Abstract
Bovine herpesvirus 4 (BoHV-4) is a gammaherpesvirus that has a worldwide distribution in the population of cattle. Many factors make human contamination by BoHV-4 likely to occur. In this study, we performed in vitro experiments to assess the risk and the consequences of human infection by BoHV-4. First, by using a recombinant BoHV-4 strain expressing enhanced green fluorescent protein under the control of the human cytomegalovirus immediate-early gene promoter, we tested 21 human cell lines for their sensitivity and their permissiveness to BoHV-4 infection. These experiments revealed that human cell lines from lymphoid and myeloid origins were resistant to infection, whereas epithelial cells, carcinoma cells, or adenocarcinoma cells isolated from various organs were sensitive but poorly permissive to BoHV-4 infection. Second, by using the HeLa cell line as a model of human cells sensitive but not permissive to BoHV-4 infection, we investigated the resistance of infected cells to apoptosis and the persistence of the infection through cellular divisions. The results obtained can be summarized as follows. (i) BoHV-4 nonpermissive infection of HeLa cells protects them against tumor necrosis factor alpha-induced apoptosis. (ii) BoHV-4 infection of HeLa cells persists in cell culture; however, the percentage of infected cells decreases with time due to erratic transmission of the viral genome through cell division. (iii) BoHV-4 infection has no effect on the rate of HeLa cell division. Altogether, these data suggest that BoHV-4 could infect humans. This study also stresses the importance of considering the insidious effects of nonpermissive infection when the biosafety of animal gammaherpesviruses for humans is being considered.
Bovine herpesvirus 4 (BoHV-4) belongs to the Herpesviridae family, the Gammaherpesvirinae subfamily, and the Rhadinovirus genus (66). BoHV-4 has been isolated throughout the world from healthy cattle, as well as from cattle exhibiting a variety of diseases. The biological life cycle of BoHV-4 relies, as for all Herpesviridae, on the existence of two types of infection: lytic (or replicative) and latent infections. The former leads to the production of progeny virions and generally to the lysis of the infected cell. During a lytic infection, the expression of herpesvirus proteins is temporally regulated. The proteins are classified into three kinetic classes depending on the order of their synthesis during the infection (49). These proteins are expressed chronologically as immediate-early (IE), early (E), and late (L) proteins. IE proteins are expressed directly after release of the viral genome from the capsid into the nucleus. Although E protein expression occurs after synthesis of IE proteins, L protein expression depends on the expression of both IE and E proteins and viral DNA synthesis. Latent infection consists of a dormant state associated with the expression of a limited number of viral genes which might affect the biology of the infected cell. Various stimuli can turn the latent infection into a lytic infection, a phenomenon known as reactivation.
The replication of most gammaherpesviruses is restricted to their natural host species. BoHV-4 is one of the few exceptions to this rule. Indeed, it has been shown that BoHV-4 is able to replicate in a broad range of host species both in vivo and in vitro. In addition to cattle, isolates of BoHV-4 have been recovered from other ruminant species such as zebu (Bos indicus) (42), American bison (Bison bison) (56), African buffalo (Syncerus caffer) (50), and sheep (59). Sporadic isolations were reported in the lion, the cat, and the owl monkey (Aotus trivirgatus) (1, 7). Experimentally, BoHV-4 was also shown to infect goats (42), guinea pigs, and rabbits (18). In vitro, BoHV-4 is able to replicate in primary cell cultures or cell lines from various animal species such as cattle, sheep, goats, swine, cats, dogs, rabbits, minks, horses, turkeys, ferrets, chickens, hamsters, rats, mice, and monkeys (1, 2, 11, 17, 20, 32, 33, 47, 53). In addition, preliminary studies have shown that some human cell lines support BoHV-4 replication, leading to the hypothesis that this virus could represent a risk to human health (11, 12, 17).
The risk assessment associated with the cross-species transmission of a virus needs to address two main factors: the risk of transmission and the consequences for the infected host in case of transmission. The risk of transmission depends on the virus prevalence in the environment, on the existence of events permitting the transmission of the virus and, finally, on the capacity of the virus to infect the nonnatural host. In relation to these factors, several observations support the existence of a risk of BoHV-4 transmission to humans. First, BoHV-4 is highly prevalent in the population of cattle (54), and no eradication scheme is directed against this virus. Second, many facts enable the transmission of the virus from cattle to humans: (i) infected animals excrete BoHV-4 in nasal and vaginal discharges both after primary infection and after reactivation (54), making human contamination possible for people having contact with infected cattle; (ii) BoHV-4 has been found in the milk of cows with mastitis (13, 62, 63), as well as from apparently healthy cows, suggesting possible human contamination by the oral route; and (iii) BoHV-4 is frequently isolated from bovine serum (7), which is used abundantly in food and pharmacological preparations, making human contamination by BoHV-4 possible by enteral or parenteral routes. Third, as mentioned above, earlier studies have revealed the capacity of some human cell lines to support BoHV-4 replication, suggesting the ability of the virus to infect humans (11, 12, 17).
The second factor to be addressed when the risk assessment associated with the cross-species transmission of a virus is being considered is the consequence(s) for the infected host. If cells of the nonnatural infected host are permissive to the infection, lesions can be induced by replication of the virus. Alternatively, nonpermissive infection, resulting from latent infection or from infection of cells that are sensitive but not permissive, can lead to dramatic effects such as oncogenesis or disregulation of the immune system. Cross-species transmission of some gammaherpesviruses has been shown to cause such phenomena. For example, alcelaphine herpesvirus 1 induces a lethal proliferative disease when it infects ruminants other than wildebeest (Connochaetes taurinus) (48). The pathogenesis of such diseases relies on the expression of a restricted number of viral genes able to immortalize, to transform, or to confer resistance to apoptosis to the host cell. In comparison to other gammaherpesviruses, BoHV-4 encodes a low number of genes susceptible to affect the biology of the infected cells (66). However, BoHV-4 possesses two genes that could protect the infected cells against apoptosis: ORF16 and ORF71 encoding a v-Bcl-2 and a v-FLIP, respectively. BoHV-4 ORF71 was previously shown to inhibit Fas- and tumor necrosis factor alpha (TNF-α)-induced apoptosis when overexpressed transiently in human HeLa cells (61).
Because many factors make human contamination by BoHV-4 likely to occur, we performed in vitro experiments to assess the risk and the consequences of human infection by BoHV-4. The present study suggests that human contamination by BoHV-4 could lead to viral replication in permissive cells and/or to nonpermissive persistent infection protecting the infected cells from apoptosis in cells that are sensitive but not permissive to BoHV-4 infection. The present study highlights the importance of assessing the risk and the consequences of human contamination by animal gammaherpesviruses. It also stresses the point that those who assess the biosafety of animal gammaherpesviruses, as an expression vector in vivo, need to take into consideration the insidious effect of nonpermissive infection on the biology of the infected cell.
MATERIALS AND METHODS
Cell lines and virus strains.
In addition to Madin-Darby bovine kidney cells (MDBK) (ATCC CCL-22), 21 human cell lines were used in the present study. These are listed in Table 1. Unless otherwise stated, all cell lines were cultured according to the recommendations of the American Type Culture Collection (http://www.atcc.org). The BoHV-4 V.test strain initially isolated from a case of orchitis (55) and a derived recombinant strain, called V.test EGFP XhoI, were used throughout the present study. The latter carries an enhanced green fluorescent protein (EGFP) expression cassette under control of the human cytomegalovirus IE gene promoter/enhancer (37). The EGFP cassette was inserted in the far left XhoI site of BoHV-4 L-DNA, a region which does not contain any open reading frames (ORFs) (66). Comparison of the parental V.test strain and the derived V.test EGFP XhoI strain revealed no difference in viral replication both by single-round and multiple-step assays (M. Lambot, unpublished data).
TABLE 1.
