Abstract
Behavioral evidence suggests that memory for context (i.e., source memory) is more vulnerable to age-related decline than item memory. It is not clear, however, whether this pattern reflects a specific age-related deficit in context memory or a more general effect of task difficulty. In the present study, we used event-related fMRI with healthy younger and older adults to dissociate the effects of age, task (item vs. source memory), and task difficulty (one vs. two study presentations) on patterns of BOLD signal changes during memory retrieval. Behavioral performance was similar in both age groups, but was sensitive to task and difficulty (item > source; easy > difficult). Data-driven multivariate analyses revealed age differences consistent with age-related over-recruitment of frontoparietal regions during difficult task conditions, and age-related functional reorganization in bilateral frontal and right-lateralized posterior regions that were sensitive to difficulty in younger adults, but to task (i.e., context demand) in older adults. These findings support the hypothesis of a specific context memory deficit in older adults.
Keywords: Aging, context memory, cognitive control, fMRI, diffusion modeling
1. Introduction
Recognizing a person to whom you were recently introduced (item recognition) is generally easier than remembering who introduced you to that person (source memory). Both tasks involve episodic long-term memory, but the second requires memory for the contextual details of the central event, and is typically associated with worse performance. Normal aging takes a disproportionate toll on context-dependent tasks (e.g., Spaniol et al., 2006). Similar observations were first made nearly 30 years ago (e.g., Burke & Light, 1981) and have since been explained in terms of either general deficits, such as age-related declines in self-initiated processing (e.g., Craik, 1986), frontal lobe functioning (e.g., Glisky et al., 2001), working memory (e.g., Park & Payer, 2006), perceptual-motor speed (e.g., Siedlecki et al., 2005), and dopaminergic neuromodulation (Li & Sikström, 2002), or in terms of specific deficits, such as age-related declines in recollection (e.g., Jacoby, 1999), memory binding (e.g., Chalfonte & Johnson, 1996), and associative encoding (e.g., Naveh-Benjamin, 2000). In the current study we used functional magnetic resonance imaging (fMRI) to test the hypothesis that aging is associated with specific losses in context processing that can be distinguished from general decrements in response to task difficulty.
Neuroimaging studies have recently started to shed light on the neural underpinnings of age-related changes in context memory, at both encoding and retrieval stages. A recent study (Dennis et al., 2008) examined brain activity linked to successful encoding of item-context associations (face-scene pairings) using event-related functional magnetic resonance imaging (fMRI). Successful encoding was measured by the ability to later recognize correct face-scene pairings, and brain activity during encoding of remembered stimuli was compared to that for forgotten stimuli. Compared with younger adults, older adults showed reduced recruitment of hippocampal and bilateral dorsolateral prefrontal cortex (PFC) regions during encoding of remembered pairs. This age-related deficit was unique to associative encoding, and was not seen in contrasts capturing successful encoding of item information (faces or scenes). Furthermore, connectivity analyses showed an age-related increase in frontal-hippocampal coupling, along with an age-related decrease in hippocampal connectivity with posterior (e.g., visual processing) regions. The authors interpreted these findings as evidence for an age-related shift toward reliance on frontally mediated control processes during encoding of item-context associations, possibly to compensate for decline in visual processing regions (see also Daselaar et al., 2006; Davis et al., 2008). Age-related reduction in activation of visual regions during successful encoding of visuospatial source information was also reported by Kukolja and colleagues (Kukolja et al., 2009).
Neuroimaging studies of age differences in context memory at the retrieval end have yielded mixed findings. In a working-memory version of a source monitoring task, Mitchell and colleagues (Mitchell et al., 2006) observed increased left dorsolateral PFC activation during source memory (format decisions: picture vs. word) compared with item recognition in younger adults, but not in older adults. The authors attributed this finding to an age-related deficit in the monitoring of specific source information during retrieval. Morcom and colleagues (Morcom et al., 2007), on the other hand, reported age-related activation increases in bilateral anterior PFC and parietal regions during correct conceptual source memory decisions (remembering which of two encoding tasks had been performed for a test item). Morcom and colleagues proposed an interpretation of these findings whereby aging is associated with a loss in the efficiency with which brain regions support cognitive performance. In contrast, a third study (Duverne et al., 2008) reported that correct spatial source memory decisions were accompanied by activation in a similar network of regions in younger and older adults when the groups were matched on overall performance, with little evidence for substantial cortical under- or over-recruitment in the older group. Consistent with this finding, Kukolja and colleagues (2009) reported largely similar activation patterns in younger and older adults during successful retrieval of spatial source information. One exception was left anterior hippocampus, where activation was associated with correct spatial source retrieval in younger adults, but with incorrect spatial source retrieval in older adults.
Finally, a more recent study (Rajah et al., 2010) examined spatial and temporal context retrieval for faces in younger and older adults. Contrasted with item recognition, both types of context retrieval were associated with deactivation in medial anterior PFC and with activation in right dorsolateral PFC in younger adults, but not in older adults. Rajah and colleagues suggested that the first finding may reflect failure on the part of older adults to silence task-irrelevant ruminations, whereas the second may reflect failure to engage in retrieval monitoring that contributes to successful task performance.
Source memory is thought to depend more strongly on recollection than familiarity (e.g., Yonelinas, 2002, but see Mitchell & Johnson, 2009, for a discussion of differences between source-monitoring and dual-process theories). Source memory tasks are sometimes referred to as objective recollection tasks because successful performance probes memory for experimenter-specified (“objective”) contextual details such as the spatial, temporal, or conceptual properties of an event. Two neuroimaging studies have compared younger and older adults in a so-called subjective recollection paradigm, the remember-know procedure (Tulving, 1985). Using this approach in the context of a word recognition task, Daselaar and colleagues (2006) observed reduced recollection-related hippocampal activity in older adults, as well as increased familiarity-related activity in rhinal cortex. Of note, behavioral recollection estimates were lower in older than younger adults in this study, although the two age groups were matched on overall old-new recognition performance. Duarte and colleagues (2008) directly compared objective and subjective recollection in younger and older adults, using a picture recognition task that incorporated spatial and temporal source-memory components as well as remember-know ratings. High-functioning older adults, who matched the younger adults on item recognition and subjective recollection, demonstrated impaired performance on objective recollection as well as reduced recollection-related activity in dorsolateral PFC. Low-functioning older adults, whose performance on all behavioral memory indices was impaired compared to that of younger adults, showed reduced subjective recollection effects in posterior brain regions, in addition to reduced prefrontal activations.
In summary, some neuroimaging studies have shown age-related decrease in the engagement of the hippocampus and of posterior brain regions that support visuospatial processing, during both encoding and retrieval of source information. Additionally, several studies have demonstrated age-related change in anterior, ventrolateral, and dorsolateral PFC activation during retrieval of source information. However, the direction of this change (i.e., age-related increase vs. decrease) has been inconsistent across different studies.
One issue that complicates the interpretation of the existing neuroimaging studies of context memory in younger and older adults, and which may account for some of the inconsistency in the literature, is that of group differences in task difficulty. When difficulty is not controlled, group differences in brain activity cannot be unequivocally attributed to impaired context memory per se, but may simply reflect differences in effort and executive control processes. In several of the above-reviewed studies, younger and older adults’ performance was matched by adjusting task demands (Daselaar et al., 2006; Dennis et al., 2008; Duverne et al., 2008; Morcom et al., 2007). The disadvantages of this approach are that the age groups are not receiving equal experimental treatment, and performance matching typically focuses on one performance index (e.g., accuracy) while ignoring another (e.g., response time). Other strategies involved the selection of performance-matched younger and older samples (e.g., Daselaar et al., 2006), and the division of older adult participants into high and low performers (e.g., Duarte et al., 2008). The former technique is potentially problematic due to selection bias, whereas the latter artificially reduces the variability within the older adult group via dichotomization.
