-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Everett, R. D., P. Barlow, A. Milner, B. Luisi, A. Orr, G. Hope, and D. Lyon. 1993. A novel arrangement of zinc-binding residues and secondary structure in the C3HC4 motif of an alpha herpes virus protein family. J. Mol. Biol. 234:1038-1047. [DOI] [PubMed] [Google Scholar]
- 45.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Everett, R. D., W. C. Earnshaw, J. Findlay, and P. Lomonte. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 18:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Everett, R., P. O'Hare, D. O'Rourke, P. Barlow, and A. Orr. 1995. Point mutations in the herpes simplex virus type 1 Vmw110 RING finger helix affect activation of gene expression, viral growth, and interaction with PML-containing nuclear structures. J. Virol. 69:7339-7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freemont, P. S. 1993. The RING finger. A novel protein sequence related to the zinc finger. Ann. N. Y. Acad. Sci. 684:174-192. [DOI] [PubMed] [Google Scholar]
- 49.Freemont, P. S., I. M. Hanson, and J. Trowsdale. 1991. A novel cysteine-rich sequence motif. Cell 64:483-484. [DOI] [PubMed] [Google Scholar]
- 50.Früh, K., K. Ahn, H. Djaballah, P. Sempé, P. M. van Endert, R. Tampé, P. A. Peterson, and Y. Yang. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375:415-418. [DOI] [PubMed] [Google Scholar]
- 51.Früh, K., E. Bartee, K. Gouveia, and M. Mansouri. 2002. Immune evasion by a novel family of viral PHD/LAP-finger proteins of gamma-2 herpesviruses and poxviruses. Virus Res. 88:55-69. [DOI] [PubMed] [Google Scholar]
- 52.Ganiatsas, S., R. Dow, A. Thompson, B. Schulman, and D. Germain. 2001. A splice variant of Skp2 is retained in the cytoplasm and fails to direct cyclin D1 ubiquitination in the uterine cancer cell line SK-UT. Oncogene 20:3641-3650. [DOI] [PubMed] [Google Scholar]
- 53.Garber, D. A., S. M. Beverly, and D. M. Coen. 1993. Demonstration of circularization of herpes simplex virus DNA following infection using pulsed field gel electrophoresis. Virology 197:459-462. [DOI] [PubMed] [Google Scholar]
- 54.Gelman, I. H., and S. Silverstein. 1985. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc. Natl. Acad. Sci. USA 82:5265-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gelman, I. H., and S. Silverstein. 1986. Co-ordinate regulation of herpes simplex virus gene expression is mediated by the functional interaction of two immediate early gene products. J. Mol. Biol. 191:395-409. [DOI] [PubMed] [Google Scholar]
- 56.Gibson, W., and B. Roizman. 1971. Compartmentalization of spermine and spermidine in the herpes simplex virion. Proc. Natl. Acad. Sci. USA 68:2818-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibson, W., and B. Roizman. 1973. The structural and metabolic involvement of polyamines with herpes simplex virus, p. 121-135. In D. H. Russell (ed.), Polyamines in normal and neoplastic growth. Raven, New York, N.Y.