Susceptibility of human cell lines to BoHV-4 infectiona
| Group | Source | Cell line | ATCC no. | Origin | BoHV-4 gene expression (%)
|
||
|---|---|---|---|---|---|---|---|
| EGFP | MAb 35 α-E/L | MAb 29 α-L | |||||
| Control | Bovine | MDBK | CCL-22 | Kidneyb | 71.1 | 39.8 | 38.8 |
| Group 1 | Human | U-266 | ACC 9 | Multiple myeloma | 1.2 | 0 | 0 |
| Human | U-937 | CRL-1593.2 | Histiocytic lymphoma | 0 | 0 | 0 | |
| Human | KARPAS-45 | ACC 105 | T-cell leukemia | 1 | 0 | 0 | |
| Human | MOLT-3 | CRL-1552 | T-cell leukemia | 0 | 0 | 0 | |
| Human | MOLT-4 | CRL-1582 | T-cell leukemia | 0 | 0 | 0 | |
| Human | Jurkat | ACC 282 | T-cell leukemia | 0 | 0 | 0 | |
| Human | L-540 | ACC 72 | Hodgkin's lymphoma | 0 | 0 | 0 | |
| Human | KM-H2 | ACC 8 | Hodgkin's lymphoma | 0 | 0 | 0 | |
| Human | DOHH-2 | ACC 47 | B-cell lymphoma | 0 | 0 | 0 | |
| Human | GRANTA-519 | ACC 342 | B-cell lymphoma | 0 | 0 | 0 | |
| Human | NALM-6 | ACC 128 | B-cell leukemia | 0 | 0 | 0 | |
| Human | Daudi | CCL-213 | Burkitt's lymphoma | 0 | 0 | 0 | |
| Human | Raji | CCL-86 | Burkitt's lymphoma | 0 | 0 | 0 | |
| Group 2 | Human | 293 | CRL-1573 | Kidneyc | 72 | 6 | 3 |
| Human | MCF7 | HTB-22 | Breast adenocarcinoma | 13.3 | 2.9 | 1.5 | |
| Human | MDA-MB-231 | HTB-26 | Breast adenocarcinoma | 19 | 2.7 | 0.3 | |
| Human | SK-BR-3 | HTB-30 | Breast adenocarcinoma | 37.2 | 3 | 1.2 | |
| Human | Hs 578T | HTB-126 | Breast carcinoma | 50 | 5 | 3.8 | |
| Human | DU-145 | ACC 261 | Prostate carcinoma | 7 | 2 | 1 | |
| Human | PC-3 | CRL-1435 | Prostate adenocarcinoma | 10 | 0.8 | 0.7 | |
| Human | HeLad | CCL-2 | Cervix adenocarcinoma | 35 | 0.2 | 0.1 | |
A total of 21 human cell lines and MDBK cells (used as a positive control) were infected at an MOI of 0.5 PFU/cell with the strain V.test EGFP XhoI of BoHV-4. At 30 h postinfection, the cells were harvested and treated as described in Materials and Methods for the detection of EGFP, MAb 35 epitope, and MAb 29 epitope expressed as IE, E-L, and L proteins, respectively. The numbers indicated in the right three columns represent the percentages of positive cells (n = 10,000 cells). Based on the results obtained, the cell lines can be classified into two groups: group 1 cell lines are resistant or poorly sensitive to BoHV-4 infection. Group 2 cell lines are sensitive but poorly permissive to BoHV-4 infection.
That is, normal epithelial cells.
That is, adenovirus 5 DNA-transformed epithelial cells.
HeLa cells were kindly provided by H. Wajant (University of Stuttgart, Stuttgart, Germany).
MAbs.
Two mouse monoclonal antibodies (MAbs) raised against BoHV-4 were used in the present study. MAb 35 recognizes the early-late (E-L) glycoprotein complex gp6/gp10/gp17 (16). The E-L classification of the latter complex is based on the fact that a precursor of two components (gp10/gp17) is expressed in the E phase, whereas the mature form (gp6/gp10/gp17) is expressed in the L phase (16). MAb 35 recognizing both the precursor and the mature forms is therefore used as a marker of E-L protein expression (16). MAb 29 recognizes the L glycoprotein gp11/vp24 (15).
Indirect immunofluorescence staining for flow cytometry.
Adherent cells were harvested with trypsin-EDTA and fixed for 30 min on ice in phosphate-buffered saline (PBS; 3 mM KCl, 1.5 mM KH2PO4, 0.14 M NaCl, 6.5 mM Na2HPO4 [pH 7.2]) containing 1% (wt/vol) paraformaldehyde (Merck, Darmstadt, Germany). After being washed with PBS, the samples were permeabilized in 70% (70:30 [vol/vol]) ethanol at 4°C for 15 min. The samples were then extensively washed with PBS. Immunofluorescence staining (incubation and washes) was performed in PBS containing 10% fetal calf serum (BioWhittaker, Verviers, Belgium). The samples were incubated at 37°C for 45 min with MAb 29 (diluted 1/500) or 35 (diluted 1/1,000) as primary antibody. After three washes, the samples were incubated at 37°C for 30 min with R-phycoerythrin (PE)-conjugated F(ab′)2 goat anti-mouse immunoglobulins (PE-GAM; 5 μg/ml; Dako, Glostrup, Denmark) as secondary conjugate. After a final wash, the cells were analyzed by flow cytometry.
Cell viability.
Cell viability was assayed with the cell-impermeant dye propidium iodide (PI; Sigma-Aldrich, St. Louis, Mo.). Just before flow cytometry analysis, PI was added to the samples at a final concentration of 0.1 μg/ml.
Induction and detection of apoptosis in HeLa cells.
HeLa cells were grown in RPMI 1640 (Invitrogen Corp., Carlsbad, Calif.) supplemented with 10% fetal calf serum as recommended by the ATCC. To induce apoptosis, cells were incubated in RPMI 1640 containing 2% horse serum (BioWhittaker), 10 ng of human TNF-α (hTNF-α; Roche, Mannheim, Germany)/ml, and 1 μg of cycloheximide (CHX; Sigma-Aldrich)/ml as described elsewhere (60). After 24 h, the cells were harvested with trypsin-EDTA and stained with annexin V-PE (Becton Dickinson, Erembodegem, Belgium) for the detection of apoptosis by flow cytometry according to the recommendations of the manufacturer.
Quantitative measurement of the number of dividing cells in the S phase.
The number of cells going through the S phase per hour was estimated by measuring the incorporation of 5-bromo-2′-deoxyuridine (BrdU; Becton Dickinson) as described elsewhere with minor modifications (29). Briefly, cells were pulse-labeled for 60 min at 37°C by the addition of BrdU (final concentration, 20 μM) to the culture medium. At the end of the incubation period, the cells were harvested with trypsin-EDTA, washed with ice-cold PBS, and incubated overnight at 4°C in PBS containing 1% (wt/vol) paraformaldehyde and 0.05% (wt/vol) NP-40 (Fluka, Buchs, Switzerland). After being washed with PBS containing 1% (wt/vol) glycine (Sigma-Aldrich), partial DNA denaturation was induced by incubation in PBS supplemented with 1 mg of DNase I (Sigma DN-25; 400 to 600 Kunitz units/mg; Sigma-Aldrich)/ml, 0.005 M CaCl2, and 0.01 M MgCl2. After an additional wash with PBS, the cells were resuspended in 50 μl of PBS containing 0.1% (wt/vol) bovine serum albumin (Sigma-Aldrich) and 0.5% (wt/vol) NP-40. BrdU staining was then performed by adding of 50 μl of anti-BrdU-R-PE-conjugated mouse MAb (Becton Dickinson) for 45 min at room temperature. Finally, cells were washed twice with PBS plus 0.1% (wt/vol) bovine serum albumin prior to flow cytometry analysis.
Cell membrane labeling.
For long-term labeling and tracking of cells, cellular membranes were loaded with the nontoxic lipophilic fluorescent marker DilC18(5)-DS (Molecular Probes, Leiden, The Netherlands) according to the instructions of the manufacturer. Briefly, cells were harvested with tryspin-EDTA, washed with PBS, and then resuspended in 1 ml of PBS containing DilC18(5)-DS at a final concentration of 2 μg/ml. The cells were incubated for 5 min at 37°C and then for an additional 5 min at 4°C. Finally, they were transferred in 10 ml of PBS at 4°C, centrifuged, and resuspended in the appropriate medium for cell culture.
Confocal microscopy analysis.
Confocal microscopy analyses were performed with a TCS SP confocal microscope (Leica, Heerbrugg, Switzerland) as described previously (58).
Flow cytometry.
Flow cytometry analyses were performed by using a Becton Dickinson fluorescence-activated cell sorter (FACStar Plus) equipped with an argon ion laser (Innova Technology with a 100-mW excitation line at 488 nm) as described elsewhere (57). Cell sorting was performed with the same flow cytometer by using an automatic cell deposit unit (Becton Dickinson).
Detection of viral DNA by PCR in sorted cells.