The goal of the current study was to overcome these limitations by manipulating context demands and (context-independent) task difficulty factorially, in the same participants. Conceptual source memory and item recognition served as high and low context demand conditions, respectively. The conceptual source task involved deciding whether a test item had been studied in the context of an animacy judgment task or in the context of a pleasantness judgment task (for neuroimaging studies of source memory using similar tasks, see Dobbins et al., 2002; Dobbins & Wagner, 2005; Morcom et al., 2007). One versus two study presentations served as high and low difficulty conditions, respectively.
We sought to test the following hypotheses. With respect to context demand (source memory vs. item recognition), we expected to replicate patterns reported in previous studies of objective recollection (for a recent meta-analysis, see Spaniol et al., 2009), including greater activity for source memory than item recognition in left dorsolateral, ventrolateral, and anterior PFC. These regions are believed to support cognitive control processes involved in the selection, maintenance, and organization of specific contextual details during retrieval (for reviews, see Badre & Wagner, 2007, and Simons & Spiers, 2003). We also expected greater activity for source memory than item recognition in superior parietal cortex, thought to facilitate top-down attentional processes involved in context retrieval (e.g., Cabeza et al., 2008). Although some studies have reported medial-temporal activations for source retrieval, these activations are more reliably observed during subjective recollection, as well as during successful encoding of source information (see Spaniol et al., 2009). We therefore did not strongly expect to find activation in the hippocampus or neighboring regions as a function of context demand.
With respect to task difficulty (one vs. two study presentations; henceforth referred to as “hard” vs. “easy”), we expected to see modulation of activity in prefrontal and parietal cognitive-control regions which typically come online when decisions (mnemonic or otherwise) have to be made on the basis of weak or ambiguous information (e.g., Dobbins & Han, 2006).
The critical question motivating our study was how age would affect the whole-brain activations associated with context demand and difficulty. If aging selectively impairs context memory, this would lead to the prediction of dissociable age differences in the neural patterns responsive to context demand (source vs. item memory) and retrieval difficulty (easy vs. hard). If, on the other hand, the age-related memory deficit is general rather than specific to context memory, one would predict a common pattern of age differences in the responses to context demand and difficulty.
2. Methods
2.1 Participants
Participants in the study were 16 right-handed younger adults (8 females) and 15 right-handed older adults (see Table 1). All participants were recruited from the research volunteer pool of the Rotman Research Institute and received monetary compensation. All participants had normal or corrected-to-normal vision and hearing. They were screened using a detailed health questionnaire to exclude health problems and/or medications that might affect cognitive function and brain activity, including strokes and cardiovascular disease. The structural MRIs also were inspected to rule out severe white matter changes or other abnormalities. The younger adults had significantly lower vocabulary scores than did the older adults, t(29) = 3.79, p < .001. There was no age difference in mean scores on a test of mental status (MMSE; Folstein et al., 1975). All participants gave informed consent for their participation, following the guidelines of the Research Ethics Board at Baycrest and the University of Toronto.
Table 1.
Participant Information
| Younger | Older | |
|---|---|---|
| N | 16 | 15 |
| Age (yrs) | 23.6 (2.7) | 65.3 (3.8) |
| Education (yrs) | 17.0 (2.1) | 17.7 (3.1) |
| Vocabulary | 17.9 (3.1) | 22.5 (3.6)* |
| MMSE | 29.3 (0.9) | 29.2 (0.9) |
Standard deviations are shown in parentheses.
indicates a significant age difference at p < .05.
2.2 Stimuli and apparatus
The stimuli consisted of 384 English nouns, ranging from 4 to 8 letters in length (M = 6) and from 9 to 431 in Ku era and Francis (1967) word frequency (Md = 27). Half of the words denoted living objects (e.g., “rabbit”), the other half denoted nonliving objects (e.g., “mirror”). For counterbalancing purposes, the words were divided into 12 sets of 32 words. Each set contained half living, half non-living words, and the lists were equated for mean word length, word frequency, and thematic variability.
The experimental tasks were created with Presentation software (Neurobehavioral Systems, Albany, CA). Stimulus presentation was controlled by a 2.8 GHz processor, Pentium(R) 4 laptop computer with a 15-in., flat-panel LCD. All stimuli and instructions appeared centrally in white 24-pt Arial font against a black background.
2.3 Procedure
Practice session
One week before the fMRI session, participants completed paper-pencil measures and practiced the memory tasks. The practice stimuli were not used in the actual experiment.
Study phase
The study phase took place in a soundproof room adjacent to the scanner suite. Nine of the 12 word sets were presented in continuous sequence, with participants unaware of the list boundaries. Three sets provided words that would serve as target stimuli during the item memory tests. Six sets provided words that would serve as test stimuli during the source memory tests. The assignment of specific word sets to item-memory lists and source-memory lists was counterbalanced across participants, as was the list presentation order. Following presentation of the nine lists, half of the words from each list (henceforth referred to as “easy words”) were presented a second time, in newly randomized order. Half of the “easy words” were living, half were non-living. Likewise, half of the “hard words” were living, half were non-living. In summary, participants studied 288 unique words. “Hard words” were presented once, whereas “easy words” were presented twice, yielding a total of 412 study trials.
Two encoding tasks were used: animacy and pleasantness judgments. Prior to each word presentation, a task cue was shown. The cue remained onscreen during the word presentation. To minimize task-switching demands, the task cue changed predictably for every second trial. For easy words, the second presentation involved the same cue as the first presentation, and participants were instructed to give a fresh answer to the question rather than trying to recall their first answer.
Test phase
The test phase took place during functional scanning, starting approximately 30 min after the end of the study phase. It included three item memory runs and three source memory runs. The presentation order of the runs was counterbalanced across participants. Each run started with a 28-s instruction screen that announced the task: ‘Old/New Judgments’ (item memory) or ‘Task Judgments’ (source memory). After a 12-s fixation screen, the test trials were presented. Each test trial comprised a 3-s presentation of the test word and the two response options, followed by a 1-s fixation screen. Thirty-two blank trials were pseudo-randomly intermixed with the test trials to jitter the event onsets. During blank trials, the central fixation mark remained on the screen for the entire duration of the trial. The trial sequence followed a pseudo-random order. The assignment of responses to left and right buttons, within both item-memory and source-memory runs, was counterbalanced across participants.
In each item-memory run, words from one of the three lists which had not been presented during the study phase served as distractor words. 32 studied (target) words were intermixed with 32 distractor words, and participants responded ‘old’ or ‘new’ to each word. Half of the target words were easy, half were difficult.
During source-memory runs, 64 studied words were presented, and participants were asked to make a source judgment for each word. The sources corresponded to the two study tasks used during the study phase. Each combination of source (animacy vs. pleasantness) and difficulty (hard vs. easy) occurred equally often in each run.
Visual stimuli were projected onto a mirror placed above the participant’s head inside the scanner bore. Participants who normally wore glasses received MR compatible goggles fitted with prescription lenses. If necessary, mirror and goggles were adjusted until the participant was able to read a series of test screens without strain. Responses were collected with the Rowland USB Response Box (RURB).