- 58.Gius, D., and L. A. Laimins. 1989. Activation of human papillomavirus type 18 gene expression by herpes simplex virus type 1 viral transactivators and a phorbol ester. J. Virol. 63:555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goebl, M. G., L. Goetsch, and B. Byers. 1994. The Ubc3 (Cdc34) ubiquitin-conjugating enzyme is ubiquitinated and phosphorylated in vivo. Mol. Cell. Biol. 14:3022-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu, H., and B. Roizman. 2003. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc. Natl. Acad. Sci. USA 100:8963-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu, W., Q. Huang, and G. S. Hayward. 1995. Multiple tandemly repeated binding sites for the YY1 repressor and transcription factors AP-1 and SP-1 are clustered within intron-1 of the gene encoding the IE110 transactivator of herpes simplex virus type 1. J. Biomed. Sci. 2:203-226. [DOI] [PubMed] [Google Scholar]
- 62.Hagglund, R., and B. Roizman. 2002. Characterization of the novel E3 ubiquitin ligase encoded in exon 3 of herpes simplex virus-1-infected cell protein 0. Proc. Natl. Acad. Sci. USA 99:7889-7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagglund, R., and B. Roizman. 2003. Herpes simplex virus 1 mutant in which ICP0 HUL-1 E3 ubiquitin ligase site is disrupted stabilizes cdc34 but degrades D-type cyclins and exhibits diminished neurotoxicity. J. Virol. 77:13194-13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hagglund, R., C. Van Sant, P. Lopez, and B. Roizman. 2002. Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 99:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halford, W. P., C. D. Kemp, J. A. Isler, D. J. Davido, and P. A. Schaffer. 2001. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J. Virol. 75:6143-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Halford, W. P., and P. A. Schaffer. 2000. Optimized viral dose and transient immunosuppression enable herpes simplex virus ICP0-null mutants to establish wild-type levels of latency in vivo. J. Virol. 74:5957-5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halford, W. P., and P. A. Schaffer. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J. Virol. 75:3240-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harada, J. N., A. Shevchenko, A. Shevchenko, D. C. Pallas, and A. J. Berk. 2002. Analysis of the adenovirus E1B-55K-anchored proteome reveals its link to ubiquitination machinery. J. Virol. 76:9194-9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Härle, P., B. Sainz, Jr., D. J. J. Carr, and W. P. Halford. 2002. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-α/β. Virology 293:295-304. [DOI] [PubMed] [Google Scholar]
- 70.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 71.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 72.Hobbs, W. E., II, and N. A. DeLuca. 1999. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J. Virol. 73:8245-8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Honess, R. W., and B. Roizman. 1973. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J. Virol. 12:1347-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Honess, R. W., and B. Roizman. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc. Natl. Acad. Sci. USA 72:1276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imai, N., N. Matsuda, K. Tanaka, A., Nakano, S. Matsumoto, and W. Kang. 2003. Ubiquitin ligase activities of Bombyx mori nucleopolyhedrovirus RING finger proteins. J. Virol. 77:923-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishov, A. M., A. G. Sotnikov, D. Negorev, O. V. Vladimirova, N. Neff, T. Kamitani, E. T. H. Yeh, J. F. Strauss III, and G. G. Maul. 1999. PML is critical for ND10 formation and recruits the PML-interacting protein daxx to this nuclear structure when modified by SUMO-1. J. Cell Biol. 147:221-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jackson, P. K., A. G. Eldridge, E. Freed, L. Furstenthal, J. Y. Hsu, B. K. Kaiser, and J. D. R. Reimann. 2000. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 10:429-439. [DOI] [PubMed] [Google Scholar]
- 79.Jackson, S. A., and N. A. DeLuca. 2003. Relationship of herpes simplex virus genome configuration to productive and persistent infections. Proc. Natl. Acad. Sci. USA 100:7871-7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jensen, K., C. Shiels, and P. S. Freemont. 2001. PML protein isoforms and the RBCC/TRIM motif. Oncogene 20:7223-7233. [DOI] [PubMed] [Google Scholar]