Cells positive or negative for EGFP expression were sorted from a cell culture infected with the BoHV-4 V.test EGFP XhoI strain. DNA was then purified from sorted cells by using the Easy-DNA kit (Invitrogen Corp.). The presence of BoHV-4 DNA was investigated by PCR assays targeting three ORFs of the viral genome: the Bo5 encoding IE 1 protein, ORF22 encoding glycoprotein H, and the EGFP ORF. In order to control the presence of cellular DNA in each sample, a sequence encoding cellular membrane cofactor protein (MCP) was amplified. The primers used for the amplifications are described in Table 2.
TABLE 2.
Primers used in this study
| Sequence | GenBank accession no. | Orientation | Primera | Expected size of PCR product (bp) |
|---|---|---|---|---|
| Viral sequences | ||||
| Bo5 | AF318573 | Forward | 5′-GCTACAGAAAATGGCCAGTAAAG-3′ | 1,149 |
| Reverse | 5′-TCATGTCCTGAGTGGGTCTATG-3′ | |||
| ORF22 | AF318573 | Forward | 5′-CCGGGTGAAACAAGTTCCTG-3′ | 563 |
| Reverse | 5′-GGTCAGAGAACATATGATAACATC-3′ | |||
| EGFP | U55762.1 | Forward | 5′-GGAATTCCATGGTGAGCAAGGGCGAGG-3′ | 734 |
| Reverse | 5′-GAAGCTTTTACTTGTACAGCTCGTCCATG-3′ | |||
| Cellular sequence (MCP) | NM_172361 | Forward | 5′-TGTGAGGAGCCACCAACA-3′ | 182 |
| Reverse | 5′-ATAACAGGCGTCATCTGA-3′ |
The pairs of primers used in this study were designed based on the sequences available in the GenBank database in order to amplify BoHV-4 or the cellular ORFs listed in the left column.
Statistical analysis.
A Student t test was used to evaluate the significance of the results (P ≤ 0.01).
RESULTS
The risk assessment and the consequences of human infection by BoHV-4 need to be addressed for several reasons. First, as mentioned above, human contact with BoHV-4 is likely to happen. Second, BoHV-4, in contrast to most gammaherpesviruses, has a broad range of host species, and preliminary studies have revealed the ability of some human cell lines to support BoHV-4 replication. Third, cross-species transmission of some gammaherpesviruses has been proven to be the cause of serious diseases even when the virus is nonpathogenic for its natural host species. For all of these reasons, we performed in vitro experiments to address the risk and the consequences of human infection by BoHV-4 as precisely defined in the introduction.
Susceptibility of human cell lines to BoHV-4 infection.
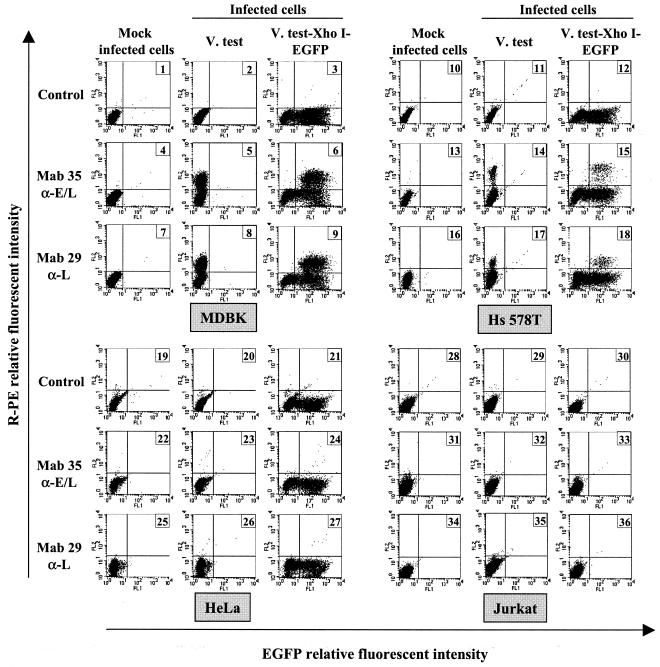
The risk of human infection after contact with BoHV-4 relies first of all on the capacity of human cells to support BoHV-4 entry (cell sensitivity to the infection) and eventually BoHV-4 replication (cell permissivity to the infection). To address this issue in detail, 21 human cell lines from different origins were infected with the BoHV-4 recombinant V.test EGFP XhoI strain expressing EGFP as an IE reporter gene (Table 1 and Fig. 1). Indirect immunofluorescence staining of infected cells with MAb 35 (anti-E-L) or MAb 29 (anti-L), followed by flow cytometry analysis, was conducted, allowing the identification of cells supporting BoHV-4 entry (cells expressing EGFP), E-L (MAb 35-positive cells), and L (MAb 29-positive cells) protein expression. The data presented in Table 1 show that the cell lines tested can be classified into two groups. A first group consisted of cell lines that are resistant or very poorly sensitive (U-266 and KARPAS-45 cell lines) to BoHV-4 infection (Table 1, group 1). The cell lines belonging to this group are lymphoid or myeloid cell lines. The possibility that the absence of EGFP expression in these cell lines resulted from the nonexpression of the EGFP expression cassette encoded by the BoHV-4 V.test EGFP XhoI strain, rather than from the absence of infection, was excluded by transfection studies. Transfection of BoHV-4 V.test EGFP XhoI DNA in these cells by electroporation led to EGFP-expressing cells (data not shown). A second group comprised cell lines that are sensitive but poorly permissive to BoHV-4 infection (Table 1, group 2). The percentage of EGFP-expressing cells in this group varied from 72% (293 cell line) to 7% (DU-145 cell line), whereas E-L and L protein-expressing cells fluctuated between 6 and 0.2% (293-HeLa cell lines) and between 3.8 and 0.1% (Hs 578T-HeLa cell lines), respectively. When compared to the results obtained with MDBK cells (Table 1 and panels 1 to 9 of Fig. 1), these data indicate that BoHV-4 replication is restricted at a postentry stage in these human cell lines. Titration of infectious particles in the supernatant of infected cells confirmed that BoHV-4 replication was extremely low (for example, the titer of the supernatant of HeLa infected cells was 0.8 PFU/ml). The cell lines belonging to this second group are either kidney epithelial cells or carcinoma or adenocarcinoma cells isolated from various organs (Table 1).
FIG. 1.
Viral gene expression in BoHV-4-infected cells. MDBK (panels 1 to 9), Hs 578T (panels 10 to 18), HeLa (panels 19 to 27) and Jurkat (panels 28 to 36) cells were mock infected or were infected at a multiplicity of infection (MOI) of 0.5 PFU/cell with the BoHV-4 strain V.test or V.test EGFP XhoI. At 30 h postinfection, the cells were harvested and treated for detection of viral gene expression by indirect immunofluorescence staining as described in Materials and Methods. MAb 35 raised against the E-L glycoprotein complex gp6/gp10/gp17 and MAb 29 raised against the L glycoprotein gp11/vp24 were used as the first antibody and were revealed by using PE-GAM. Cells were analyzed by flow cytometry for simultaneous detection of EGFP and PE emissions.
Effect of nonpermissive BoHV-4 infection on the resistance of human cells to TNF-α-induced apoptosis.
The results presented above revealed that some human cell lines (Table 1, group 2) are sensitive but poorly permissive to BoHV-4 infection. For some gammaherpesviruses, nonpermissive infection has been proven to have important consequences on the biology of the infected cells such as immortalization, transformation, or resistance to apoptotic signals. These effects are due to the expression of a restricted number of viral genes. BoHV-4 genome has been sequenced completely (66). In comparison to other gammaherpesviruses, BoHV-4 encodes a low number of genes likely to affect the biology of the infected cells. However, BoHV-4 possesses two genes that could protect the infected cells against apoptosis: ORF16 and ORF71 encoding a v-Bcl-2 and a v-FLIP, respectively. BoHV-4 ORF71 was previously shown to inhibit Fas- and TNF-α-induced apoptosis when overexpressed transiently in human HeLa cells (61). ORF16 and ORF71 are expressed as E and IE genes, respectively (F. Minner, unpublished data). Reverse transcriptase PCR experiments performed on BoHV-4-infected HeLa cell culture 10 days postinfection showed that mRNAs encoding v-Bcl-2 and v-FLIP are produced (data not shown). Altogether, these data suggest that nonpermissive infection of human cells by BoHV-4 could protect them against apoptosis.