2.4 Image acquisition
Scanning was conducted on at 3T GE scanner with a standard head coil. For each participant, we collected a sagittal localizer, T1-weighted anatomical volumetric images (124 slices, 1.4-mm thick, FOV = 22 cm), and T2*-weighted functional images. During functional imaging, we acquired twenty-six 5-mm-thick contiguous axial slices using a T2*-weighted pulse sequence with spiral in–out readout (TR = 2000 msec, TE = 30 msec, FOV = 20 cm, 64 X 64 matrix, 70° flip angle).
The first 14 image volumes of each run were discarded. Correction for head motion, slice timing, and physiological motion were performed using 3dregistration, 3dTshift, and 3dretroicor functions provided in the Analysis of Functional Neuroimages software (AFNI; Cox, 1996). Residual head motion and scanner artifacts were detected and removed using Multivariate Exploratory Linear Optimized Decomposition (MELODIC, http://www.fmrib.ox.ac.uk/analysis/research/melodic/), an implementation for the estimation of a Probabilistic Independent Component Analysis model (Beckmann & Smith, 2004). The final pre-processing steps were performed with Statistical Parametric Mapping (SPM99; www.fil.ion.ucl.ac.uk/spm). Images were normalized to MNI space using a linear transformation with sinc interpolation, and smoothed with an 8-mm Gaussian filter. The resulting voxel size after processing was 4 × 4 × 4 mm.
2.5 Analysis of functional neuroimaging data
For statistical analysis we used a multivariate approach, Spatiotemporal Partial Least Squares, or PLS (McIntosh, 1999; McIntosh et al., 1996, 2004), in order to identify whole brain patterns of activity. PLS operates on the covariance between brain voxels and the experimental design to identify a new set of variables (so-called latent variables or LVs) that optimally relate the two sets of measurements. PLS can be used to assess a priori contrasts or to assess data-driven effects, in which case the algorithm extracts LVs in order of the amount of covariance explained between conditions and brain activity (with the LV accounting for the most covariance extracted first). Each LV resulting from either kind of analysis contains a spatial activity pattern depicting the brain regions that show the strongest relation to (i.e., are covariant with) the task contrast identified by the LV.
We carried out a series of analyses to examine the effects of task, difficulty and group. The first two analyses used pre-specified contrasts to assess the main effects of task (item vs. source memory) and difficulty (easy vs hard), and any age differences in these effects (quantitative differences). A third analysis (without a priori contrasts) was carried out on all conditions in both groups to assess the patterns of differences across groups inherent in the data (potentially qualitative differences). For the analyses reported here we averaged across all trials with correct responses in each of the item and source memory conditions. Trials consisting of distractor items in the item memory condition, as well as those associated with incorrect responses, were excluded from the analyses. For the source memory condition, we collapsed across the two sources (animacy and pleasantness). All participants had at least 25 trials included for each combination of task and difficulty, with the exception of three older adults, who had between 15 and 20 trials included for the hard item condition.
The analysis included 8 post-stimulus time points, or lags, for each event (i.e., 16 sec), and activity at each time point was normalized to activity in the first lag of the trial. In event-related PLS, there is no baseline condition per se; rather, because data from all time points in each event are normalized to first time point in the event, the changes in signal represent either increases or decreases of activity relative to the beginning of each trial. PLS as applied to event-related data results in a set of brain regions that are reliably related to the task contrasts for each TR on each LV, thus providing temporal as well as spatial information (McIntosh et al., 2004). Each brain voxel has a weight, known as a salience, which is proportional to the covariance of activity with the task contrast at each time point on each LV. Multiplying the BOLD signal value in each brain voxel for each subject by the salience for that voxel, and summing across all voxels, gives a “brain score” for each subject on a given LV. To characterize brain activity across the conditions, we plotted the mean brain score at each TR for each condition (referred to here as the temporal brain scores, which are analogous to a hemodynamic response function for a given region).
The significance for each LV as a whole was determined by using a permutation test (McIntosh et al., 1996). As 500 permutations were used, the smallest p value obtainable for each LV was p < 0.002. In addition to the permutation test, a second and independent step was used to determine the reliability of the saliences for the brain voxels characterizing each pattern identified by the LVs. To do this, all saliences for each TR were submitted to a bootstrap estimation of the standard errors (Efron & Tibshirani, 1986). Reliability for each voxel was determined from the ratio of its salience value to the standard error for that voxel, and clusters of at least 10 contiguous voxels with a bootstrap ratio > 3.0 were identified. A ratio of 3.0 approximates p < 0.005 (Sampson et al., 1989). The local maximum for each cluster was defined as the voxel with a bootstrap ratio higher than any other voxel in a 2-cm cube centered on that voxel. Cluster maxima are reported for the time points where the hemodynamic response was at a peak (i.e., at TR2 or TR3, 4–8 sec post-stimulus) and locations of these maxima are reported in terms of coordinates in MNI space. Confidence intervals (95%) for the brain scores (mean-centered and collapsed across all 8 time points) in each condition also were calculated from the bootstrap, and the reliability of differences in activity between conditions and groups was determined via a lack of overlap in these confidence intervals.
3. Results
3.1 Behavioral results
Accuracy and reaction time (RT) results are shown in Table 2. The age groups differed in behavioral performance only in the hard item memory condition, with older adults showing a reduced hit rate, t(29) = 2.65, p = .013, and longer RTs, t(29) = 2.56, p = .016. However, accuracy and RT are in a trade-off relationship, which makes it difficult to interpret either measure in isolation. We therefore used diffusion modeling (Ratcliff, 1978) to estimate, from each participant’s accuracy and RT data, a set of parameters that accounted for behavioral performance in each experimental condition. A full description of the model and of the estimation procedures can be found in (Voss & Voss, 2008). Similar applications of diffusion modeling to item and source memory retrieval in younger and older adults were described in detail elsewhere (e.g., Spaniol et al., 2006, 2008). Briefly, the diffusion model (Ratcliff, 1978) assumes that information that drives two-choice decisions (e.g., the mnemonic information that leads participants to decide “old” vs. “new, or “Source A” vs. “Source B”) accumulates gradually and in a noisy manner. Once the information reaches a decision boundary, a response is initiated. In the diffusion model, accuracy and RT data depend on the quality of the information driving the decision (“drift rate”), cautiousness, response bias, and perceptual-motor speed, as well as on between-trial variability in each of these processes. Of particular interest in the current context are drift rate and response bias, which roughly correspond to d’ and criterion in signal detection theory (Green & Swets, 1966). However, signal detection theory accounts for accuracy only, whereas the diffusion model accounts for both accuracy and RT.
Table 2.
Behavioral Performance
| Condition | Younger | Older |
|---|---|---|
| Item Memory | ||
| Hits | ||
| Easy | .85 (.09); 1304 (261) | .79 (.12); 1432 (232) |
| Hard | .69 (.12); 1370 (211) | .58 (.13)*; 1563 (209)* |
| False alarms | .21 (.10); 1748 (386) | .16 (.09); 1720 (395) |
| Source Memory | ||
| Correct responses | ||
| Easy | .73 (.11); 1591 (256) | .69 (.09); 1758 (309) |
| Hard | .64 (.08); 1701 (243) | .60 (.08); 1866 (316) |
Mean proportion of responses and mean reaction time (in ms). Standard deviations are shown in parentheses.
indicates a significant age difference at p < .05.
