Abstract
We report the initial characterization of the product of the vaccinia virus A28L gene, which is highly conserved in all sequenced poxviruses. Our studies showed that the A28 protein is expressed at late times during the virus replication cycle and is a membrane component of the intracellular mature virion. An N-terminal hydrophobic sequence, present in all poxvirus A28 orthologs, anchors the protein in the virion surface membrane so that most of it is exposed to the cytoplasm. The cytoplasmic domain contains four conserved cysteines, which form two intramolecular disulfide bonds. Disulfide bond formation depended on the expression of three viral proteins, E10, A2.5, and G4, which together comprise a conserved cytoplasmic redox pathway. A28 is the third identified substrate of this pathway; the others are the L1 and F9 proteins. We constructed a conditional-lethal recombinant vaccinia virus with an inducible A28L gene. The recombinant virus was propagated in the presence of inducer but was unable to replicate and spread in its absence. During a single round of an abortive infection in the absence of inducer, the synthesis and processing of viral proteins, assembly of intra- and extracellular virions, and formation of actin tails occurred normally. In another paper (T. Senkevich, B. M. Ward, and B. Moss, J. Virol. 78:2357-2366, 2004), we have demonstrated that virions assembled without A28 cannot carry out a second round of infection because they are defective in cell penetration.
Poxviruses comprise a family of large, complex cytoplasmic DNA viruses with members that replicate in a variety of vertebrate and insect species, including mammals (10). Vaccinia virus, the best-characterized poxvirus, encodes nearly 200 proteins with roles in transcription, replication, virion assembly, and host defense. The most abundant infectious particle, known as the intracellular mature virion (IMV), is composed of a compact core surrounded by a lipoprotein membrane. The core contains the viral genome, enzymes involved in mRNA synthesis and modification, and additional proteins, presumably with structural roles. The IMV membrane appears to be composed of one bilayer (7) but may consist of two that are tightly opposed (13, 21). We still do not understand how the IMV membrane is formed. De novo formation of the viral membrane has been proposed (4), but subsequent studies suggested an origin from the endoplasmic reticulum-Golgi intermediate compartment (13, 21). Recent data, demonstrating that cargo transport from the endoplasmic reticulum to the endoplasmic reticulum-Golgi intermediate compartment is unnecessary for IMV formation, points to the endoplasmic reticulum as a possible source of viral precursor membranes or a novel membrane origin (8).
More than a dozen proteins are associated with the IMV membrane, including some with and others without transmembrane domains. One transmembrane protein, L1, and the structurally related but less well-characterized F9 protein, have intramolecular disulfide bonds in their cytoplasmic domains that are formed by vaccinia virus-encoded thiol-disulfide oxidoreductases (19, 20, 25). Here, we present the initial characterization of the protein encoded by the vaccinia virus A28L open reading frame (ORF) and show that this protein is also a substrate of the virus-specific cytoplasmic disulfide bond formation pathway. (Vaccinia virus ORFs are designated by a capital letter indicating a HindIII restriction endonuclease fragment, a number indicating the position in the HindIII fragment, and a letter [L or R] indicating the direction of transcription, e.g., A28L. The corresponding protein is designated by a capital letter and number, e.g., A28.) The A28 protein contains two disulfide bonds, is localized to the IMV membrane, and is essential for virus reproduction in cell culture.
MATERIALS AND METHODS
Cells and viruses.
Standard procedures for preparation and maintenance of BS-C-1 cells (ATCC CCL-26) and propagation, titration, and purification of vaccinia virus were used (6). Recombinant vaccinia viruses were derived from the WR strain (ATCC VR-1354).
Construction of vA28-HA and vA28-HAi.
vA28-HA was derived from vaccinia virus WR by attaching the sequence encoding the influenza hemagglutinin (HA) epitope with an additional C-terminal serine codon (YPYDVPDYAS) to the 3′ terminus of the A28L gene, as previously described for the construction of vE10-HA (18). In brief, recombinant PCR (Expand High Fidelity PCR system; Roche Applied Science) was used to assemble a DNA segment containing the A28L gene with the HA epitope tag sequence, followed by the Escherichia coli β-glucuronidase (GUS) gene under the control of the vaccinia virus strong early-late synthetic promoter (3), and flanking viral DNA sequences of ∼500 bp at both ends. The final PCR product was transfected into cells infected with vaccinia virus WR, where homologous recombination occurred. Plaques containing recombinant viruses expressing the GUS gene, which stained blue, were picked, and the recombinant virus was purified by three cloning cycles.
vA28-HAi was derived from vT7lacOI (1), which contains an IPTG (isopropyl-β-d-thiogalactoside)-inducible bacteriophage T7 RNA polymerase gene and the E. coli lac repressor gene inserted into the nonessential thymidine kinase locus, by using the procedure described previously for the construction of vE10i (18). The final product was assembled by recombinant PCR and inserted into vT7lacOI by homologous recombination. In addition to the lac repressor and IPTG-inducible T7 polymerase of the parental vT7lacOI, vA28-HAi contains an HA epitope-tagged A28L gene under the control of a lac operator-regulated T7 promoter.
Expression vectors.
To express A28 under the control of a vaccinia virus promoter, the A28L ORF was amplified by PCR using DNA from the cosmid pWR 120-150 (22) as a template and inserted into the pGEM-T easy vector (Promega). The strong late P11 promoter sequence (GAATTTCATTTTGTTTTTTTCTATGCTATAAATG) (the start codon is underlined) was included at the 5′ end of the forward PCR primer, and the HA epitope coding sequence was included at the 3′ end of the reverse PCR primer. For in vitro synthesis of A28 under the control of the bacteriophage T7 promoter, the A28L ORF with a C-terminal HA epitope coding sequence was inserted into the pVOTE vector (24). All constructs were verified by sequencing.