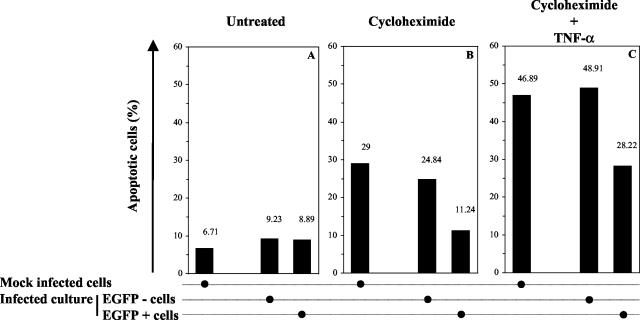
To test this hypothesis, HeLa cells were infected with the recombinant BoHV-4 strain V.test EGFP XhoI. HeLa cells were selected for this experiment since the induction of apoptosis has been studied extensively in this cell line (60, 61). At 24 h postinfection, apoptosis was induced by CHX-hTNF-α treatment as described in Materials and Methods. After an additional 24 h, the cells were analyzed by flow cytometry for simultaneous detection of EGFP expression and apoptosis. Analysis of infected HeLa cell culture treated with CHX-hTNF-α showed that EGFP-expressing cells were more resistant to apoptosis than EGFP-negative cells of the same culture (P ≤ 0.01) (Fig. 2C). The latter cells exhibited the same sensitivity to apoptosis as mock-infected cells treated identically (P ≤ 0.01). Control cultures (Fig. 2B), treated with CHX only, confirmed that EGFP-expressing cells were more resistant to apoptosis than were EGFP-negative cells from the same culture or from a mock-infected culture. No difference of sensitivity to apoptosis was observed between untreated cell cultures (Fig. 2A). Altogether, these results showed that nonpermissive infection of HeLa cells by BoHV-4 protects infected cells against TNF-α-induced apoptosis.
FIG. 2.
Effect of BoHV-4 infection on the susceptibility of HeLa cells to TNF-α-induced apoptosis. HeLa cells were mock infected or were infected with the recombinant strain V.test EGFP XhoI of BoHV-4 at an MOI of 0.5 PFU/cell. At 24 h after infection, apoptosis was induced by CHX-hTNF-α treatment as described in Materials and Methods. The resulting apoptotic effect was assayed 24 h later by using Annexin V-PE labeling, followed by flow cytometry analysis. Panels A, B, and C represent cultures mock treated, treated with CHX, and treated with CHX-hTNF-α, respectively. For infected cultures, the percentage of apoptotic cells was determined for EGFP-positive and -negative cells. Each value represents the percentage of apoptotic cells based on an analysis of 10,000 cells.
Persistence and viability of EGFP-expressing cells in HeLa cell cultures infected with the BoHV-4 strain V.test EGFP XhoI.
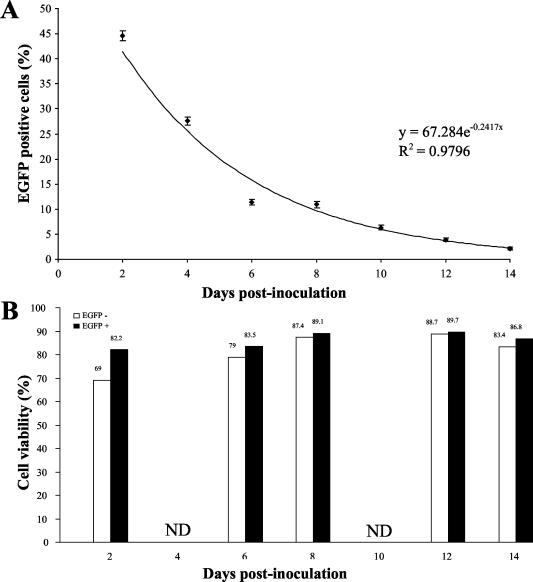
The results presented above revealed that nonpermissive infection of HeLa cells by BoHV-4 protects infected cells against TNF-α-induced apoptosis. If this phenomenon occurs in vivo, its relevance will depend on the viability of the infected cell and on its persistence in the infected organism. In order to address these parameters in vitro, HeLa cells were infected with the recombinant BoHV-4 strain V.test EGFP XhoI and cultured for 14 days as described in Fig. 3. At different time points postinfection, the percentage of EGFP-positive cells and the viability of EGFP-positive and -negative cells were determined by flow cytometry. As shown in Fig. 3A, the percentage of EGFP-positive cells in the HeLa infected cell culture decreased exponentially [y = 67.284 e−0.2417(X), R2 = 0.9796] with time. This reduction could not be explained by a reduced viability of EGFP-expressing cells as shown in panel B (Fig. 3). Indeed, in the course of this experiment the viability of EGFP-expressing cells was comparable to or even higher (at 2 days postinfection [P ≤ 0.01]) than that observed for EGFP-negative cells.
FIG. 3.
Persistence and viability of infected cells in HeLa cell cultures infected with the BoHV-4 strain V.test EGFP XhoI. HeLa cells were infected with the BoHV-4 V.test EGFP XhoI strain at an MOI of 0.5 PFU/cell. The cells were then passaged every other day (1:2 split ratio). At the indicated times, the percentages of EGFP-expressing cells (A) and cell viability (B) were determined by flow cytometry as described in Materials and Methods. Cell viability was estimated for EGFP-positive and -negative cells. Each value represents the percentage of EGFP-positive cells (A) or viable cells (B) based on an analysis of 10,000 cells.
Effect of BoHV-4 infection on the rate of division of HeLa cells.
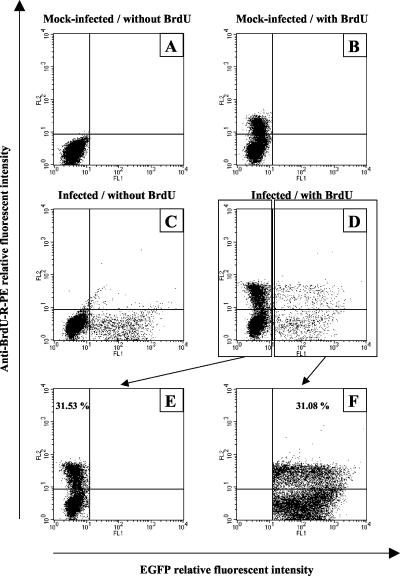
The results presented above revealed that the exponential reduction of EGFP-positive cells in a BoHV-4-infected HeLa cell culture could not be explained by a deleterious effect of the infection on cell viability. Alternatively, the reduction of EGFP-positive cells could be explained by a slower rate of division of infected cells in comparison to the noninfected cells (EGFP-negative cells) of the culture. To test this hypothesis, the number of cells going through the S phase per hour was estimated for EGFP-positive and EGFP-negative cells (Fig. 4). Flow cytometry analysis of 10,000 EGFP-negative (Fig. 4E) or EGFP-positive (Fig. 4F) cells from the same culture for BrdU incorporation revealed that both cell types have a similar rate of division (P ≤ 0.01).
FIG. 4.
Rate of cellular division of EGFP-positive and EGFP-negative cells in HeLa cell culture infected with the BoHV-4 V.test EGFP XhoI strain. HeLa cells were mock infected (A and B) or infected with the BoHV-4 V.test EGFP XhoI strain at an MOI of 0.5 PFU/cell (C and D). The cells were then passaged every other day for 8 days (1:2 split ratio). At 9 days postinfection, the cells were mock pulsed (A and C) or pulsed with BrdU (B and D) for 1 h as described in Materials and Methods. Cells positive for the incorporation of BrdU were then revealed by immunofluorescence staining with anti-BrdU-R-PE and analyzed by flow cytometry for the emission of green (EGFP) and red (anti-BrdU-R-PE) signals. By using the sample of infected cells pulsed with BrdU (D), the rate of BrdU-positive cells was estimated for 10,000 EGFP-negative (E) or EGFP-positive (F) cells.
Persistence of EGFP expression in the progeny cells of EGFP-expressing HeLa cells.