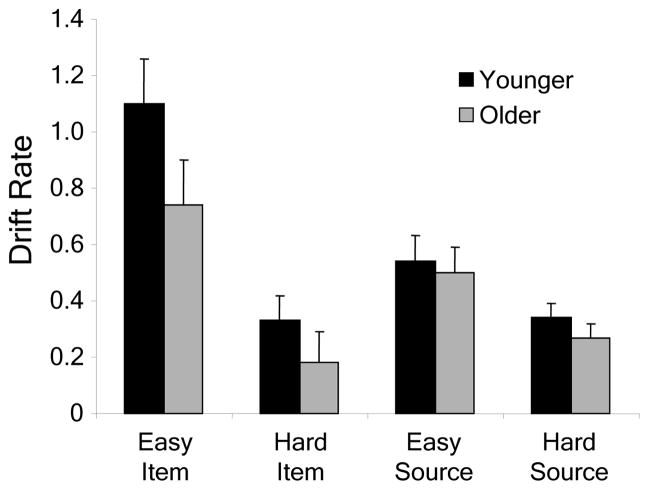
Average drift rate parameters are shown in Figure 1. Higher drift rates indicate better memory. A mixed analysis of variance (ANOVA) on drift rate with between-subjects factor group (younger vs. older) and within-subjects factors task (item vs. source) and difficulty (easy vs. hard) revealed a main effect of task, F(1, 29) = 4.38, p = .045, a main effect of difficulty, F(1, 29) = 60.16, p < .001, and a Task X Difficulty interaction, F(1, 29) = 16.29, p < .001. Follow-up t tests probing the interaction showed that the difficulty manipulation had a significant effect on both item memory, t(30) = 6.75, p < .001, and source memory, t(30) = 4.17, p < .001, but the effect was larger for item memory. No effects involving age were significant. It should be noted, however, that the small sample size of our study (N = 31) severely limited the statistical power of the group comparison. Specifically, the power to detect a medium between-subjects effect was only 27% as calculated with GPower (Faul et al., 2007). Nevertheless, inspection of the drift rate results (see Fig. 1) shows that, across the four experimental conditions, older adults’ drift rates were consistently lower than those of younger adults.
Figure 1.
The response-bias measure is scaled such that a value of .5 indicates unbiased responding. Values greater than .5 indicate a bias to respond “old” (item memory task) or “animacy” (source memory task), whereas values smaller than .5 indicate a bias to respond “new” (item memory task) or “pleasantness” (source memory task). In the item memory task, younger adults (M = .57, SD = .09) showed a significant bias to respond “old,” t(15) = 3.06, p < .01, whereas older adults (M = .55, SD = .12) did not; at the same time, response bias was not significantly different for the two groups. In the source memory task, neither group showed a significant response bias (younger: M = .54, SD = .09; older: M = .52, SD = .09).
3.2 fMRI results: Pre-specified contrasts
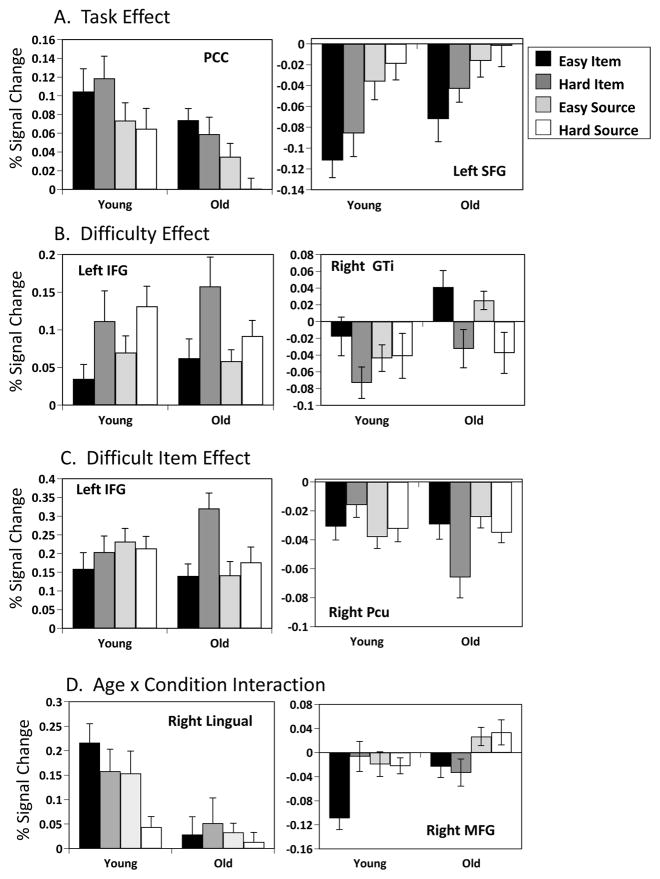
The analysis using pre-specified task contrasts to assess main effects of task and difficulty showed a marginally significant effect of task (p=0.058, Figure 2). Areas identified by this analysis showed more activity for item memory or source memory, regardless of difficulty, in both age groups (Figure 2b and c). Increased activity for item memory was found bilaterally in the inferior frontal gyrus, extending into the operculum, bilateral caudate/putamen and thalamus, posterior cingulate cortex, and left parietal cortex (Figure 2a and Table 3). More activity during source memory was seen in the superior frontal gyri, bilateral temporal cortex and orbitofrontal cortex. There were no age differences in this pattern of activity (Figure 2b). Examples of activity in representative regions are illustrated in Figure 6a. The posterior cingulate had increased activity over baseline and showed more activity for item than for source memory. However, in a number of regions showing a main effect of task, activity was reduced below baseline levels, only more so for item memory. An example of a region with this pattern of activity is the left superior frontal gyrus.
Figure 2.
Table 3.
Brain Areas with Modulations of Activity Differentiating Item from Source Memory
| Region | BA | X | Y | Z | BSR |
|---|---|---|---|---|---|
| Item > Source | |||||
| R inferior frontal gyrus | 47 | 36 | 24 | −12 | 4.3 |
| L insula/inferior frontal | −44 | 4 | −4 | 4.8 | |
| L cingulate gyrus | 23 | −4 | −28 | 28 | 6.6 |
| L angular gyrus | 39 | −40 | −76 | 32 | 5.3 |
| L superior parietal lobe | 7 | −40 | −68 | 56 | 4.4 |
| R caudate | 12 | 12 | 0 | 5.8 | |
| R thalamus | 8 | −16 | 0 | 4.3 | |
| L putamen | −16 | 4 | 4 | 6.7 | |
| Source > Item | |||||
| L superior frontal gyrus | 10 | −16 | 60 | 28 | −8.4 |
| L superior frontal gyrus | 6 | −12 | 20 | 60 | −4.4 |
| R orbitofrontal cortex | 11 | 40 | 44 | −8 | −4.1 |
| L orbitofrontal cortex | 10 | −20 | 44 | −4 | −4.1 |
| R middle/superior temporal gyrus | 22 | 32 | −56 | 16 | −6.3 |
| L middle temporal gyrus | 21 | −36 | −48 | 8 | −4.8 |
Activity from italicized regions is shown in Figure 6.
Figure 6.