- 81.Joazeiro, C. A. P., S. S. Wing, H. Huang, J. D. Leverson, T. Hunter, and Y.-C. Liu. 1999. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286:309-312. [DOI] [PubMed] [Google Scholar]
- 82.Kato, K., Y. Kawaguchi, M. Tanaka, M. Igarashi, A. Yokoyama, G. Matsuda, M. Kanamori, K. Nakajima, Y. Nishimura, M. Shimojima, H. T. Phung, E. Takahashi, and K. Hirai. 2001. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1δ (EF-1δ): EF-1δ is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 82:1457-1463. [DOI] [PubMed] [Google Scholar]
- 83.Katz, J. P., E. T. Bodin, and D. M. Coen. 1990. Quantitative polymerase chain reaction analysis of herpes simplex virus DNA in ganglia of mice infected with replication-incompetent mutants. J. Virol. 64:4288-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1998. Eukaryotic elongation factor 1δ is hyperphosphorylated by the protein kinase encoded by the UL13 gene of herpes simplex virus 1. J. Virol. 72:1731-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kawaguchi, Y., K. Kato, M. Tanaka, M. Kanamori, Y. Nishiyama, and Y. Yamanashi. 2003. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1δ. J. Virol. 77:2359-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawaguchi, Y., M. Tanaka, A. Yokoymama, G. Matsuda, K. Kato, H. Kagawa, K. Hirai, and B. Roizman. 2001. Herpes simplex virus 1 α regulatory protein ICP0 functionally interacts with cellular transcription factor BMAL1. Proc. Natl. Acad. Sci. USA 98:1877-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawaguchi, Y., R. Bruni, and B. Roizman. 1997. Interaction of herpes simplex virus 1 α regulatory protein ICP0 with elongation factor 1δ: ICP0 affects translational machinery. J. Virol. 71:1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawaguchi, Y., T. Matsumura, B. Roizman, and K. Hirai. 1999. Cellular elongation factor 1δ is modified in cells infected with representative alpha-, beta-, or gammaherpesviruses. J. Virol. 73:4456-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lees-Miller, S. P., M. C. Long, M. A. Kilvert, V. Lam, S. A. Rice, and C. A. Spencer. 1996. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator ICP0. J. Virol. 70:7471-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leib, D. A., D. M. Coen, C. L. Bogard, K. A. Hicks, D. R. Yager, D. M. Knipe, K. L. Tyler, and P. A. Schaffer. 1989. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J. Virol. 63:759-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levine, A. J. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323-331. [DOI] [PubMed] [Google Scholar]
- 94.Lium, E. K., and S. Silverstein. 1997. Mutational analysis of the herpes simplex virus type 1 ICP0 C3HC4 zinc ring finger reveals a requirement for ICP0 in the expression of the essential α27 gene. J. Virol. 71:8602-8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lomonte, P., K. F. Sullivan, and R. D. Everett. 2001. Degradation of nucleosome-associated centromeric histone H3-like protein CENP-A induced by herpes simplex virus type 1 protein ICP0. J. Biol. Chem. 276:5829-5835. [DOI] [PubMed] [Google Scholar]
- 96.Lopez, P., C. Van Sant, and B. Roizman. 2001. Requirements for the nuclear-cytoplasmic translocation of infected-cell protein 0 of herpes simplex virus 1. J. Virol. 75:3832-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lopez, P., R. J. Jacob, and B. Roizman. 2002. Overexpression of promyelocytic leukemia protein precludes the dispersal of ND10 structures and has no effect on accumulation of infectious herpes simplex virus 1 or its proteins. J. Virol. 76:9355-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lorick, K. L., J. P. Jensen, S. Fang, A. M. Ong, S. Hatakeyama, and A. M. Weissman. 1999. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc. Natl. Acad. Sci. USA 96:11363-11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mansouri, M., E. Bartee, K. Gouveia, B. T. H. Nerenberg, J. Barrett, L. Thomas, G. Thomas, G. McFadden, and K. Früh. 2003. The PHD/LAP-domain protein M153R of myxomavirus is a ubiquitin ligase that induces the rapid internalization and lysosomal destruction of CD4. J. Virol. 77:1427-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marsden, H. S., N. D. Stow, V. G. Preston, M. C. Timbury, and N. M. Wilkie. 1978. Physical mapping of herpes simplex virus-induced polypeptides. J. Virol. 28:624-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maul, G. G., A. M. Ishov, and R. D. Everett. 1996. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology 217:67-75. [DOI] [PubMed] [Google Scholar]