Transfection of vaccinia virus-infected cells with expression plasmids.
BS-C-1 cells in a 24-well plate were infected with 5 PFU of virus per cell and 2 h later were transfected with 0.25 μg of plasmid that had been incubated with 2 μl of Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The cells were incubated for 15 to 24 h after infection without changing the medium.
Protein analysis.
Infected or infected and transfected cells were collected by centrifugation, solubilized in nonreducing sodium dodecyl sulfate (SDS) loading buffer (Invitrogen) containing 20 mM N-ethylmaleimide (NEM; Sigma) or 20 mM 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS; Molecular Probes) or NuPage sample reducing agent (Invitrogen). In some cases, the proteins were reduced with Tris-(2-carboxyethyl) phosphine prior to alkylation, as described previously (20). All lysates were sonicated and boiled. Proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) in 10 to 20% polyacrylamide gels in Tris-glycine or 12% NuPage gels (Invitrogen). The proteins were transferred to a nitrocellulose membrane; incubated with peroxidase-conjugated rat high-affinity anti-HA monoclonal antibody (3F10; Roche Molecular Biochemicals) or other antibodies, followed by secondary peroxidase-conjugated antibodies; and detected with a chemiluminescence detection kit (Pierce).
Protein sequence analysis.
The nonredundant protein sequence database (National Center for Biotechnology Information, National Institutes of Health, Bethesda, Md.) was searched using the BLASTP program, and iterative searches were performed using the PSI-BLAST program (2). Multiple sequence alignments were constructed using the CLUSTAL-X program (23). Protein secondary structure was predicted using the PHD program, with multiple sequence alignments submitted as queries (14).
Electron microscopy.
Infected cells were fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer, washed in 0.1 M sodium cacodylate buffer, postfixed with reduced osmium tetroxide, and washed in buffer. The cells were dehydrated in a series of ethyl alcohol dilutions (50, 70, and 100%), followed by propylene oxide. The cells were then embedded in EMbed 812. Sections were obtained using the Leica Ultracut S ultramicrotome. Thin sections were stained with 7% uranyl acetate in 50% ethanol and then with 0.01% lead citrate and analyzed on the FEI CM100 transmission electron microscope.
Purified virus was adsorbed onto Formvar-coated, carbon-treated copper mesh grids and incubated with a mouse monoclonal anti-HA antibody (HA.11; Covance), followed by a secondary rabbit anti-mouse immunoglobulin G antibody and protein A conjugated to 10-nm-diameter colloidal gold. The virions were stained briefly with uranyl acetate and examined.
Fluorescence microscopy.
HeLa cells were grown on coverslips and infected with vaccinia virus at a multiplicity of 10 PFU per cell. The cells were fixed with 4% paraformaldehyde in phosphate-buffered saline for 20 min at 4°C, followed by 40 min at room temperature. After being washed three times with cold phosphate-buffered saline, the cells were incubated with the anti-B5 monoclonal antibody 19C2 (15), followed by fluorescein isothiocyanate-conjugated donkey anti-rat antibody (Jackson Immunoresearch). After being washed three times in cold phosphate-buffered saline, the cells were permeabilized in 0.05% saponin in phosphate-buffered saline and stained with 5 μg of diamidino-2-phenylindole dihydrochloride (Molecular Probes) per ml for 5 min. Filamentous actin was visualized by staining the cells with Alexa Fluor 568 phalloidin (Molecular Probes) according to the manufacturer's directions. Images were collected on a Leica TCS-NT/SP2 inverted confocal microscope system with an attached argon ion laser (Coherent Inc.).
RESULTS
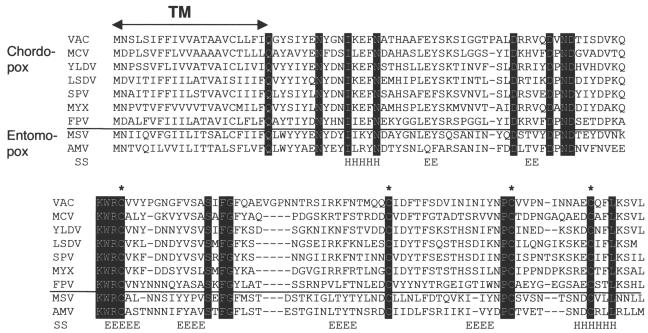
A28 is highly conserved in all sequenced poxvirus genomes. The ORF designated A28L in the Copenhagen strain of vaccinia virus and VACWR151 in the WR strain is predicted to encode a 16.3-kDa protein, which is conserved in all sequenced poxvirus genomes. We could find no significant sequence similarity between A28 and any nonpoxvirus protein, in spite of extensive attempts using iterative database searches with the PSI-BLAST program. All A28 orthologs contain a highly hydrophobic N-terminal region, which could function as a signal peptide or a transmembrane anchor; four conserved cysteines potentially involved in disulfide bond formation; and several invariant charged and polar residues (Fig. 1). The nonhydrophobic portion of A28 was predicted to have primarily a β-sheet structure, with six putative β-strands and two flanking α-helices (Fig. 1).
FIG. 1.