The second contrast, assessing activity related to the difficulty of the memory task, revealed a significant effect of difficulty in both age groups (p < 0.005, Figure 3). Hard memory decisions were accompanied by more activity in bilateral medial and lateral prefrontal regions and left inferior parietal lobe (Figure 3a and Table 4). Easy memory decisions were associated with more activity in bilateral posterior insula and right occipital cortex. Again, there were no age differences in the expression of this difficulty related pattern (Figure 3b). Examples of regions with a main effect of difficulty are shown in Figure 6b. One of these, the left inferior frontal gyrus, showed an increase over baseline and more activity for the more difficult tasks in both groups. The right inferior temporal gyrus showed a more complex pattern of increased and decreased activity in the two groups, but generally more activity for the easier conditions.
Figure 3.
Table 4.
Brain Areas with Modulations of Activity Differentiating Easy from Hard Memory Conditions
| Region | BA | X | Y | Z | BSR |
|---|---|---|---|---|---|
| Hard > Easy | |||||
| R medial frontal gyrus | 6 | 8 | 20 | 60 | 4.7 |
| L medial frontal gyrus | 6 | −8 | 32 | 40 | 5.7 |
| R inferior frontal gyrus | 45 | 44 | 24 | 20 | 5.8 |
| L inferior frontal gyrus | 45 | −44 | 8 | 32 | 5.6 |
| L inferior frontal gyrus | 45/46 | −56 | 36 | 16 | 5.0 |
| L superior frontal gyrus | 10 | −12 | 60 | 32 | 4.9 |
| L angular gyrus | 39 | −40 | −64 | 36 | 4.8 |
| Easy > Hard | |||||
| Rectal gyrus | 11 | 0 | 28 | −12 | −4.3 |
| R insula | 22 | 44 | −8 | −12 | −4.5 |
| L insula | 22 | −40 | 0 | −12 | −5.1 |
| L parahippocampal gyrus | 30 | −16 | −36 | −8 | −4.6 |
| R superior temporal gyrus | 42 | 64 | −12 | 12 | −4.4 |
| R inferior temporal gyrus | 37 | 56 | −60 | −8 | −4.2 |
Activity from italicized regions is shown in Figure 6.
3.3 fMRI results: Data-driven analysis
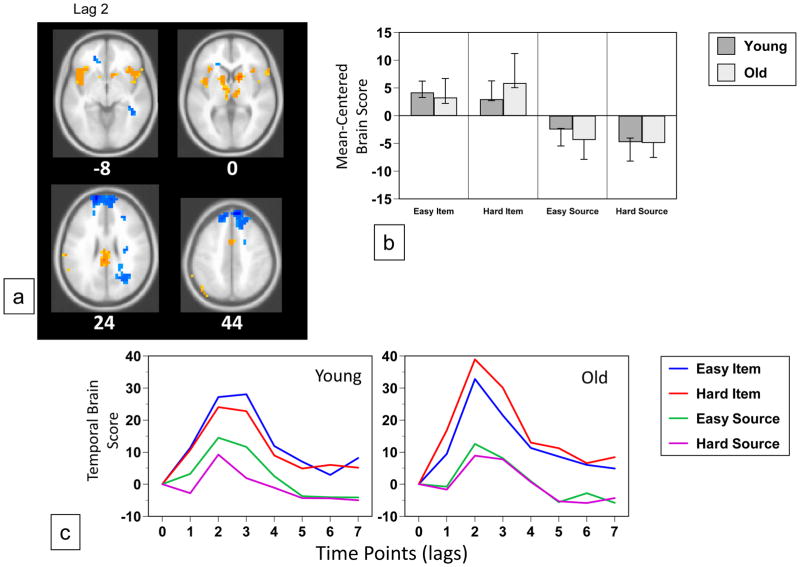
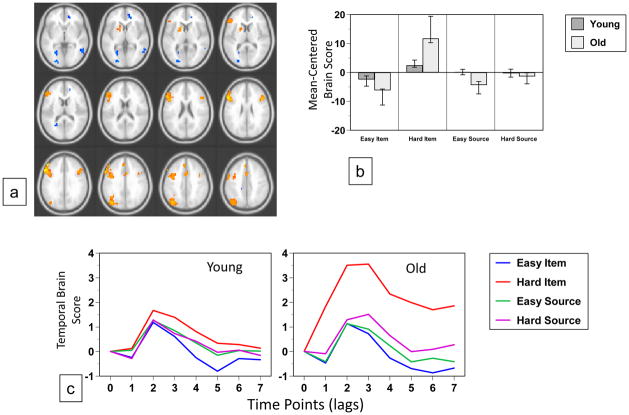
The data-driven analysis of the fMRI images revealed two significant patterns of brain activity. The first (p < 0.002, accounting for 27% of the covariance) identified a set of lateral and medial prefrontal regions, and left lateral parietal cortex (Figure 4a, Table 5) where activity was increased during the harder item condition, relative to the other conditions, in both groups (Figure 4b and c). However, this increase was much larger in older adults, suggesting that this pattern of activity was driven mainly by the older group. Areas showing the reverse effect of less activity during the hard item condition relative to other conditions included a number of occipital regions (Figure 4a, Table 5). This pattern of activity was similar, but not identical to that seen in Figure 3, which reflected a difficulty effect across both groups and memory tasks. Examples are shown in Figure 6c, of increased activity during the hard item condition in left inferior frontal gyrus and decreased activity in this condition in the precuneus, both of which were seen mainly in older adults.
Figure 4.
Table 5.
Brain Areas with Modulations of Activity Differentiating Easy from Hard Item Memory
| Region | BA | X | Y | Z | BSR |
|---|---|---|---|---|---|
| Hard Item > Easy Item | |||||
| R inferior frontal gyrus | 45 | 44 | 20 | 24 | 5.9 |
| L inferior frontal gyrus | 45 | −56 | 24 | 28 | 8.3 |
| L middle frontal gyrus | 9 | −48 | 40 | 32 | 7.4 |
| L medial frontal gyrus | 9 | −4 | 40 | 36 | 4.7 |
| Medial frontal gyrus (pre-SMA) | 6 | 0 | 12 | 52 | 4.5 |
| L inferior/superior parietal lobe | 40/7 | −44 | −64 | 52 | 6.8 |
| L caudate/putamen | −16 | 12 | 4 | 4.1 | |
| Easy Item > Hard Item | |||||
| Anterior cingulate | 25 | 0 | 28 | −8 | −5.3 |
| L insula | −44 | 0 | 8 | −5.5 | |
| L calcarine | 17 | −24 | −68 | 4 | −7.1 |
| R middle temporal gyrus | 21 | 56 | −48 | 0 | −5.0 |
| L superior temporal gyrus | 22 | −60 | 0 | 4 | −4.8 |
| R precuneus | 7 | 16 | −44 | 48 | −5.5 |
Activity from italicized regions is shown in Figure 6.