- 102.Maul, G. G., and R. D. Everett. 1994. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 75:1223-1233. [DOI] [PubMed] [Google Scholar]
- 103.Maul, G. G., H. H. Guldner, and J. G. Spivak. 1993. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0). J. Gen. Virol. 74:2679-2690. [DOI] [PubMed] [Google Scholar]
- 104.Mavromara-Nazos, P., S. Silver, J. Hubenthal-Voss, J. L. C. McKnight, and B. Roizman. 1986. Regulation of herpes simplex virus 1 genes: α gene sequence requirements for transient induction of indicator genes regulated by β or late (γ2) promoters. Virology 149:152-164. [DOI] [PubMed] [Google Scholar]
- 105.McGeoch, D. J., A. Dolan, S. Donald, and F. J. Rixon. 1985. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J. Mol. Biol. 181:1-13. [DOI] [PubMed] [Google Scholar]
- 106.McGeoch, D. J., C. Cunningham, G. McIntyre, and A. Dolan. 1991. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J. Gen. Virol. 72:3057-3075. [DOI] [PubMed] [Google Scholar]
- 107.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 108.Meredith, M., A. Orr, and R. Everett. 1994. Herpes simplex virus type 1 immediate-early protein Vmw110 binds strongly and specifically to a 135-kDa cellular protein. Virology 200:457-469. [DOI] [PubMed] [Google Scholar]
- 109.Meredith, M., A. Orr, M. Elliott, and R. Everett. 1995. Separation of sequence requirements for HSV-1 Vmw110 multimerisation and interaction with a 135-kDa cellular protein. Virology 209:174-187. [DOI] [PubMed] [Google Scholar]
- 110.Mitchell, C., J. A. Blaho, A. L. McCormick, and B. Roizman. 1997. The nucleotidylylation of herpes simplex virus 1 regulatory protein α22 by human casein kinase II. J. Biol. Chem. 272:25394-25400. [DOI] [PubMed] [Google Scholar]
- 111.Mitchell, C., J. A. Blaho, and B. Roizman. 1994. Casein kinase II specifically nucleotidylylates in vitro the amino acid sequence of the protein encoded by the α22 gene of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA 91:11864-11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morse, L. S., L. Pereira, B. Roizman, and P. A. Schaffer. 1978. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 × HSV-2 recombinants. J. Virol. 26:389-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nabel, G. J., S. A. Rice, D. M. Knipe, and D. Baltimore. 1988. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science 239:1299-1302. [DOI] [PubMed] [Google Scholar]
- 115.Natarajan, R., S. Deshmane, T. Valyi-Nagy, R. Everett, and N. W. Fraser. 1991. A herpes simplex virus type 1 mutant lacking the ICP0 introns reactivates with normal efficiency. J. Virol. 65:5569-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Negorev, D., and G. G. Maul. 2001. Cellular proteins localized at and interacting within ND10/PML nuclear bodies/PODs suggest functions of a nuclear depot. Oncogene 20:7234-7242. [DOI] [PubMed] [Google Scholar]
- 117.Ogle, W. O., T. I. Ng, K. L. Carter, and B. Roizman. 1997. The UL13 protein kinase and the infected cell type are determinants of posttranslational modification of ICP0. Virology 235:406-413. [DOI] [PubMed] [Google Scholar]
- 118.O'Hare, P., and G. S. Hayward. 1985. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early gene proteins of herpes simplex virus in the transactivation of delayed-early promoters. J. Virol. 53:751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Panagiotidis, C. A., E. K. Lium, and S. J. Silverstein. 1997. Physical and functional interactions between herpes simplex virus immediate-early proteins ICP4 and ICP27. J. Virol. 71:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parkinson, J., and R. D. Everett. 2000. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 affect cellular structures and proteins. J. Virol. 74:10006-10017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parkinson, J., and R. D. Everett. 2001. Alphaherpesvirus proteins related to herpes simplex virus type 1 ICP0 induce the formation of colocalizing, conjugated ubiquitin. J. Virol. 75:5357-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parkinson, J., S. P. Lees-Miller, and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J. Virol. 73:650-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perry, L. J., F. J. Rixon, R. D. Everett, M. C. Frame, and D. J. McGeoch. 1986. Characterization of the IE110 gene of herpes simplex virus type 1. J. Gen. Virol. 67:2365-2380. [DOI] [PubMed] [Google Scholar]