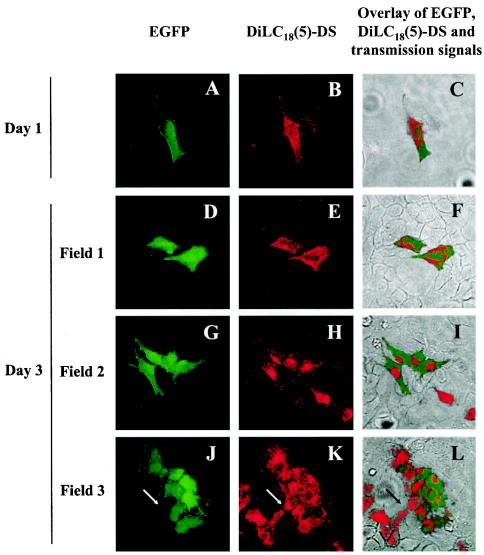
The results presented above demonstrate that the reduction of EGFP-expressing cells in a HeLa cell culture infected with the BoHV-4 V.test EGFP XhoI strain cannot be explained either by a reduced viability or by a slower rate of division of BoHV-4-infected cells. A third hypothesis to explain this reduction could be that the transmission of BoHV-4 genome to daughter cells during cell division is an erratic process. If this hypothesis is true, the division of EGFP-positive cells should generate some EGFP-negative cells free of BoHV-4 genome. To address this hypothesis, the membranes of HeLa cells infected with the BoHV-4 V.test EGFP XhoI strain were loaded with the nontoxic lipophilic fluorescent marker DilC18(5)-DS. This marker allows long-term labeling and tracking of labeled cells because it does not diffuse from the labeled cells to neighboring cells (even after days of culture) and because it is distributed equally between daughter cells during the cellular division. Infected cells labeled with DilC18(5)-DS were then mixed (1:100) with mock-infected unlabeled HeLa cells and grown in culture (Fig. 5). All fluorescent cells observed 1 day postlabeling were singletons surrounded by negative cells. Fluorescent cells were either double positive [labeled with DilC18(5)-DS and expressing EGFP] (Fig. 5A to C) or single positive [labeled with DilC18(5)-DS] (data not shown). Examination of the cell monolayer 3 days postlabeling revealed clones derived from fluorescent double-positive cells (Fig. 5D to L). Interestingly, most of the clones contained EGFP-negative cells, indicating that BoHV-4-infected HeLa cells can generate noninfected cells through cell division. Cells positive for EGFP expression, but not labeled with DilC18(5)-DS, were never observed, indicating that BoHV-4 replication in the culture was extremely low if existent.
FIG. 5.
Persistence of EGFP expression in the progeny cells of HeLa cells infected with the BoHV-4 V.test EGFP XhoI strain. HeLa cells were infected with the BoHV-4 V.test EGFP XhoI strain at an MOI of 0.5 PFU/cell and then passaged every other day for 6 days (1:2 split ratio). At 6 days postinfection, infected cells were harvested and treated for long-term membrane labeling with DilC18(5)-DS as described in Materials and Methods. Labeled infected cells were then mixed (1:100 ratio) with mock-infected unlabeled cells and grown on glass coverslips. At the indicated times after DilC18(5)-DS labeling, the cells were examined by confocal microscopy for EGFP (A, D, G, and J) and DilC18(5)-DS (B, E, H, and K) signals. Panels C, F, I, and L show the merged EGFP, DilC18(5)-DS, and transmission signals. The side of each panel corresponds to 250 μm of the specimen. The arrows in panels J, K, and L identify the very same point of the examined field.
Persistence of BoHV-4 genome in the progeny cells of HeLa-infected cells.
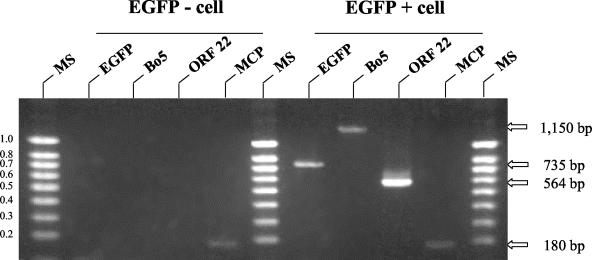
The results presented in Fig. 5 suggest that the emergence of EGFP-negative cells in the descendant of BoHV-4-infected HeLa cells derives from the loss of the BoHV-4 genome. To control this explanation and exclude other possible causes, the following experiment was performed (Fig. 6). HeLa cells were infected with the BoHV-4 V.test EGFP XhoI strain. At 6 days postinfection, a pure population of EGFP-expressing cells (99.9% as determined by the analysis of 10,000 sorted cells) was obtained by cell sorting. Sorted cells were then cultured for 10 days in order to allow the emergence of EGFP-negative cells. The DNA extracted from four sorted cells negative or positive for EGFP expression were then analyzed by PCR for the detection of BoHV-4 genome (Fig. 6). The results presented in Fig. 6 show that BoHV-4 genome was not detected in EGFP-negative cells despite the use of three different pairs of primers (listed in Table 1). In contrast, all three PCRs were positive when performed on the DNA of EGFP-positive cells. Control PCRs raised against cellular MCP were positive for both EGFP-positive and -negative cells. Altogether, these results demonstrate that the EGFP-negative cells derived from EGFP-positive cells are free of BoHV-4 genome.
FIG. 6.
Persistence of BoHV-4 genome in the progeny cells of infected HeLa cells. HeLa cells were infected with the BoHV-4 V.test EGFP XhoI strain at an MOI of 0.5 PFU/cell. The cells were then passaged every other day for 6 days (1:2 split ratio). At 6 days postinfection, EGFP-positive cells were sorted and cultured for 10 days in order to allow the emergence of EGFP-negative cells. Four EGFP-negative or positive cells were then sorted by automatic cell deposit unit as described in Materials and Methods. After DNA purification from those single cells, single-cell PCR assays were performed in order to detect the BoHV-4 ORFs EGFP, Bo5, and ORF22 or cellular ORF MCP. The primers used for the PCRs are described in Table 2. The data presented in this figure are representative of results obtained for 20 single sorted EGFP-positive and -negative cells. Marker sizes (MS) in kilobase pairs are indicated on the left. Expected molecular sizes of the amplicons are indicated on the right.
DISCUSSION
Herpesviruses have a complex life cycle relying on both replicative (or lytic) and nonreplicative (or latent) phases (49). Each of these phases may be the source of specific diseases. The diseases associated with the replicative phase occur after primary infection and/or after reactivation from latency. Their pathogenesis relies mainly on the destruction of permissive cells caused by the replication and spread of the virus. The pathogenesis of the diseases associated with the latent phase results from a more insidious effect of the infection. Indeed, during latency only a limited number of viral genes are expressed. However, some latent genes of some herpesviruses (mainly gammaherpesviruses) may affect the biology of the infected cells in a harmful manner for the host. For example, by influencing B-cell survival mechanisms, Epstein-Barr virus may induce tumors such as B-lymphoproliferative disease and Hodgkin's disease (4, 38). Similarly, Kaposi sarcoma-associated herpesvirus (KSHV or HHV8) expresses seven latent genes with a demonstrated effect on host cell growth (14). These genes are commonly recognized as the Kaposi sarcoma pathogenesis effectors. These examples illustrate the fact that expression of a limited number of gammaherpesvirus genes during latency may be sufficient to induce a malignant pathology.
In addition to latency, restricted expression of gammaherpesvirus genes may also result from cross-species abortive infection. Indeed, when a nonnatural host is infected, some herpesviruses may initiate an abortive infection. The virus succeeds in entering cells and in expressing a few genes, but it fails to replicate. Interestingly, this type of cross-species abortive infection associated with very little, if any, replication may be the cause of lethal diseases. For example, alcelaphine herpesvirus 1 and ovine herpesvirus 2, which infect asymptomatically wildebeest and sheep, respectively, induce a lethal lymphoproliferative syndrome known as malignant catarrhal fever when transmitted to nonnatural ruminant host species (48). Similarly, saimiriine herpesvirus 2 (SaHV-2), ateline herpesvirus 2, and murid herpesvirus 4 have been shown to induce lymphoproliferative diseases when transmitted to nonnatural host species (22, 45). These examples demonstrate that cross-species transmission of gammaherpesviruses to nonpermissive and nonnatural host species may nevertheless be the origin of serious diseases.