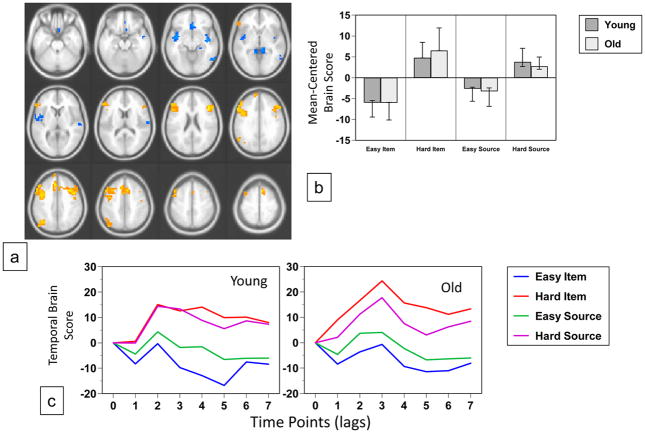
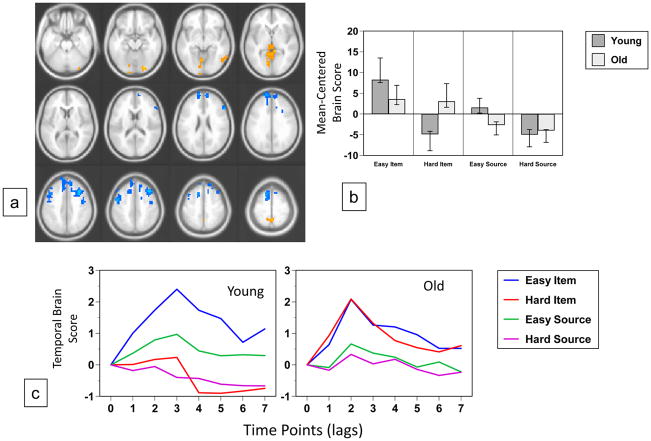
The second significant pattern of activity revealed by the data-driven analysis (p < 0.002, accounting for 21% of the covariance, Figure 5a) identified regions where the pattern of activity across the conditions was different in the two groups. In young adults more activity in occipital regions was found in the two easy memory conditions, especially in the easy item condition (Figure 5b and c). In contrast, older adults showed more activity in these regions for item memory regardless of difficulty. More activity in a mostly frontal group of regions was seen for hard memory decisions in younger adults, including bilateral dorsolateral prefrontal cortex and rostral frontal regions (Table 6); these regions were more active for source memory in older adults, relative to item memory, regardless of difficulty. Examples of activity from regions showing this age difference are illustrated in Figure 6d. Activity in the right lingual gyrus was greater during the easy conditions in young adults, and showed only small modulations in older adults, with a trend for more activity during item memory. Conversely, the right middle frontal region in younger adults showed a marked reduction of activity during the easy item condition, whereas in older adults this region showed more activity for source than item memory, regardless of difficulty.
Figure 5.
Table 6.
Brain Areas with Condition x Age Interactions
| Region | BA | X | Y | Z | BSR |
|---|---|---|---|---|---|
| More Activity for Easy Memory in Young and Item Memory in Old | |||||
| R inferior frontal gyrus | 47 | 36 | 20 | −8 | 4.7 |
| R superior temporal gyrus | 22 | 56 | −8 | 8 | 4.4 |
| R inferior temporal gyrus | 37 | 60 | −56 | −12 | 4.2 |
| R lingual gyrus | 18 | 24 | −84 | −20 | 4.8 |
| Precuneus | 7 | 0 | −52 | 56 | 5.1 |
| R caudate/putamen | 16 | 0 | 12 | 4.6 | |
| Cerebellar vermis | 0 | −60 | −8 | 4.8 | |
| More Activity for Hard Memory in Young and Source Memory in Old | |||||
| R middle frontal gyrus | 8 | 40 | 24 | 44 | −6.6 |
| R inferior frontal gyrus | 45 | 56 | 28 | 16 | −5.0 |
| R superior frontal gyrus | 8 | 12 | 44 | 44 | −5.5 |
| L superior frontal gyrus | 10 | −16 | 64 | 24 | −7.1 |
| L superior frontal gyrus | 6 | −12 | 20 | 56 | −7.0 |
| L precentral gyrus | 6 | −48 | −4 | 40 | −5.8 |
| R precentral gyrus | 6 | 44 | −4 | 40 | −5.3 |
Activity from italicized regions is shown in Figure 6.
4. Discussion
Theories of cognitive aging that postulate a specific age-related deficit in context processing (e.g., due to declines in associative binding or recollection) predict that high context demand should render memory retrieval disproportionately difficult for older adults. To test this hypothesis, and to disentangle the effects of context demand from those of general (context-independent) difficulty, we factorially manipulated the context demand (item memory vs. source memory) and the difficulty (one vs. two study presentations) of retrieval in younger and older adults. Using a multivariate approach, Partial Least Squares (PLS; McIntosh, 1999; McIntosh et al., 1996, 2004), we found evidence for dissociable activation patterns related to context demand and task difficulty that were shared by younger and older adults. We also found age differences in neural recruitment patterns, mostly in prefrontal and visual processing regions, which were sensitive to difficulty in younger adults, but to task (i.e., context demand) in older adults. Before examining these findings more closely, we first turn to a discussion of the behavioral results.
4.1 Behavioral findings
The raw accuracy and RT data were submitted to a diffusion model analysis (Ratcliff, 1978) that provided individual estimates of memory (drift rate) and response bias. The model results suggested that participants adopted a slightly liberal response bias on the item memory test (although this pattern was not significant for the older adults), with no evidence of response bias on the source memory test. Drift rate was sensitive to both task and difficulty (item recognition > source memory; easy > difficult). The difficulty effect was particularly pronounced for item memory, suggesting that repeated presentation of items during study had less impact on the strength of item-source bindings than it did on the strength of the items themselves. A possible explanation for this finding is the fact that there were many items but only two sources. The interference among the memory representations of item-source bindings may have minimized the impact of repeated presentations on source memory.
Although context-dependent memory tasks, such as source memory, typically produce robust age differences (e.g., Spaniol et al., 2006; Spencer & Raz, 1995), we found no significant effect of age on drift rate in this study, most likely because of low statistical power. It is also possible that the age-related behavioral deficit in source memory was minimized by the selection of highly educated samples (see Table 1). The matched performance of the two groups across the four task conditions was incidental, but it made the interpretation of the fMRI results more straightforward. Specifically, when behavioural performance is equated and analysis is restricted to correct responses, age differences in the neuroimaging data cannot be attributed to differences in the frequency of retrieval success, error monitoring, or guessing (e.g., Morcom et al., 2007).
4.2 fMRI findings: Age-invariant task effects
Similar to previous fMRI studies of source memory in younger and older adults, our data revealed frontal and posterior activations that were shared by younger and older adults (e.g., Daselaar et al., 2006; Dennis et al., 2008; Duarte et al., 2008; Duverne et al., 2008; Kukolja et al., 2009; Morcom et al., 2007; Rajah et al., 2010). Both age groups showed greater activation for source memory, compared with item memory, in bilateral medial anterior PFC (BA 10), extending into orbitofrontal cortex. These activations actually represented reduced deactivations from baseline (see Fig. 6). Medial anterior PFC is hypothesized to form part of the brain’s ‘default network’ (Raichle et al., 2001; Raichle & Snyder, 2007) which is activated during daydreaming and self-referential thought, and deactivated during challenging task conditions. Anterior PFC activation (or reduced deactivation) is frequently seen during context retrieval (e.g., Dobbins & Wagner, 2005; Simons et al., 2005a, 2005b; see also Mitchell & Johnson, 2009). The precise functional significance of anterior PFC for source memory is still under debate, but our results are consistent with the idea that medial anterior PFC mediates the retrieval of internally generated contextual details, such as the thoughts and associations produced during semantic encoding (e.g., Dobbins & Wagner, 2005; Simons et al., 2005a, 2005b, 2008). This hypothesis would explain why the source memory task was associated with less deactivation of anterior PFC than the item memory task, which would require retrieval of fewer details.