- 124.Poffenberger, K. L., and B. Roizman. 1985. A noninverting genome of a viable herpes simplex virus 1: presence of head-to-tail linkages in packaged genomes and requirements for circularization after infection. J. Virol. 53:587-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Poon, A. P. W., S. J. Silverstein, and B. Roizman. 2002. An early regulatory function required in a cell type-dependent manner is expressed by the genomic but not the cDNA copy of the herpes simplex virus 1 gene encoding infected cell protein 0. J. Virol. 76:9744-9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Poon, A. P. W., Y. Liang, and B. Roizman. Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J. Virol. 77:12671-12678. [DOI] [PMC free article] [PubMed]
- 127.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA 90:6701-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Querido, E., P. Blanchette, Q. Yan, T. Kamura, M. Morrison, D. Boivin, W. G. Kaelin, R. C. Conaway, J. W. Conaway, and P. E. Branton. 2001. Degradation of p53 by adenovirus E4orf6 and E1B55K proteins occurs via a novel mechanism involving a cullin-containing complex. Genes Dev. 23:3104-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Quinlan, M. P., and D. M. Knipe. 1985. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol. Cell. Biol. 5:957-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Regad, T., and M. K. Chelbi-Alix. 2001. Role and fate of PML nuclear bodies in response to interferon and viral infections. Oncogene 20:7274-7286. [DOI] [PubMed] [Google Scholar]
- 131.Roizman, B., and A. E. Sears. 1987. An inquiry into the mechanisms of herpes simplex virus latency. Annu. Rev. Microbiol. 41:543-571. [DOI] [PubMed] [Google Scholar]
- 132.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams, & Wilkins, Philadelphia, Pa.
- 133.Russell, A., J. Hendley, and D. Germain. 1999. Inhibitory effect of p21 in MCF-7 cells is overcome by its coordinated stabilization with D-type cyclins. Oncogene 18:6454-6459. [DOI] [PubMed] [Google Scholar]
- 134.Russell, A., M. A. Thompson, J. Hendley, L. Trute, J. Armes, and D. Germain. 1999. Cyclin D1 and D3 associate with the SCF complex and are coordinately elevated in breast cancer. Oncogene 18:1983-1991. [DOI] [PubMed] [Google Scholar]
- 135.Russell, J., N. D. Stow, E. C. Stow, and C. M. Preston. 1987. Herpes simplex virus genes involved in latency in vitro. J. Gen. Virol. 68:3009-3018. [DOI] [PubMed] [Google Scholar]
- 136.Sacks, W. R., and P. A. Schaffer. 1987. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J. Virol. 61:829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sandri-Goldin, R. M. 2001. Nuclear export of herpes virus RNA. Curr. Top. Microbiol. Immunol. 259:2-23. [PubMed] [Google Scholar]
- 138.Sawtell, N. M. 1997. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J. Virol. 71:5423-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sawtell, N. M. 1998. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J. Virol. 72:6888-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 141.Sears, A. E., I. W. Haliburton, B. Meignier, S. Silver, and B. Roizman. 1985. Herpes simplex virus 1 mutant deleted in the α22 gene: growth and gene expression in permissive and restrictive cells and establishment of latency in mice. J. Virol. 55:338-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Seeler, J.-S., and A. Dejean. 2001. SUMO: of branched proteins and nuclear bodies. Oncogene 20:7243-7249. [DOI] [PubMed] [Google Scholar]