In the present study, because human contact with BoHV-4 is likely to occur, we performed in vitro experiments to assess the risk and consequences of putative human infection by BoHV-4. In view of the arguments presented above, we first tested the permissiveness, and also the sensitivity, of 21 human cell lines to BoHV-4 infection. These experiments revealed that human cell lines from lymphoid and myeloid origins were resistant to the infection, whereas epithelial cells and carcinoma or adenocarcinoma cells isolated from various organs were sensitive but poorly permissive to BoHV-4 infection. Next, by using the HeLa cell line as a model for human cells sensitive but not permissive to BoHV-4 infection, we analyzed the effect of the infection on the resistance of cells to apoptosis and the persistence of the infection through cellular divisions. The results obtained can be summarized as follows: (i) nonpermissive infection of HeLa cells by BoHV-4 protects them against TNF-α-induced apoptosis; (ii) BoHV-4 infection of HeLa cells persists in cell culture, although the percentage of infected cells decreases with time due to erratic transmission of the viral genome through cell division; and (iii) BoHV-4 infection has no effect on the rate of HeLa cell division.
Consistent with earlier reports, the present study confirms that BoHV-4 can replicate in some human cell lines (11, 17). Nevertheless, the fluorescence-activated cell sorting (FACS) approach adopted here revealed that the percentage of L protein-expressing cells in permissive cultures was extremely low (between 0.1 and 3.8%, Table 1), indicating that in the in vitro conditions of the present study, human cells are poorly permissive to BoHV-4 infection. However, analysis of EGFP expression (used as an IE reporter gene) revealed that these human cell lines are sensitive to BoHV-4 infection (Table 1, group 2) and, consequently, that viral replication is blocked in these cells at a postentry stage. This blockage could represent latent infection induced in vitro as a default pathway (as shown recently for KSHV [3]) and/or abortive infection resulting from the cross-species infection. To test the former hypothesis, the effect of sodium butyrate and dexamethasone was tested on BoHV-4-infected HeLa cell cultures. Both agents failed to induce BoHV-4 reactivation (data not shown), thus supporting the hypothesis that the postentry blockage of BoHV-4 infection in human cells could represent an abortive infection resulting from the inappropriate species of the host cell. Such a blockage has been described for different herpesviruses (3, 6, 9, 34, 44, 46) and was shown to have occurred at different stages of the infection.
Nonpermissive infection of HeLa cells by BoHV-4 protects them against TNF-α-induced apoptosis. BoHV-4 encodes at least two inhibitors of apoptosis, ORF16 and ORF71, which encode the v-Bcl-2 and v-FLIP homologues, respectively (66). ORF71 was previously shown to inhibit Fas- and TNF-α-induced apoptosis when overexpressed transiently in human HeLa cells (61). Different observations suggest that ORF71 is the gene conferring the observed resistance to apoptosis of BoHV-4-infected HeLa cells (Fig. 2). First, reverse transcriptase PCR experiments revealed that ORF71 is expressed in BoHV-4-infected HeLa cells (data not shown). Second, the experiments presented in Fig. 2 have recently been repeated with a recombinant BoHV-4 strain deleted for ORF71. The results demonstrated that deletion of ORF71 drastically reduces the property of BoHV-4 to protect HeLa cells against TNF-α-induced apoptosis (F. Minner, unpublished data).
Nonpermissive infection of HeLa cells by BoHV-4 persists in cell culture. However, the percentage of infected cells decreased with time (Fig. 3). A similar phenomenon was also observed in cell cultures persistently infected with SaHV-2 (52). The results presented in Fig. 3 to 6 demonstrated that this reduction is explained by the nontransmission of BoHV-4 genome to both daughter cells upon cell division rather than by the reduction of infected cell viability or the slowing down of their division rate. Nonpermissive persistent infection by herpesviruses relies on the maintenance of the double-stranded DNA genome as multiple copies of closed circular episomes that are distributed between daughter cells during cell division (49). This process has been studied extensively for the gammaherpesviruses KHSV and SaHV-2. It has been shown for these viruses that two viral components are sufficient to mediate the long-term episome maintenance in infected cells (21, 24, 30, 35). One is the latency-associated nuclear antigen 1 (LANA1), encoded by ORF73, and the other is a cis-acting DNA sequence (latent origin of replication [oriP]) (24) in the 5′ end of the genome (terminal-repeat sequence). If we assume that BoHV-4 persistence in HeLa cells relies on a similar mechanism, two hypotheses that are not mutually exclusive could explain the loss of BoHV-4 genome through cell division. First, BoHV-4 oriP could be inefficient in HeLa cells. This hypothesis is not consistent with the fact that EGFP-positive cells could be maintained for more than 4 months in culture by repeated sorting of EGFP-positive cells (data not shown). Second, the homologue of LANA encoded by BoHV-4 ORF73 could be inefficient. In relation to this hypothesis, it is interesting that BoHV-4 ORF73 product, in contrast to its homologues in KHVS and SaHV-2, does not contain any acidic internal repeated region (26, 36). The absence of this domain could lead to a less efficient tethering of the viral episome to host chromosome during cell division. However, Grundhoff and Ganem (26) have shown recently that the C-terminal region of KSHV LANA, which is conserved in the BoHV-4 ORF73 product, is sufficient for replication of plasmid containing a terminal repeat.
Herpesviruses have mainly evolved by cospeciation with their host (10, 39-41). For many herpesviruses, the cospeciation process has restricted the viral tropism to the natural host species and has led to the selection of viruses able to complete their biological cycle asymptomatically. The fact that the latter property represents adaptation of the virus to its host is supported by the observation that herpesviruses apathogenic for their natural host can be pathogenic for nonnatural host species. Cases of herpesvirus transmission from animals to humans are surprisingly rare (51). Skinner et al. (51) postulated that there are two ways to explain this observation. First, humans could be naturally resistant to animal herpesviruses transmitted through natural routes of infection. Second, it is possible that human infection by animal herpesviruses happen but are not diagnosed. Even if humans are naturally resistant to herpesvirus transmission from animals through natural routes of infection, it does not mean by default that iatrogenic parenteral infections are harmless. This concern is particularly important for gammaherpesviruses. Indeed, some gammaherpesviruses, including BoHV-4 and SaHV-2, have been proposed as viral vectors for gene therapy (11, 23, 28, 52, 65). The results presented here demonstrate that, in vitro, human cells can be infected by BoHV-4 in a nonpermissive manner protecting them against apoptosis. If such infection occurs in vivo, they will allow the viral genome to persist for a prolonged period of time within the infected host, which might end in recombination events, either with genes derived from the host cell or with genes derived from other coinfecting viruses. In addition, such infections could also allow the infected cells to accumulate mutations, leading to transformation. The fate and the relevance of such incidents are unknown and need to be addressed. However, the present study stresses the importance of assessing the safety of herpesviruses candidates as vectors. In theory, any viral gene susceptible to affect the biology of the infected cells in a harmful manner for the host should be deleted from the viral genome. Alternatively, iatrogenic transmission of animal gammaherpesviruses to humans could also result from xenotransplantations of infected organs (5, 25, 27, 43). Indeed, both pigs and baboons have been shown to be reservoir of various gammaherpesviruses (8, 19, 31, 64). This biosafety issue will need to be addressed in the future.
In conclusion, we performed in vitro experiments to assess the risks and the consequences of human infection by BoHV-4. Even though the present study was restricted to in vitro investigations, our results suggest that human contamination by BoHV-4 could lead to viral replication in permissive cells and/or to nonpermissive persistent infection in cells sensitive to but not permissive to the infection. Further studies are required to determine the existence of human infections by BoHV-4 and to identify their pathogenic role in humans. The present study highlights the importance of evaluating the risk and consequences of human contamination by animal gammaherpesviruses. It also stresses the point that, when one assesses the biosafety of animal gammaherpesviruses as an expression vector in vivo, one needs to consider the insidious effect of nonpermissive infection on the biology of the infected cell.
Acknowledgments
L. Gillet, A. Vanderplasschen, and L. Willems are Research Fellow, Senior Research Associate, and Research Director, respectively, of the “Fonds National Belge de la Recherche Scientifique.” This study was supported by a Concerted Action awards program of the French Community of Belgium (ARC 98/03-220).