Source memory was also associated with activations in left and right lateral temporal cortices, similar to what was reported by Dobbins and Wagner (2005). These activations may reflect reinstatement, during source retrieval, of semantic and visuospatial properties evaluated during animacy and pleasantness judgments at encoding (for reviews of the contribution of these regions to semantic and perceptual processing, see Binder et al., 2009, and Grill-Spector, 2003).
A different set of regions was more active for item than source memory in both age groups. These regions included bilateral ventrolateral PFC, bilateral caudate/putamen and thalamus, posterior cingulate cortex, and left dorsal parietal cortex. All of these areas are routinely found to be activated during episodic long-term memory retrieval (see Spaniol et al., 2009). However, left anterior ventrolateral PFC (BA 47) is usually more active during source memory than during item recognition (for a review, see Badre & Wagner, 2007), and has been hypothesized to mediate controlled semantic retrieval in the service of contextual recollection (see also Dobbins & Wagner, 2005). It is thus unclear why, in our study, bilateral ventrolateral PFC was more active for item memory than for source memory. Although speculative, one explanation may be that the item memory task gave rise to spontaneous retrieval of semantic and visuospatial details, unconstrained by the specific requirements of the source memory task. This phenomenon is sometimes referred to as noncriterial recollection (Yonelinas & Jacoby, 1996), and it highlights the general point that item and source memory tasks do not provide ‘process-pure’ measures of familiarity and recollection, respectively.
4.3 fMRI findings: Age-invariant difficulty effects
For both younger and older adults, hard memory decisions were accompanied by activity in prefrontal cognitive-control regions including left dorsomedial (BA 6) and bilateral ventrolateral (BA 45) PFC. Left mid-ventrolateral PFC is theorized to play a role in the control of post-retrieval selection from multiple competing semantic representations (Badre & Wagner, 2007), whereas activation in its right-hemisphere homologue is thought to support the control of visuospatial attention during retrieval (Dobbins & Wagner, 2005). Hard memory decisions were also associated with activation in inferior parietal cortex (BA 39). According to current theories of the parietal role in memory (Cabeza et al., 2008; Ciaramelli et al., 2008), this area is thought to support bottom-up attention to retrieved contents (e.g., during relatively effortless, recollection-based, and high-confidence retrieval). Our finding of greater inferior parietal activity during hard, compared to easy, memory decisions can thus not be easily accommodated by these theories.
Finally, easy memory decisions were accompanied by activity in bilateral posterior insula, right lateral temporal cortex, and left posterior parahippocampal gyrus, the latter possibly indicating an enhanced medial-temporal recollection signal for twice-studied items (for a review, see, e.g., Eichenbaum et al., 2007).
4.4 fMRI findings: Age-related over-recruitment of fronto-parietal regions in response to task difficulty
The data-driven analyses revealed two separate activation patterns that showed significant age differences. The first of these patterns differentiated easy from hard item memory, with older adults showing an enhancement of this effect. It encompassed a set of regions similar to those seen in the age-invariant difficulty pattern, but additionally included activations in left dorsolateral PFC (BA 9) and the superior parietal lobe (BA 7), both associated with hard memory decisions. Dorsolateral PFC has been shown to subserve domain-general, rather than memory-specific, executive-control processes related to classification and decision making (Dobbins & Han, 2006; Han et al., 2009). The superior parietal lobe is believed to mediate top-down attention to memory when the mnemonic evidence is weak or ambiguous (Cabeza et al., 2008; Ciaramelli et al., 2008). The existence of this pattern suggests that the age-invariant difficulty pattern, reported above, does not tell the whole story. In older adults, maintaining task performance at young-adult levels, in the face of increased retrieval difficulty, was associated with activity in dorsolateral prefrontal and parietal executive-control regions to a greater degree than in younger adults. This finding is consistent with other reports of overrecruitment of brain regions during episodic retrieval, particularly in the frontal cortex, in older adults (e.g., Daselaar et al., 2003; Davis et al., 2008; Cabeza et al., 1997; Grady et al., 1994; Madden et al., 1999). It is often difficult to know how to interpret such over-recruitment, and interpretations include age-related compensation (e.g., Vallesi et al., 2010; Velanova et al., 2007), less efficient use of neural resources (Morcom et al., 2007; Zarahn et al., 2007; Rypma et al., 2007), and a reflection of poor performance in older adults (Duverne et al., 2009; for a review, see Grady, 2008). We found that the over-recruitment in older adults was driven almost exclusively by the item memory condition, which showed a stronger effect of difficulty than source memory, in both age groups. Given that performance was low in the hard item condition, the increased activity in frontal and parietal regions during this condition in older adults may be due more to an increased demand on resources, or less effective use of these resources, than to compensation, but our data do not speak directly to either interpretation.
4.5 fMRI findings: Evidence of functional reorganization in response to context demand
The data-driven analysis revealed a second pattern best described as an interaction of age with task and difficulty. First, there was greater activity in older relative to younger adults in bilateral frontal regions (medial anterior, right posterior ventrolateral and dorsolateral, and bilateral motor cortex) for source memory, regardless of difficulty. In contrast, in younger adults, activity in these areas was associated with difficult memory decisions, regardless of task. Second, there was greater activity in older relative to younger adults in right-lateralized posterior regions (anterior ventrolateral PFC, striatum, temporal and extrastriate cortex, as well as precuneus) for item memory, regardless of difficulty. In contrast, in younger adults, activity in these areas was associated with easy memory decisions, regardless of task.
The age differences captured in this pattern are best characterized in terms of functional reorganization rather than compensatory overrecruitment (e.g., Vallesi et al., 2010; Velanova et al., 2007) or dedifferentiation (e.g., Morcom et al., 2007), because the same frontal executive-control regions recruited by older adults during source memory decisions were also recruited by younger adults during difficult memory decisions. Even though behavioral performance in this experiment did not show an Age X Task interaction, the fMRI results suggest that younger and older adults differed in how they prioritized the allocation of frontal controlled processing resources to optimize performance across different task conditions. Younger adults prioritized difficult retrieval conditions (i.e., those in which the mnemonic information was relatively weak), whereas older adults prioritized context-demanding retrieval conditions (i.e., those which required the recovery of source information). This pattern thus provides the strongest support, within the current set of results, of a specific context memory deficit in older adults, albeit one that was not expressed behaviorally.
4.6 Conclusions
What is the cause of the age-related context memory deficit? Short of answering this question directly, our findings do at least provide a hint. The bilateral frontal regions differentially engaged during source memory in older adults were associated with task difficulty in younger adults, and indeed were similar, though not identical, to those participating in the age-invariant difficulty pattern. Furthermore, difficulty was manipulated by varying the number of presentations during study, and hence, the strength of the resulting mnemonic representations. Our findings are thus consistent with the idea that older adults’ context memory deficit is caused at least partly by a reduction in the quality or strength of bound item-context representations (e.g., Dennis et al., 2008; Naveh-Benjamin, 2000), rather than by prefrontal dysfunction (e.g., Mitchell et al., 2006; Rajah et al., 2010) or by changes in the connectivity of prefrontal and medial-temporal regions (e.g., Daselaar et al., 2008; Dennis et al., 2008). A promising avenue for future neuroimaging studies of aging and context memory may therefore involve investigations of specific item-context binding processes, and their substrates in medial-temporal and posterior representational regions, during episodic encoding (see also Mitchell & Johnson, 2009).