- 143.Sekulovich, R. E., K. Leary, and R. M. Sandri-Goldin. 1988. The herpes simplex virus type 1 α protein ICP27 can act as a trans-repressor or a trans-activator in combination with ICP4 and ICP0. J. Virol. 62:4510-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seol, J. H., R. M. R. Feldman, W. Zachariae, A. Shevchenko, C. C. Correll, S. Lyapina, Y. Chi, M. Galova, J. Claypool, S. Sandmeyer, K. Nasmyth, A. Shevchenko, and R. J. Deshaies. 1999. Cdc53/cullin and the essential Hrt1 RING-H2 subunit of SCF define a ubiquitin ligase module that activates the E2 enzyme Cdc34. Genes Dev. 13:1614-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sourvinos, G., and R. D. Everett. 2002. Visualization of parental HSV-1 genomes and replication compartments in association with ND10 in live infected cells. EMBO J. 21:4989-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Spatz, S. J., E. C. Nordby, and P. C. Weber. 1996. Mutational analysis of ICP0R, a transrepressor protein created by alternative splicing of the ICP0 gene of herpes simplex virus type 1. J. Virol. 70:7360-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Spear, P. G., and B. Roizman. 1972. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J. Virol. 9:143-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 149.Tang, Q., L. Li, A. M. Ishov, V. Revol, A. L. Epstein, and G. G. Maul. 2003. Determination of minimum herpes simplex virus type 1 components necessary to localize transcriptionally active DNA to ND10. J. Virol. 77:5821-5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Telford, E. A. R., M. S. Watson, K. McBride, and A. J. Davision. 1992. The DNA sequence of equine herpesvirus-1. Virology 189:304-316. [DOI] [PubMed] [Google Scholar]
- 151.Van Sant, C., P. Lopez, S. J. Advani, and B. Roizman. 2001. Role of cyclin D3 in the biology of herpes simplex virus 1 ICP0. J. Virol. 75:1888-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Van Sant, C., R. Hagglund, P. Lopez, and B. Roizman. 2001. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E3 ubiquitin-conjugating enzyme, and possesses in vitro E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. USA 98:8815-8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Van Sant, C., Y. Kawaguchi, and B. Roizman. 1999. A single amino acid substitution in the cyclin D binding domain of the infected cell protein no. 0 abrogates the neuroinvasiveness of herpes simplex virus without affecting its ability to replicate. Proc. Natl. Acad. Sci. USA 96:8184-8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weber, P. C., and B. Wigdahl. 1992. Identification of dominant-negative mutants of the herpes simplex virus type 1 immediate-early protein ICP0. J. Virol. 66:2261-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weber, P. C., J. J. Kenny, and B. Wigdahl. 1992. Antiviral properties of a dominant negative mutant of the herpes simplex virus type 1 regulatory protein ICP0. J. Gen. Virol. 73:2955-2961. [DOI] [PubMed] [Google Scholar]
- 156.Weber, P. C., S. J. Spatz, and E. C. Nordby. 1999. Stable ubiquitination of the ICP0R protein of herpes simplex virus type 1 during productive infection. Virology 253:288-298. [DOI] [PubMed] [Google Scholar]
- 157.Wilcox, C. L., R. L. Smith, R. D. Everett, and D. Mysofski. 1997. The herpes simplex virus type 1 immediate-early protein ICP0 is necessary for the efficient establishment of latent infection. J. Virol. 71:6777-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wirth, U. V., C. Fraefel, B. Vogt, C. Vlcek, V. Paces, and M. Schwyzer. 1992. Immediate-early RNA 2.9 and early RNA 2.6 of bovine herpesvirus 1 are 3′ coterminal and encode a putative zinc finger transactivator protein. J. Virol. 66:2763-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yao, F., and P. A. Schaffer. 1994. Physical interaction between the herpes simplex virus type 1 immediate-early regulatory proteins ICP0 and ICP4. J. Virol. 68:8158-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yao, F., and P. A. Schaffer. 1995. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J. Virol. 69:6249-6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]
- 162.Yu, Z.-K., J. L. M. Gervais, and H. Zhang. 1998. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc. Natl. Acad. Sci. USA 95:11324-11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhong, S., S. Müller, S. Ronchetti, P. S. Freemont, A. Dejean, and P. P. Pandolfi. 2000. Role of SUMO-1-modified PML in nuclear body formation. Blood 95:2748-2752. [PubMed] [Google Scholar]