Multiple alignment of A28 orthologs. The alignment includes one representative sequence from each genus of Chordopoxvirinae for which such information is available and the two complete Entomopoxvirinae sequences. VAC, vaccinia virus (Orthopoxvirus); MCV, molluscum contagiosum virus (Molluscipoxvirus); YLDV, Yaba-like disease virus (Yatapoxvirus); LSDV, lumpy skin disease virus (Capripoxvirus); SPV, swinepox virus (Suipoxvirus); MYX, myxoma virus (Leporipoxvirus); FPV, fowlpox virus (Avipoxvirus); MSV, Melanoplus sanguinipes entomopoxvirus (Entomopoxvirus B); AMV, Amsacta moorei entomopoxvirus (Entomopoxvirus B). Invariant amino acid residues are shown by white letters against a black background, and conserved cysteines are indicated by asterisks. TM, predicted transmembrane domain. The predicted secondary structure (SS), consisting of six putative β-strands (E) and two flanking α-helices (H), is shown.
Synthesis of A28 during the vaccinia virus replicative cycle.
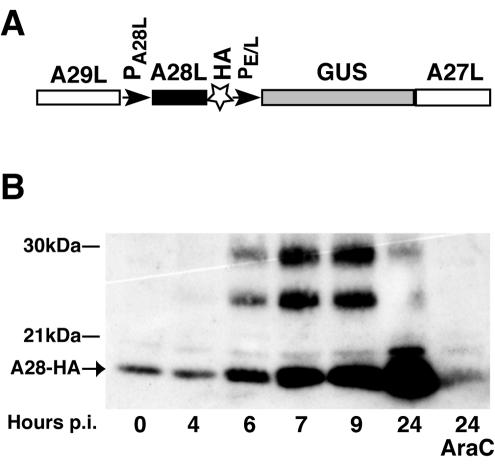
The start codon of the A28L ORF forms part of a TAAATG sequence, which is typical of promoters of late genes in poxviruses (5, 17). To monitor the synthesis of A28 during the vaccinia virus replicative cycle, we constructed a recombinant vaccinia virus in which the coding sequence for a 9-amino-acid influenza virus HA epitope was linked to the 3′ terminus of the A28L gene, which accordingly remained under the control of the native promoter (Fig. 2A). The GUS gene under the control of an early-late vaccinia virus promoter was coinserted into the vaccinia virus genome to allow screening for recombinant virus plaques. The recombinant virus, called vA28-HA, was clonally purified and characterized. Neither the virus yield nor the plaque size of the recombinant virus was impaired compared to wild-type virus (data not shown).
FIG. 2.
Temporal synthesis of A28. (A) Construction of vA28-HA. The DNA used for the insertion of the HA tag coding sequence at the 3′ end of the A28L ORF by homologous recombination was constructed by PCR and is shown schematically. A29L and A27L are ORFs flanking the A28L ORF. PA28L and PE/L are the native A28L promoter and a strong early-late synthetic promoter, respectively. The GUS gene was used as a reporter for the screening procedure, and clonally pure vA28-HA was isolated by repeated plaque isolations. (B) Time course of A28-HA synthesis in infected cells. Replicate cell cultures were infected with 5 PFU of vA28-HA per cell for 1 h; unadsorbed virus was removed by washing the cell monolayers three times with medium, and the incubation continued. Additional cells were infected in the presence of AraC (40 μg/ml). Cells were collected at the indicated times postinfection (p.i.) and disrupted in SDS-PAGE loading buffer without reducing agent and in the presence of 20 mM NEM. Proteins from total cell extract were resolved by SDS-PAGE; A28-HA was detected by Western blotting with peroxidase-conjugated anti-HA rat monoclonal antibody. The position of the 17-kDa A28-HA protein is indicated by an arrow. The 27- and 24-kDa bands migrate between the 30- and 21-kDa markers shown on the left.
Cells were infected with vA28-HA in the absence or presence of cytosine arabinoside (AraC), an inhibitor of DNA replication, and the lysates were analyzed by SDS-PAGE under nonreducing conditions. A 17-kDa band was detected by sensitive Western blotting with anti-HA antibody at zero time, reflecting the presence of A28-HA in the inoculum (Fig. 2B), which was not seen in lysates of uninfected cells (not shown). The amount of A28-HA increased from 4 h after infection, the usual time of onset of viral late-protein synthesis. A28-HA was not elevated above the zero time background when viral DNA replication was inhibited with AraC, as expected for a late protein (Fig. 2B). Prominent bands of 24 and 27 kDa were also detected in cells infected with vA28-HA (Fig. 2B) but not in cells infected with the wild-type virus lacking the epitope tag (not shown). The 24- and 27-kDa species accumulated with the same kinetics as the 17-kDa protein during the first 7 h of infection but did not increase thereafter. Only the 17-kDa form of A28-HA was incorporated into virus particles, as can be seen in the zero time lane (Fig. 2B) and by analysis of purified virions (16a). We presume that the 24- and 27-kDa proteins represent modified or tight complexes of A28-HA, and their identification by mass spectroscopy is planned.
A28-HA contains two intramolecular disulfide bonds.