Acknowledgments
Julia Spaniol, Department of Psychology, Ryerson University; Cheryl L. Grady, Rotman Research Institute at Baycrest and Departments of Psychology and Psychiatry, University of Toronto. This work was supported by CIHR grant # MOP14036, the Canada Research Chairs program, the Ontario Research Fund, and the Canadian Foundation for Innovation. Additional support was provided by an NSERC Discovery Grant and by the Faculty of Arts at Ryerson University. We are grateful to Dr. Andreas Voss for providing assistance with the diffusion model, and to Maroquine Aziz, Hua Han, Kimberly Chiew, and Charisa Ng for technical assistance.
Footnotes
Disclosure Statement
There are no conflicts of interest to declare. All study procedures approved by the local Research Ethics Board.
References
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, Light LL. Memory and aging: The role of retrieval processes. Psychological Bulletin. 1981;90:513–546. [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: An attentional account. Nature Reviews Neuroscience. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FI. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. Journal of Neuroscience. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory & Cognition. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craik FIM, editor. Human memory and cognitive capabilities: Mechanisms and performances. Elsevier; Amsterdam: 1986. A functional account of age differences in memory; pp. 409–422. [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cerebral Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Qué PASA? the posterior-anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Isolating rule- versus evidence-based prefrontal activity during episodic and lexical discrimination: A functional magnetic resonance imaging investigation of detection theory distinctions. Cerebral Cortex. 2006;16:1614–1622. doi: 10.1093/cercor/bhj098. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cerebral Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Duarte A, Henson RN, Graham KS. The effects of aging on the neural correlates of subjective and objective recollection. Cerebral Cortex. 2008;18:2169–2180. doi: 10.1093/cercor/bhm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiology of Aging. 2008;29:1902–1916. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cerebral Cortex. 2009;19:733–744. doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science. 1986;1:54–75. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Glisky EL, Rubin SR, Davidson PS. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. Journal of Neuroscience. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. Wiley; Oxford: 1966. [Google Scholar]
- Grill-Spector K. The neural basis of object perception. Current Opinion in Neurobiology. 2003;13:159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Han S, Huettel SA, Dobbins IG. Rule-dependent prefrontal cortex activity across episodic and perceptual decisions: An fMRI investigation of the criterial classification account. Journal of Cognitive Neuroscience. 2009;21:922–937. doi: 10.1162/jocn.2009.21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL. Ironic effects of repetition: Measuring age-related differences in memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:3–22. doi: 10.1037//0278-7393.25.1.3. [DOI] [PubMed] [Google Scholar]
- Kuçera H, Francis WN. Computational analysis of present-day American English. Brown University Press; Providence, RI: 1967. [Google Scholar]
- Kukolja J, Thiel CM, Wilms M, Mirzazade S, Fink GR. Ageing-related changes of neural activity associated with spatial contextual memory. Neurobiology of Aging. 2009;30:630–645. doi: 10.1016/j.neurobiolaging.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Li S, Sikström S. Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. Neuroscience & Biobehavioral Reviews. 2002;26:795–808. doi: 10.1016/s0149-7634(02)00066-0. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Gottlob LR, Denny LL, Turkington TG, Provenzale JM, Hawk TC, Coleman RE. Aging and recognition memory: Changes in regional cerebral blood flow associated with components of reaction time distributions. Journal of Cognitive Neuroscience. 1999;11:511–520. doi: 10.1162/089892999563571. [DOI] [PubMed] [Google Scholar]
- McIntosh AR. Mapping cognition to the brain through neural interactions. Memory. 1999;7:523–548. doi: 10.1080/096582199387733. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. NeuroImage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB. Spatiotemporal analysis of event-related fMRI data using partial least squares. NeuroImage. 2004;23:764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: What have we learned from fMRI about the neural mechanisms of source memory? Psychological Bulletin. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Johnson MK, Greene EJ. An fMRI investigation of short-term source memory in young and older adults. NeuroImage. 2006;30:627–633. doi: 10.1016/j.neuroimage.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: Increased cortical recruitment with matched performance. Cerebral Cortex. 2007;17:2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Park DC, Payer D. Working memory across the adult lifespan. In: Bialystok E, Craik FIM, editors. Lifespan cognition: Mechanisms of change. Oxford University Press; New York: 2006. pp. 128–142. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. NeuroImage. 2007;37:1083–90. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Rajah MN, Languay R, Valiquette L. Age-related changes in prefrontal cortex activity are associated with behavioural deficits in both temporal and spatial context memory retrieval in older adults. Cortex. 2010;46:535–549. doi: 10.1016/j.cortex.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. A theory of memory retrieval. Psychological Review. 1978;85:59–108. [Google Scholar]
- Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation-performance relations in delayed-response tasks: A multiple component analysis. Cortex. 2007;43:65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Barr HM, Bookstein FL. Neurobehavioral effects of prenatal alcohol: Part II. partial least squares analysis. Neurotoxicology and Teratology. 1989;11:477–491. doi: 10.1016/0892-0362(89)90025-1. [DOI] [PubMed] [Google Scholar]
- Siedlecki KL, Salthouse TA, Berish DE. Is there anything special about the aging of source memory? Psychology and Aging. 2005;20:19–32. doi: 10.1037/0882-7974.20.1.19. [DOI] [PubMed] [Google Scholar]
- Simons JS, Gilbert SJ, Owen AM, Fletcher PC, Burgess PW. Distinct roles for lateral and medial anterior prefrontal cortex in contextual recollection. Journal of Neurophysiology. 2005a;94:813–820. doi: 10.1152/jn.01200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Henson RN, Gilbert SJ, Fletcher PC. Separable forms of reality monitoring supported by anterior prefrontal cortex. Journal of Cognitive Neuroscience. 2008;20:447–457. doi: 10.1162/jocn.2008.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Owen AM, Fletcher PC, Burgess PW. Anterior prefrontal cortex and the recollection of contextual information. Neuropsychologia. 2005b;43:1774–1783. doi: 10.1016/j.neuropsychologia.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Madden DJ, Voss A. A diffusion model analysis of adult age differences in episodic and semantic long-term memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:101–117. doi: 10.1037/0278-7393.32.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Voss A, Grady CL. Aging and emotional memory: Cognitive mechanisms underlying the positivity effect. Psychology and Aging. 2008;23:859–872. doi: 10.1037/a0014218. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: A meta-analysis. Psychology and Aging. 1995;10:527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology/Psychologie canadienne. 1985;26:1–12. [Google Scholar]
- Vallesi A, McIntosh AR, Stuss DT. Overrecruitment in the aging brain as a function of task demands: Evidence for a compensatory view. Journal of Cognitive Neuroscience. 2010 doi: 10.1162/jocn.2010.21490. in press. [DOI] [PubMed] [Google Scholar]
- Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cerebral Cortex. 2007;17:1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- Voss A, Voss J. A fast numerical algorithm for the estimation of diffusion model parameters. Journal of Mathematical Psychology. 2008;52:1–9. [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Jacoby LL. Noncriterial recollection: Familiarity as automatic, irrelevant recollection. Consciousness and Cognition: An International Journal. 1996;5:131–141. [PubMed] [Google Scholar]
- Zarahn E, Rakitin B, Abela D, Flynn J, Stern Y. Age-related changes in brain activation during a delayed item recognition task. Neurobiology of Aging. 2007;28:784–798. doi: 10.1016/j.neurobiolaging.2006.03.002. [DOI] [PubMed] [Google Scholar]