REFERENCES
- 1.Barahona, H. H., L. V. Meléndez, N. W. King, M. D. Daniel, C. E. O. Fraser, and A. C. Preville. 1973. Herpesvirus aotus type 2: a new viral agent from owl monkeys (Aotus trivirgatus). J. Infect. Dis. 127:171-178. [DOI] [PubMed] [Google Scholar]
- 2.Bartha, A., M. Juhasz, and H. Liebermann. 1966. Isolation of a bovine herpesvirus from calves with respiratory disease and keratoconjunctivitis: a preliminary report. Acta Vet. Acad. Sci. Hung. 16:357-358. [PubMed] [Google Scholar]
- 3.Bechtel, J. T., Y. Liang, J. Hvidding, and D. Ganem. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 77:6474-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop, G. A., and L. K. Busch. 2002. Molecular mechanisms of B-lymphocyte transformation by Epstein-Barr virus. Microbes Infect. 4:853-857. [DOI] [PubMed] [Google Scholar]
- 5.Boneva, R. S., T. M. Folks, and L. E. Chapman. 2001. Infectious disease issues in xenotransplantation. Clin. Microbiol. Rev. 14:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borchers, K., J. Brackmann, and O. Kershaw. 2002. The mouse is not permissive for equine herpesvirus 2 (EHV-2), however viral DNA persisted in lung and spleen depending on the inoculation route. Arch. Virol. 147:1437-1444. [DOI] [PubMed] [Google Scholar]
- 7.Bublot, M., J. Dubuisson, M. F. Van Bressem, S. Danyi, P. P. Pastoret, and E. Thiry. 1991. Antigenic and genomic identity between simian herpesvirus aotus type 2 and bovine herpesvirus type 4. J. Gen. Virol. 72:715-719. [DOI] [PubMed] [Google Scholar]
- 8.Chmielewicz, B., M. Goltz, K. H. Lahrmann, and B. Ehlers. 2003. Approaching virus safety in xenotransplantation: a search for unrecognized herpesviruses in pigs. Xenotransplantation 10:349-356. [DOI] [PubMed] [Google Scholar]
- 9.Dahlberg, J. E., D. V. Ablashi, J. S. Rhim, A. Fladger, and S. Z. Salahuddin. 1988. Replication of Herpesvirus saimiri is semipermissive in human cells because of a block in the synthesis of certain late proteins. Intervirology 29:234-277. [DOI] [PubMed] [Google Scholar]
- 10.Davison, A. J. 2002. Evolution of the herpesviruses. Vet. Microbiol. 86:69-88. [DOI] [PubMed] [Google Scholar]
- 11.Donofrio, G., S. Cavirani, T. Simone, and V. L. van Santen. 2002. Potential of bovine herpesvirus 4 as a gene delivery vector. J. Virol. Methods 101:49-61. [DOI] [PubMed] [Google Scholar]
- 12.Donofrio, G., S. Cavirani, and V. L. van Santen. 2000. Establishment of a cell line persistently infected with bovine herpesvirus-4 by use of a recombinant virus. J. Gen. Virol. 81:1807-1814. [DOI] [PubMed] [Google Scholar]
- 13.Donofrio, G., C. F. Flammini, F. Scatozza, and S. Cavirani. 2000. Detection of bovine herpesvirus 4 (BoHV-4) DNA in the cell fraction of milk of dairy cattle with history of BoHV-4 infection. J. Clin. Microbiol. 38:4668-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dourmishev, L. A., A. L. Dourmishev, D. Palmeri, R. A. Schwartz, and D. M. Lukac. 2003. Molecular genetics of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) epidemiology and pathogenesis. Microbiol. Mol. Biol. Rev. 67:175-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubuisson, J., D. Boulanger, M. Bublot, E. Thiry, and P. P. Pastoret. 1989. Proteins specified by bovine herpesvirus type 4: structural proteins of the virion and identification of two major glycoproteins by using monoclonal antibodies. J. Gen. Virol. 70:1743-1753. [DOI] [PubMed] [Google Scholar]
- 16.Dubuisson, J., P. P. Pastoret, and E. Thiry. 1991. Temporal control of bovine herpesvirus type 4 glycoprotein synthesis. J. Gen. Virol. 72:1429-1434. [DOI] [PubMed] [Google Scholar]
- 17.Egyed, L. 1998. Replication of bovine herpesvirus type 4 in human cells in vitro. J. Clin. Microbiol. 36:2109-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egyed, L., J. P. Kluge, and A. Bartha. 1997. Histological studies of bovine herpesvirus type 4 infection in non-ruminant species. Vet. Microbiol. 57:283-289. [DOI] [PubMed] [Google Scholar]
- 19.Ehlers, B., S. Ulrich, and M. Goltz. 1999. Detection of two novel porcine herpesviruses with high similarity to gammaherpesviruses. J. Gen. Virol. 80:971-978. [DOI] [PubMed] [Google Scholar]
- 20.Fabricant, C. G., J. H. Gillespsie, and L. Krook. 1971. Intracellular and extracellular mineral crystal formation induced by viral infection of cell cultures. Infect. Immun. 3:416-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fejer, G., M. M. Medveczky, E. Horvath, B. Lane, Y. Chang, and P. G. Medveczky. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts preferentially with the terminal repeats of the genome in vivo and this complex is sufficient for episomal DNA replication. J. Gen. Virol. 84:1451-1462. [DOI] [PubMed] [Google Scholar]
- 22.Fickenscher, H., and B. Fleckenstein. 2001. Herpesvirus saimiri. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:545-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frolova-Jones, E. A., A. Ensser, A. J. Stevenson, S. E. Kinsey, and D. M. Meredith. 2000. Stable marker gene transfer into human bone marrow stromal cells and their progenitors using novel herpesvirus saimiri-based vectors. J. Hematother. Stem Cell Res. 9:573-581. [DOI] [PubMed] [Google Scholar]
- 24.Garber, A. C., J. Hu, and R. Renne. 2002. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277:27401-27411. [DOI] [PubMed] [Google Scholar]
- 25.Gollackner, B., N. J. Mueller, S. Houser, I. Qawi, D. Soizic, C. Knosalla, L. Buhler, F. J. Dor, M. Awwad, D. H. Sachs, D. K. Cooper, S. C. Robson, and J. A. Fishman. 2003. Porcine cytomegalovirus and coagulopathy in pig-to-primate xenotransplantation. Transplantation 75:1841-1847. [DOI] [PubMed] [Google Scholar]
- 26.Grundhoff, A., and D. Ganem. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus permits replication of terminal repeat-containing plasmids. J. Virol. 77:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunzburg, W. H., and B. Salmons. 2000. Xenotransplantation: is the risk of viral infection as great as we thought? Mol. Med. Today 6:199-208. [DOI] [PubMed] [Google Scholar]
- 28.Hall, K. T., M. S. Giles, D. J. Goodwin, M. A. Calderwood, I. M. Carr, A. J. Stevenson, A. F. Markham, and A. Whitehouse. 2000. Analysis of gene expression in a human cell line stably transduced with herpesvirus saimiri. J. Virol. 74:7331-7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holm, M., M. Thomsen, M. Hoyer, and P. Hokland. 1998. Optimization of a flow cytometric method for the simultaneous measurement of cell surface antigen, DNA content, and in vitro BrdUrd incorporation into normal and malignant hematopoietic cells. Cytometry 32:28-36. [DOI] [PubMed] [Google Scholar]
- 30.Hu, J., A. C. Garber, and R. Renne. 2002. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 76:11677-11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jenson, H. B., Y. Ench, S. J. Gao, K. Rice, D. Carey, R. C. Kennedy, J. R. Arrand, and M. Mackett. 2000. Epidemiology of herpesvirus papio infection in a large captive baboon colony: similarities to Epstein-Barr virus infection in humans. J. Infect. Dis. 181:1462-1466. [DOI] [PubMed] [Google Scholar]
- 32.Kit, S., M. Kit, H. Ichimura, R. Crandell, and S. McConnell. 1986. Induction of thymidine kinase activity by viruses with group B DNA genomes: bovine cytomegalovirus (bovine herpesvirus 4). Virus Res. 4:197-212. [DOI] [PubMed] [Google Scholar]
- 33.Kokles, V. R. 1986. Zum Vorkommen von Herpes-Orphan-Virusinfektionen des Rindes. Mh. Vet. Med. 41:551-555. [Google Scholar]