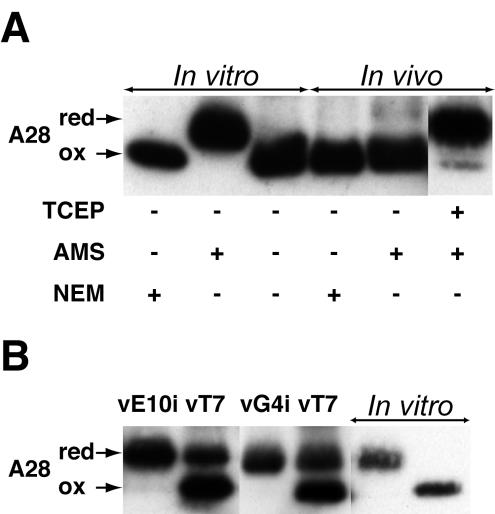
A28 and all poxvirus homologs were predicted to have four conserved cysteines within the nonhydrophobic segment of the protein (Fig. 1A). In some cases, intramolecular disulfide-bonded proteins have a compact structure and can be resolved from their reduced forms by SDS-PAGE. We therefore compared the mobilities of A28-HA proteins from infected cells under reducing and nonreducing conditions but found no difference (data not shown). To explain this result, we considered three possibilities: (i) the cysteines of A28 are not disulfide bonded, (ii) A28 has disulfide bonds that are not reducible by the methods used, or (iii) A28 has disulfide bonds but their reduction does not alter the electrophoretic mobility under the gel conditions used. To detect free cysteines, we used AMS as an alkylating agent. For each reactive cysteine, AMS increases the mass of a protein by 0.536 kDa. Because nonalkylated proteins can become alkylated with unpolymerized acrylamide during electrophoresis, we used NEM-alkylated A28 as the control. NEM increases the mass by only 0.125 kDa per reactive cysteine. Therefore, if A28 has four reactive cysteines, there would be a mass difference of 1.6 kDa between the AMS- and NEM-alkylated proteins, which would be easily discerned by SDS-PAGE. A28-HA synthesized in vitro had free cysteines, as indicated by the clear decrease in mobility when AMS was used as an alkylating agent (Fig. 3A). A28-HA synthesized in infected cells exhibited no mobility change with AMS treatment (Fig. 3A), indicating the absence of free cysteines. However, when A28-HA from infected cells was first reduced with Tris-(2-carboxyethyl) phosphine and then alkylated with AMS, the mass of the majority of the protein increased by ∼2 kDa. Therefore, the third explanation was correct: A28 from infected cells contains two reducible disulfide bonds. The same result was obtained when A28 was extracted from purified virions (data not shown).
FIG. 3.
Formation of disulfide bonds in A28-HA. (A) A28-HA contains two intramolecular disulfide bonds. In vitro, A28-HA synthesized in a coupled transcription-translation recticulocyte lysate system was captured with anti-HA antibody, and aliquots were disrupted in SDS-PAGE loading buffer containing (+) NEM or AMS or no alkylating agent (−), as indicated. In vivo, cells were infected with vA28-HA, collected 24 h after infection, and disrupted in SDS-PAGE loading buffer containing NEM, AMS, or Tris-(2-carboxyethyl) phosphine (TCEP), followed by AMS. Proteins were resolved by SDS-PAGE; A28L-HA was detected by Western blotting with anti-HA antibody. A28, A28-HA; red, reduced A28-HA; ox, oxidized A28-HA. (B) Expression of E10 and G4 is required to form disulfide bonds in A28. Cells were infected with either vE10i or vG4i without IPTG or with vT7lacOI (vT7) as a control and transfected with plasmid expressing A28-HA under the control of the strong late vaccinia virus promoter P11. Cells were collected 20 h after infection. Proteins from infected and transfected cells were allowed to react with AMS and were analyzed as for panel A. A28-HA made in vitro was treated with AMS (next to last lane on right) or NEM (last lane on right) to provide mobility markers.
A28 is a substrate of the viral disulfide bond formation pathway.
Previously, Senkevich et al. (19) described a unique poxvirus-specific pathway for disulfide bond formation that operates on the cytoplasmic side of the IMV membrane, creating intramolecular disulfide bonds in at least two vaccinia virus membrane proteins. A28, with two disulfide bonds and a presumptive membrane topology, was a good candidate for another substrate of this pathway. To determine whether the viral pathway is required for the formation of disulfide bonds in A28, we transfected the plasmid encoding A28 under the control of a strong late vaccinia virus promoter into cells infected with a vaccinia virus mutant that does not express one of the three proteins comprising this pathway (E10, A2.5, or G4) in the absence of IPTG. As a control, cells were also infected with the parental virus, vT7lacOI, which expresses all three proteins in the absence of IPTG. The results of the experiments with vE10i and vG4i are shown in Fig. 3B, and corresponding results were obtained with vA2.5i (data not shown). AMS was used to distinguish between reduced A28 with free reactive cysteines and oxidized A28 with unreactive disulfide bonds. A28 was completely reduced when expressed in cells unable to synthesize E10, A2.5, or G4 and mostly oxidized when expressed in cells making these proteins. We attribute incomplete disulfide bond formation under the latter conditions to the overexpression of A28 under the strong P11 promoter, as the same phenomenon was observed when other disulfide-bonded proteins were overexpressed (20). Thus, the expression of E10, A2.5, and G4 was required for the formation of disulfide bonds in A28.
Localization of A28 in virus particles.
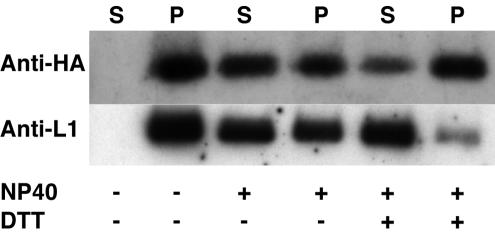
A28 was tightly associated with purified IMV and was not released by incubation in Tris (pH 7.5) buffer at 37°C for 30 min (Fig. 4). The similar sizes of A28 made in vitro and in infected cells (Fig. 3) suggested that the N-terminal hydrophobic sequence of A28 was not cleaved from the rest of the protein and most likely functions as a transmembrane anchor that holds the protein in the membrane of IMV. The distribution of A28 between nonionic-detergent-soluble and -insoluble fractions of purified virions was in agreement with its membrane association. At least 50% of A28 could be extracted from purified virions with NP-40 alone, and there was no difference, in this respect, between A28 and L1, a well-characterized IMV membrane protein (Fig. 4). Unexpectedly, but reproducibly, less A28 was extracted when dithiothreitol was included in addition to NP-40. It might be that A28 is less soluble, interacts with insoluble proteins of the core, or is more susceptible to degradation when artificially reduced in the absence of a denaturing agent.
FIG. 4.
Extraction of A28-HA from purified virions. Triplicate samples of sucrose gradient-purified virions were resuspended in Tris buffer alone (50 mM Tris, pH 7.5) or in Tris buffer containing (+) 1% NP-40 or 50 mM dithiothreitol and incubated for 30 min at 37°C. The soluble (S) and insoluble (P) fractions were separated by centrifugation and analyzed by Western blotting with anti-HA or anti-L1 polyclonal antibody (11).
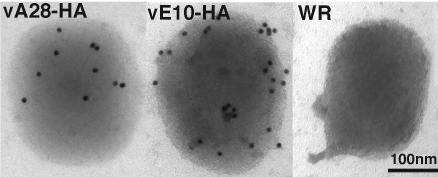
To determine the membrane orientation of the C-terminal portion of A28, purified A28-HA virions were incubated with anti-HA antibody and examined by immunoelectron microscopy. Purified E10-HA and wild-type WR virions were used as positive and negative controls, respectively. Gold particles conjugated to the secondary antibody were detected on the surfaces of both E10-HA and A28-HA virions, in contrast to the near absence of surface staining of control wild-type virions lacking an HA-tagged protein (Fig. 5). The fewer gold particles detected on A28-HA virions than on E10-HA virions probably reflected the relative amounts of the two proteins in virions. We also detected less A28-HA than E10-HA by Western blotting of extracts from the same number of infected cells or the same number of purified virions (data not shown), suggesting differences in the expression levels of the two proteins. Taken together, the data suggested that A28 is anchored in the IMV membrane via its hydrophobic N terminus, with the rest of the molecule exposed on the virion surface.
FIG. 5.
Immunoelectron microscopy of purified virions. Purified IMV preparations of A28-HA, E10-HA, or wild-type vaccinia virus WR were adsorbed to grids and stained with a mouse monoclonal anti-HA antibody, followed by anti-mouse antibody and protein A conjugated to gold particles.
A28 is essential for virus replication and plaque formation.
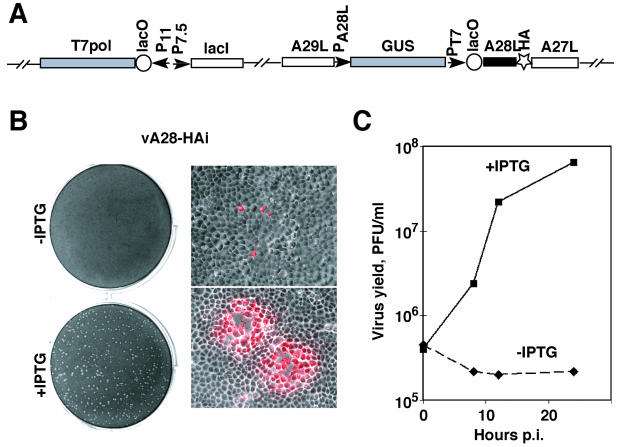
Because of the conservation of the A28L gene in all poxviruses, it seemed likely that its expression would be essential for virus replication in cell culture. To investigate this, we constructed the recombinant vaccinia virus vA28-HAi, which encodes the E. coli lac repressor continuously expressed by a vaccinia virus dual early-late promoter, the bacteriophage T7 RNA polymerase regulated by the lac operator and a vaccinia virus late promoter, and an HA epitope-tagged A28 under the control of the T7 promoter, which was also regulated by the lac operator (Fig. 6A). The lac repressor inhibited two consecutive steps, expression of T7 polymerase and of A28, ensuring high stringency of repression. The GUS gene was coinserted to allow selection of the recombinant virus, which was clonally purified. Controlled expression of A28 was achieved by addition of the desired concentration of IPTG. vA28-HAi formed plaques in the presence of IPTG (Fig. 6B, lower left) but not in its absence (Fig. 6B, upper left). At concentrations of 50 μM IPTG or higher, the plaques were similar in size to those of the parental virus, vT7lacOI (not shown). Although vA28-HAi did not form plaques in the absence of IPTG, at higher magnification we could detect single infected cells by their staining with antibody to vaccinia virus proteins (Fig. 6B, upper right). Cell-to-cell spread occurred only in the presence of IPTG (Fig. 6B, lower right). These data indicated that without IPTG, vA28-HAi exhibited a block in the formation or spread of infectious virus.
FIG. 6.
Effect of IPTG on replication of vA28-HAi. (A) Diagram of relevant portions of the vA28-HAi genome. The locations of the A29L, A28L-HA, A27L, and GUS ORFs are shown. P11, a vaccinia virus late promoter; P7.5, a vaccinia virus early-late promoter; PA28L, promoter of A28L gene; PT7, bacteriophage T7 promoter; lacO, E. coli lac operator; lacI, E. coli lac repressor ORF; T7 pol, bacteriophage T7 RNA polymerase ORF. (B) Plaque formation and virus spread. BS-C-1 cell monolayers were infected with vA28-HAi in the absence (top) or presence (bottom) of 100 μM IPTG. The cells were incubated for 48 h and then stained with crystal violet (left) or for 24 h and then fixed with 4% paraformaldehyde and stained with a high-titer rabbit polyclonal antivaccinia virus serum diluted 1:1,000 in phosphate-buffered saline containing 2% fetal bovine serum, followed by Texas Red-conjugated anti-rabbit antibody (right). The image on the right is a merge of phase-contrast and epifluorescence microscopy, with infected cells appearing red. (C) Effect of IPTG on the replication of vA28L-HAi under one-step growth conditions. Replicate samples of BS-C-1 cell monolayers were infected with 5 PFU of vA28L-HAi per cell, incubated for 1 h at 37°C, washed three times, and incubated further at the same temperature in the presence (+) or absence (−) of 100 μM IPTG. The cells were harvested at the indicated times postinfection (p.i.) and washed, and the virus titers were determined by plaque assay in the presence of 100 μM IPTG.