- 34.Lafemina, R. L., and G. S. Hayward. 1988. Differences in cell-type-specific blocks to immediate-early gene expression and DNA replication of human, simian and murine cytomegalovirus. J. Gen. Virol. 69:355-374. [DOI] [PubMed] [Google Scholar]
- 35.Lim, C., D. Lee, T. Seo, C. Choi, and J. Choe. 2003. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus functionally interacts with heterochromatin protein 1. J. Biol. Chem. 278:7397-7405. [DOI] [PubMed] [Google Scholar]
- 36.Lomonte, P., M. Bublot, V. van Santen, G. M. Keil, P. P. Pastoret, and E. Thiry. 1995. Analysis of bovine herpesvirus 4 genomic regions located outside the conserved gammaherpesvirus gene blocks. J. Gen. Virol. 76:1835-1841. [DOI] [PubMed] [Google Scholar]
- 37.MacGregor, G. R., and C. T. Caskey. 1989. Construction of plasmids that express Escherichia coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 17:2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macsween, K. F., and D. H. Crawford. 2003. Epstein-Barr virus-recent advances. Lancet Infect. Dis. 3:131-140. [DOI] [PubMed] [Google Scholar]
- 39.McGeoch, D. J. 2001. Molecular evolution of the gamma-Herpesvirinae. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:421-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGeoch, D. J., S. Cook, A. Dolan, F. E. Jamieson, and E. A. Telford. 1995. Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J. Mol. Biol. 247:443-458. [DOI] [PubMed] [Google Scholar]
- 41.McGeoch, D. J., A. Dolan, and A. C. Ralph. 2000. Toward a comprehensive phylogeny for mammalian and avian herpesviruses. J. Virol. 74:10401-10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreno-Lopez, J., M. Goltz, C. Rehbinder, K. V. Valsala, and H. Ludwig. 1989. A bovine herpesvirus (BHV-4) as passenger virus in ethmoidal tumours in Indian cattle. Zentbl. Vetmed. B 36:481-486. [DOI] [PubMed] [Google Scholar]
- 43.Mueller, N. J., R. N. Barth, S. Yamamoto, H. Kitamura, C. Patience, K. Yamada, D. K. Cooper, D. H. Sachs, A. Kaur, and J. A. Fishman. 2002. Activation of cytomegalovirus in pig-to-primate organ xenotransplantation. J. Virol. 76:4734-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakamichi, K., Y. Matsumoto, and H. Otsuka. 2001. Defective infection of bovine herpesvirus 1 in non-permissive murine cells. J. Vet. Med. Sci. 63:1139-1142. [DOI] [PubMed] [Google Scholar]
- 45.Nash, A. A., B. M. Dutia, J. P. Stewart, and A. J. Davison. 2001. Natural history of murine gamma-herpesvirus infection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nixdorf, R., J. Schmidt, A. Karger, and T. C. Mettenleiter. 1999. Infection of Chinese hamster ovary cells by pseudorabies virus. J. Virol. 73:8019-8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson, R. B., and S. M. Goyal. 1988. Propagation and quantitation of animal herpesviruses in eight cell culture systems. Comp. Immunol. Microbiol. Infect. Dis. 11:93-98. [DOI] [PubMed] [Google Scholar]
- 48.Plowright, W., R. D. Ferris, and G. R. Scott. 1960. Blue wildebeest and the etiological agent of bovine malignant fever. Nature 188:1167-1169. [DOI] [PubMed] [Google Scholar]
- 49.Roizman, B., and P. E. Pellet. 2001. The family Herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 50.Rossiter, P. B., I. D. Gumm, D. A. Stagg, P. A. Conrad, S. Mukolwe, F. G. Davies, and H. White. 1989. Isolation of bovine herpesvirus-3 from African buffaloes (Syncerus caffer). Res. Vet. Sci. 46:337-343. [PubMed] [Google Scholar]
- 51.Skinner, G. R., A. Ahmad, and J. A. Davies. 2001. The infrequency of transmission of herpesviruses between humans and animals: postulation of an unrecognized protective host mechanism. Comp. Immunol. Microbiol. Infect. Dis. 24:255-269. [DOI] [PubMed] [Google Scholar]
- 52.Stevenson, A. J., E. Frolova-Jones, K. T. Hall, S. E. Kinsey, A. F. Markham, A. Whitehouse, and D. M. Meredith. 2000. A herpesvirus saimiri-based gene therapy vector with potential for use in cancer immunotherapy. Cancer Gene Ther. 7:1077-1085. [DOI] [PubMed] [Google Scholar]
- 53.Theodoridis, A. 1985. Studies on bovine herpesviruses. Part 1. Isolation and characterization of viruses isolated from the genital tract of cattle. Onderstepoort J. Vet. Res. 52:239-254. [PubMed] [Google Scholar]
- 54.Thiry, E., P. Lomonte, A. Vanderplasschen, and P. P. Pastoret. 1997. Bovine herpesvirus 4 (Movar herpesvirus), p. 439-445. IARC Monographs on the evaluation of carcinogenic risks to human, vol. 70. Epstein-Barr virus and Kaposi's sarcoma herpesvirus/human herpesvirus 8. International Agency for Research on Cancer, Lyon, France.
- 55.Thiry, E., P. P. Pastoret, and C. M. Calberg-Bacq. 1981. Isolement d'un herpesvirus chez un taureau infertile. Ann. Med. Vet. 125:143. [Google Scholar]
- 56.Todd, W. J., and J. Storz. 1983. Morphogenesis of a cytomegalovirus from an American bison affected with malignant catarrhal fever. J. Gen. Virol. 64:1025-1030. [DOI] [PubMed] [Google Scholar]
- 57.Vanderplasschen, A., M. Goltz, J. Lyaku, C. Benarafa, H.-J. Buhk, E. Thiry, and P.-P. Pastoret. 1995. The replication in vitro of the gammaherpesvirus bovine herpesvirus 4 is restricted by its DNA synthesis dependence on the S phase of the cell cycle. Virology 213:328-340. [DOI] [PubMed] [Google Scholar]
- 58.Vanderplasschen, A., and G. L. Smith. 1997. A novel virus binding assay using confocal microscopy: demonstration that the intracellular and extracellular vaccinia virions bind to different cellular receptors. J. Virol. 71:4032-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Opdenbosch, E., G. Wellemans, and J. Oudewater. 1986. Toevallige isolatie van het boviene herpesvirus 4 uit de long van een schaap. Vlaams Diergeneesk. Tijdschr. 55:432-433. [Google Scholar]
- 60.Wajant, H., E. Haas, R. Schwenzer, F. Muhlenbeck, S. Kreuz, G. Schubert, M. Grell, C. Smith, and P. Scheurich. 2000. Inhibition of death receptor-mediated gene induction by a cycloheximide-sensitive factor occurs at the level of or upstream of Fas-associated death domain protein (FADD). J. Biol. Chem. 275:24357-24366. [DOI] [PubMed] [Google Scholar]
- 61.Wang, G. H., J. Bertin, Y. Wang, D. A. Martin, J. Wang, K. J. Tomaselli, R. C. Armstrong, and J. I. Cohen. 1997. Bovine herpesvirus 4 BORFE2 protein inhibits Fas- and tumor necrosis factor receptor 1-induced apoptosis and contains death effector domains shared with other gamma-2 herpesviruses. J. Virol. 71:8928-8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wellenberg, G. J., W. H. M. Van Der Poel, T. J. K. Van Der Vorst, P. H. R. Van Valkengoed, Y. H. Schukken, F. Wagenaar, and J. T. Van Oirschot. 2000. Bovine herpesvirus 4 in bovine clinical mastitis. Vet. Rec. 147:222-225. [DOI] [PubMed] [Google Scholar]
- 63.Wellenberg, G. J., E. R. Verstraten, S. Belak, S. B. Verschuren, F. A. Rijsewijk, R. Peshev, and J. T. Van Oirschot. 2001. Detection of bovine herpesvirus 4 glycoprotein B and thymidine kinase DNA by PCR assays in bovine milk. J. Virol. Methods 97:101-112. [DOI] [PubMed] [Google Scholar]
- 64.Whitby, D., A. Stossel, C. Gamache, J. Papin, M. Bosch, A. Smith, D. H. Kedes, G. White, R. Kennedy, and D. P. Dittmer. 2003. Novel Kaposi's sarcoma-associated herpesvirus homolog in baboons. J. Virol. 77:8159-8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitehouse, A. 2003. Herpesvirus saimiri: a potential gene delivery vector. Int. J. Mol. Med. 11:139-148. [PubMed] [Google Scholar]
- 66.Zimmermann, W., H. Broll, B. Ehlers, H. J. Buhk, A. Rosenthal, and M. Goltz. 2001. Genome sequence of bovine herpesvirus 4, a bovine rhadinovirus, and identification of an origin of DNA replication. J. Virol. 75:1186-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]