To differentiate between the above possibilities, we determined the yield of vA28-HAi in the presence or absence of IPTG under one-step growth conditions. The replication of vA28-HAi was entirely dependent on the addition of IPTG, as no increase in the amount of vA28-HAi was detected in the absence of inducer during a 24-h period (Fig. 6C). In contrast, in the presence of 100 μM IPTG, the amount of vA28-HAi increased >100-fold during this time. The kinetics of replication of vA28-HAi in the presence of IPTG was similar to that of the parental vT7lacOI (data not shown), except that the total accumulation of vA28-HAi was slightly reduced. The yield of vA28-HAi increased dramatically when the concentration of IPTG was raised from 0 to 50 μM; a further rise in the IPTG concentration to 1 mM had no effect on the virus yield (not shown). The dependence of A28 synthesis on IPTG is demonstrated elsewhere (16a).
Late-protein synthesis and processing of the major core proteins occur normally in the absence of A28.
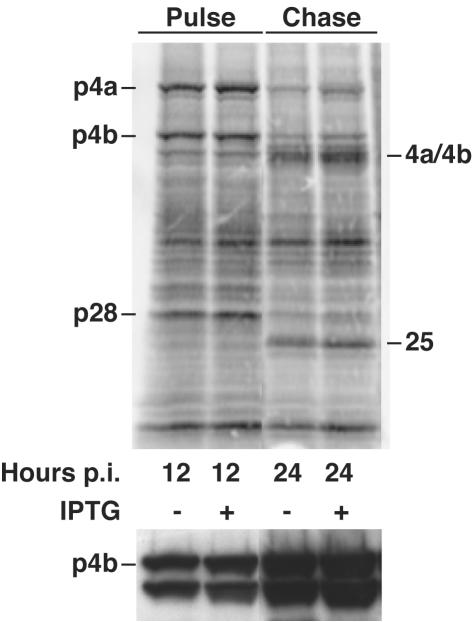
To determine whether virus replication was inhibited at an early or late stage, cells were infected with vA28-HAi in the presence or absence of inducer and were metabolically labeled with [35S]methionine at various times. The proteins were then analyzed by SDS-PAGE and autoradiography. We could discern no difference in the patterns of viral-protein bands or in their intensities in the presence or absence of IPTG at any time examined (Fig. 7, top, and data not shown). Furthermore, the p4a, p4b, and p28 core protein precursors, derived from the A10R, A3R, and L4R ORFs, were processed into their mature products, 4a, 4b, and a 25-kDa protein, during the chase (Fig. 7, top). Processing was confirmed by Western blotting with specific antibody that recognizes p4b and 4b. Both the precursor and product were detected at 12 and 24 h in the presence or absence of inducer (Fig. 7, bottom). Since the processing of core proteins is dependent on morphogenesis (9), these data suggested that morphogenesis occurred normally.
FIG. 7.
Synthesis of viral proteins in cells infected with vA28L-HAi in the presence (+) or absence (−) of IPTG. BS-C-1 cell monolayers were infected with vA28L-HAi at a multiplicity of 5 PFU per cell in the presence or absence of 100 μM IPTG as indicated. At 12 h postinfection (p.i.), duplicate cell monolayers were pulse-labeled with [35S]methionine for 30 min, and the cells were either harvested immediately (Pulse) or incubated with an excess of unlabeled methionine for an additional 12 h (Chase). The proteins were denatured with SDS and reducing agent, analyzed by electrophoresis in a 4 to 20% polyacrylamide gel, transferred to a nitrocellulose membrane, and analyzed by autoradiography (top) or Western blotting with anti-p4b/4b antibody (bottom).
Intracellular and extracellular virions are made in the absence of A28.
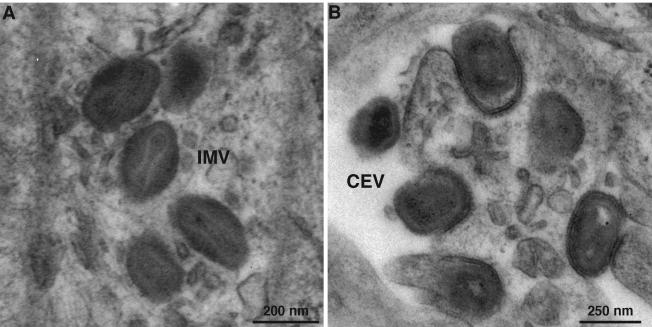
To directly examine the effect of A28 repression on morphogenesis, thin sections of cells infected with vA28-HAi in the presence or absence of IPTG were examined by transmission electron microscopy. Cells infected with vA28-HAi in the absence of IPTG showed the full range of viral structures, including IMV (Fig. 8A) and cell-associated enveloped virions (CEV) (Fig. 8B), which were indistinguishable from those produced by vA28-HAi in the presence of IPTG or wild-type virus (not shown). Our finding of mature virus particles was consistent with the observed normal processing of core proteins. Nevertheless, this result was unusual because the essential proteins of the IMV membrane that have been studied are needed for virus maturation.
FIG. 8.
Electron microscopy of cells infected with vA28-HAi in the absence of IPTG. BS-C-1 cell monolayers were infected with vA28-HAi in the absence of IPTG for 24 h, fixed, and embedded in EPON, and ultrathin sections were prepared. (A) IMV in the cytoplasm. (B) CEV at the cell surface. The increased electron density on the concave surface of the plasma membrane just under some CEV presumably represents the remains of the fused outer intracellular enveloped virion membrane.
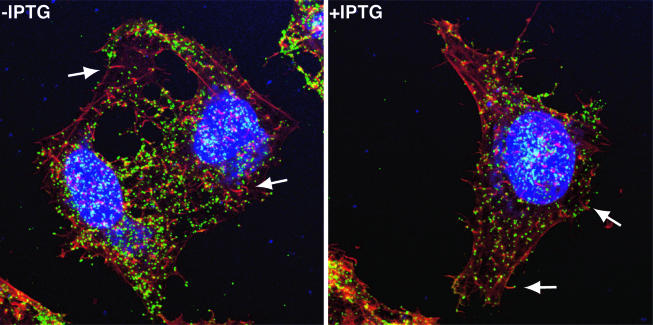
Our finding that the infection could not spread to neighboring cells in the absence of IPTG (Fig. 6B) seemed at odds with the detection of extracellular virus by electron microscopy (Fig. 8). Because cell-to-cell spread is mediated primarily by virions at the tips of actin tails, we analyzed HeLa cells that were infected with vA28-HAi in the presence or absence of IPTG by confocal microscopy. After 24 h of incubation, the cells were fixed, and the still unpermeabilized cells were stained with a monoclonal antibody to the extracellular domain of the B5 protein component of CEV and extracellular enveloped virions. After being washed, the cells were permeabilized and stained to detect actin filaments and DNA. The B5 protein was detected as punctate green fluorescence on the surfaces of cells infected in the presence and absence of IPTG (Fig. 9). Furthermore, numerous red actin tails with CEV at their tips were detected in both preparations. Thus, the failure of virus spread was not due to a defect in actin tail formation.
FIG. 9.
Detection of CEV and actin tails by confocal microscopy. HeLa cells were infected with vA28-HAi in the presence (+) or absence (−) of IPTG. After 24 h, the cells were fixed and stained with anti-B5 monoclonal antibody, followed by fluorescein isothiocyanate-conjugated goat anti-rat antibody (green). The cells were then washed and permeabilized prior to staining the DNA with diamidino-2-phenylindole dihydrochloride (blue) and staining filamentous actin with Alexa Fluor 568 phalloidin (red). The arrows point to CEV at the tips of actin tails.
DISCUSSION
Orthologs of vaccinia virus A28 are encoded in all completely sequenced poxvirus genomes, which comprise representatives from seven of the eight genera of chordopoxviruses and two distantly related entomopoxviruses. However, no homologs were detected in any nonpoxvirus genome, and our only clues to function were the presence of a typical late promoter, an N-terminal hydrophobic sequence, and four conserved cysteines. We demonstrated that A28 is expressed late in infection, is incorporated into virions, and contains two intramolecular disulfide bonds. The ability to extract A28 from purified virions with a nonionic detergent suggested that the protein is membrane associated. Moreover, immunoelectron microscopy demonstrated that the C-terminal segment is exposed on the virion surface, implying that the N-terminal hydrophobic segment anchors A28 into the viral membrane. This membrane topology places the four conserved cysteines in the relatively reducing cytoplasm. The L1 membrane protein and the less well characterized but structurally related F9 protein, which also have cytoplasmically oriented cysteines, require the functions of three conserved vaccinia virus proteins, namely, E10, A2.5, and G4, for disulfide bond formation (19). Here, we showed that E10, A2.5, and G4 were also required for the cysteines of A28 to form disulfide bonds, making it the third known substrate of the poxvirus cytoplasmic disulfide bond pathway. A28 and L1, however, differ in their topologies. The hydrophobic segment of L1 is located at the C terminus, opposite to that of A28.
The presence of A28 in all poxviruses and its high sequence conservation predicted an essential role in the virus life cycle. To investigate this role, we constructed a recombinant vaccinia virus in which the E. coli lac operator stringently regulated the expression of the A28L ORF. The recombinant virus, vA28-HAi, exhibited a conditional-lethal phenotype, as replication and virus spread were dependent on the addition of inducer. Stocks of vA28-HAi, prepared in the presence of inducer, were used to infect cells in its absence. In this way, we hoped to determine the step at which A28 is required in the poxvirus replication cycle. Previously, when this approach was used to analyze the roles of the redox proteins E10 (18), A2.5 (16), and G4 (25) or the L1 membrane protein (12), blocks in virus assembly and processing of core proteins were found. In contrast, the presence or absence of IPTG made no discernible difference in the synthesis or processing of viral proteins. Moreover, the assembly and morphogenesis of virions were unimpaired in the absence of A28, as demonstrated by electron microscopy. In the absence of IPTG, we also detected extracellular virus particles at the tips of actin tails by confocal microscopy. Although such particles are usually responsible for cell-to-cell spread of vaccinia virus, such spread did not occur, suggesting that virions made in the absence of IPTG and lacking A28 were not infectious. In another study, we purified A28-deficient virions and demonstrated that they are noninfectious because of their inability to penetrate cells for initiation of a second round of replication.
Acknowledgments
We thank Andrea Weisberg for electron microscopy and Norman Cooper for providing cells. Dennis Hruby kindly provided L1-specific polyclonal antibody.
REFERENCES
- 1.Alexander, W. A., B. Moss, and T. R. Fuerst. 1992. Regulated expression of foreign genes in vaccinia virus under the control of bacteriophage T7 RNA polymerase and the Escherichia coli lac repressor. J. Virol. 66:2934-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 21:1904-1907. [DOI] [PubMed] [Google Scholar]
- 4.Dales, S., and E. H. Mosbach. 1968. Vaccinia as a model for membrane biogenesis. Virology 35:564-583. [DOI] [PubMed] [Google Scholar]
- 5.Davison, A. J., and B. Moss. 1989. The structure of vaccinia virus late promoters. J. Mol. Biol. 210:771-784. [DOI] [PubMed] [Google Scholar]
- 6.Earl, P. L., B. Moss, L. S. Wyatt, and M. W. Carroll. 1998. Generation of recombinant vaccinia viruses, p. 16.17.1-16.17.19. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene Publishing Associates and Wiley Interscience, New York, N.Y.
- 7.Hollinshead, M., A. Vanderplasschen, G. L. Smith, and D. J. Vaux. 1999. Vaccinia virus intracellular mature virions contain only one lipid membrane. J. Virol. 73:1503-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husain, M., and B. Moss. 2003. Evidence against an essential role of COPII-mediated cargo transport to the endoplasmic reticulum-Golgi intermediate compartment in the formation of the primary membrane of vaccinia virus. J. Virol. 77:11754-11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz, E., and B. Moss. 1970. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc. Natl. Acad. Sci. USA 6:677-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moss, B. 1996. Poxviridae: the viruses and their replication, p. 2637-2671. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa.
- 11.Ravanello, M. P., C. A. Franke, and D. E. Hruby. 1993. An NH2-terminal peptide from the vaccinia virus L1R protein directs the myristylation and virion envelope localization of a heterologous fusion protein. J. Biol. Chem. 268:7585-7593. [PubMed] [Google Scholar]
- 12.Ravanello, M. P., and D. E. Hruby. 1994. Conditional lethal expression of the vaccinia virus L1R myristylated protein reveals a role in virus assembly. J. Virol. 68:6401-6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risco, C., J. R. Rodriguez, C. Lopez-Iglesias, J. L. Carrascosa, M. Esteban, and D. Rodriguez. 2002. Endoplasmic reticulum-Golgi intermediate compartment membranes and vimentin filaments participate in vaccinia virus assembly. J. Virol. 76:1839-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rost, B., C. Sander, and R. Schneider. 1994. PHD—an automatic mail server for protein secondary structure prediction. Comput. Appl. Biosci. 10:53-60. [DOI] [PubMed] [Google Scholar]
- 15.Schmelz, M., B. Sodeik, M. Ericsson, E. J. Wolffe, H. Shida, G. Hiller, and G. Griffiths. 1994. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans-Golgi network. J. Virol. 68:130-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senkevich, T., C. White, A. Weisberg, J. Granek, E. Wolffe, E. Koonin, and B. Moss. 2002. Expression of the vaccinia virus A2.5L redox protein is required for virion morphogenesis. Virology 300:296-303. [DOI] [PubMed] [Google Scholar]
- 16a.Senkevich, T., B. M. Ward, and B. Moss. 2004. Vaccinia virus entry into cells is dependent on a virion membrane surface protein encoded by the A28L gene. J. Virol. 78:2357-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senkevich, T. G., E. V. Koonin, J. J. Bugert, G. Darai, and B. Moss. 1997. The genome of molluscum contagiosum virus: analysis and comparison with other poxviruses. Virology 233:19-42. [DOI] [PubMed] [Google Scholar]
- 18.Senkevich, T. G., A. Weisberg, and B. Moss. 2000. Vaccinia virus E10R protein is associated with the membranes of intracellular mature virions and has a role in morphogenesis. Virology 278:244-252. [DOI] [PubMed] [Google Scholar]
- 19.Senkevich, T. G., C. L. White, E. V. Koonin, and B. Moss. 2002. Complete pathway for protein disulfide bond formation encoded by poxviruses. Proc. Natl. Acad. Sci. USA 99:6667-6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senkevich, T. G., C. L. White, E. V. Koonin, and B. Moss. 2000. A viral member of the ERV1/ALR protein family participates in a cytoplasmic pathway of disulfide bond formation. Proc. Natl. Acad. Sci. USA 97:12068-12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sodeik, B., R. W. Doms, M. Ericsson, G. Hiller, C. E. Machamer, W. van't Hof, G. van Meer, B. Moss, and G. Griffiths. 1993. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J. Cell Biol. 121:521-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson, C. L., and R. C. Condit. 1986. Marker rescue mapping of vaccinia virus temperature-sensitive mutants using overlapping cosmid clones representing the entire virus genome. Virology 150:10-20. [DOI] [PubMed] [Google Scholar]
- 23.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward, G. A., C. K. Stover, B. Moss, and T. R. Fuerst. 1995. Stringent chemical and thermal regulation of recombinant gene expression by vaccinia virus vectors in mammalian cells. Proc. Natl. Acad. Sci. USA 92:6773-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White, C. L., T. G. Senkevich, and B. Moss. 2002. Vaccinia virus G4L glutaredoxin is an essential intermediate of a cytoplasmic disulfide bond pathway required for virion assembly. J. Virol. 76:467-472. [DOI] [PMC free article] [PubMed] [Google Scholar]