Abstract
Goat manure (GM) is an excellent raw material for anaerobic digestion because of its high total nitrogen content and fermentation stability. Several comparative assays were conducted on the anaerobic co-digestion of GM with three crop residues (CRs), namely, wheat straw (WS), corn stalks (CS) and rice straw (RS), under different mixing ratios. All digesters were implemented simultaneously under mesophilic temperature at 35±1 °C with a total solid concentration of 8%. Result showed that the combination of GM with CS or RS significantly improved biogas production at all carbon-to-nitrogen (C/N) ratios. GM/CS (30:70), GM/CS (70:30), GM/RS (30:70) and GM/RS (50:50) produced the highest biogas yields from different co-substrates (14840, 16023, 15608 and 15698 mL, respectively) after 55 d of fermentation. Biogas yields of GM/WS 30:70 (C/N 35.61), GM/CS 70:30 (C/N 21.19) and GM/RS 50:50 (C/N 26.23) were 1.62, 2.11 and 1.83 times higher than that of CRs, respectively. These values were determined to be the optimal C/N ratios for co-digestion. However, compared with treatments of GM/CS and GM/RS treatments, biogas generated from GM/WS was only slightly higher than the single digestion of GM or WS. This result was caused by the high total carbon content (35.83%) and lignin content (24.34%) in WS, which inhibited biodegradation.
Introduction
China is one of the largest agricultural countries in the world. The production of net available crop residues (CRs) in China is estimated to be over 800 million t/yr [1], which ranks first in the world. The use of agricultural waste as a major component of renewable energy is suitable for improving energy security and decreasing environmental disruption caused by carbon emissions [2], [3]. Wheat straw (WS), rice straw (RS) and corn stalks (CS) are the top three agricultural wastes in China and account for 80.5% of the total output (15.7%, 24.2% and 40.6%, respectively) [1]. Thus, studying the energy generation potential of these three wastes is important.
Anaerobic digestion (AD) is a biological process that produces biogas from bio-degradable wastes by bacteria under poor or no oxygen conditions. In the past two decades, AD has been applied as an effective technology for solving the energy shortage and environmental pollution problems of biotechnology industries and residential activities caused by heating and electricity generation [4], [5], [6].
CRs and animal manure have recently been used together to produce biogas by AD. Compared with the single digestion of feedstock, the co-digestion of CRs and animal manures increases the rate of biogas production because of the greater balance between carbon and nitrogen [7] and improves AD efficiency [8]. Annual goat manure (GM) yield in China is approximately 3.21×108 t followed by dairy manure, swine manure and chicken manure [9]. The total nitrogen (TN) contents of fresh GM (1.01%) and chicken manure (1.03%) are significantly higher than those of dairy manure (0.35%) and swine manure (0.24%) [10]. High TN content is beneficial to co-digestion with CRs because it decreases the carbon-to-nitrogen (C/N) ratios of single CRs substrates. GM is also insensitive to acidification during anaerobic fermentation [11], [12]. Hence, GM is an excellent raw material for AD. Although various raw materials, such as agricultural waste, animal manures, sewage sludge and food waste have been reported as potentially feasible for co-digestion [7], [13], [14], [15], [16], [17], [18], [19], [20], the suitable mixing ratios of multi-component substrates between GM and various CRs are largely unknown.
We investigated the biogas-producing efficiency of anaerobic co-digestion influenced by different GM and CR mixing ratios. The best ratio between these substrates was obtained by comparing the results. Furthermore, an optimum co-digestion condition for biogas production was proposed.
Materials and Methods
Feedstocks and inocula
GM was obtained from a local livestock farm near Northwest Agriculture and Forestry University (NWAFU), Yangling Shaanxi, China. WS, CS and RS were collected from the experimental field of NWAFU. All of these straws were cut into sections at lengths of 2 cm to 3 cm by using a grinder. Inoculum was the anaerobic sludge of dairy manure, which was obtained from an anaerobic digester in a local village.
Experimental digester and design
The experiment was conducted according to Song et al. (2012) by using lab-scale anaerobic digesters fabricated from 1 L Erlenmeyer flasks. Batch reactors were used to determine the co-digestions of GM mixed with three CRs. The working volume of each digester was 700 mL, including 140 g inocula and an appropriate amount of digesting material. Deionized water was added to digesters to maintain a total solid (TS) content of 8% [5]. All reactors were gently mixed manually for approximately 1 min/d prior to biogas volume measurement.
To obtain the best mixing ratio of the co-digestion of GM supplemented with three CRs as external carbon sources, five different mixing mass ratios at 90∶10, 70∶30, 50∶50, 30∶70 and 10∶90 were tested under mesophilic condition (35±1°C) for 55 d. Unmixed GM (100∶0) and CR (0∶100) were anaerobically digested as controls. Each treatment was performed thrice with a control to investigate the effect of different mixed ratios on biogas production.
Analysis and statistics
The TS, volatile solids (VS), pH, volatile fatty acid (VFA), and TN content of the materials were determined in accordance with the Standard Methods for the Examination of Water and Wastewater of the American Public Health Association [21]. Total carbon (TC) and lignin contents were analyzed by using the method described by Cuetos et al. and Song et al. [5], [22]. The amount of biogas produced from each digester was recorded every day by using the water displacement method during the digestion period. Each batch experiment was deemed complete when a clear downward trend in daily biogas volume produced was observed for 10 d.
ANOVA was performed to determine the significant differences among each treatment by using SAS version 8.12 (SAS Institute Inc., Cary, NC, USA).
Results and Discussion
Substrate characteristics
The C/N ratios of the different substrates and substrate mixtures in AD greatly influence biogas production [23], [24]. A higher carbon content provides more carbon for CH4 production, whereas a lower nitrogen content limits microbial activity because microbes need a considerable amount of nitrogen to maintain growth [8]. The ideal C/N ratios range from 9 to 30 for anaerobic digesters [25]. The chemical characteristics of substrates used in this study are shown in Table 1. The C/N ratio of GM was 17.97, which is too low for biogas production. However, the C/N ratios of WS, CS and RS were significantly higher (91.17, 88.13 and 92.91, respectively) than that of GM (P<0.01). This result suggested that CRs increased methane production when co-digested with GM under the optimal C/N ratio.
Table 1. Chemical characterization of substrates used in the co-digestion experiments.
| GM | WS | CS | RS | |
| pH | 7.94±0.15 | ND | ND | ND |
| TS (%) | 33.65±3.23, b | 81.08±7.62, a | 81.74±7.43, a | 77.92±6.97, a |
| VS (%) | 82.21±8.93, a | 90.29±9.25, a | 91.42±9.33, a | 94.23±9.42, a |
| TC (%) | 18.22±1.14, c | 35.83±3.17, a | 28.82±2.03, b | 31.96±2.92, ab |
| TN (%) | 1.014±0.11, a | 0.393±0.02, b | 0.327±0.04, b | 0.344±0.02, b |
| C/N | 17.97±0.84, b | 91.17±3.44, a | 88.13±4.65, a | 92.91±3.10, a |
| Lignin (%) | ND | 24.34±1.89, a | 15.38±1.21, b | 9.49±0.33, c |
TS, total solid; VS, volatile solids; TC, Total carbon; TN, total nitrogen.
The values are the mean ± standard deviation of the triplicate measurements.
ND = not detected.
The ANOVA test was conducted to determine the differences between each cultivar. Values with the same letters indicate no significant difference at P<0.01.
Biogas yields and production rates at different GM/CR ratios
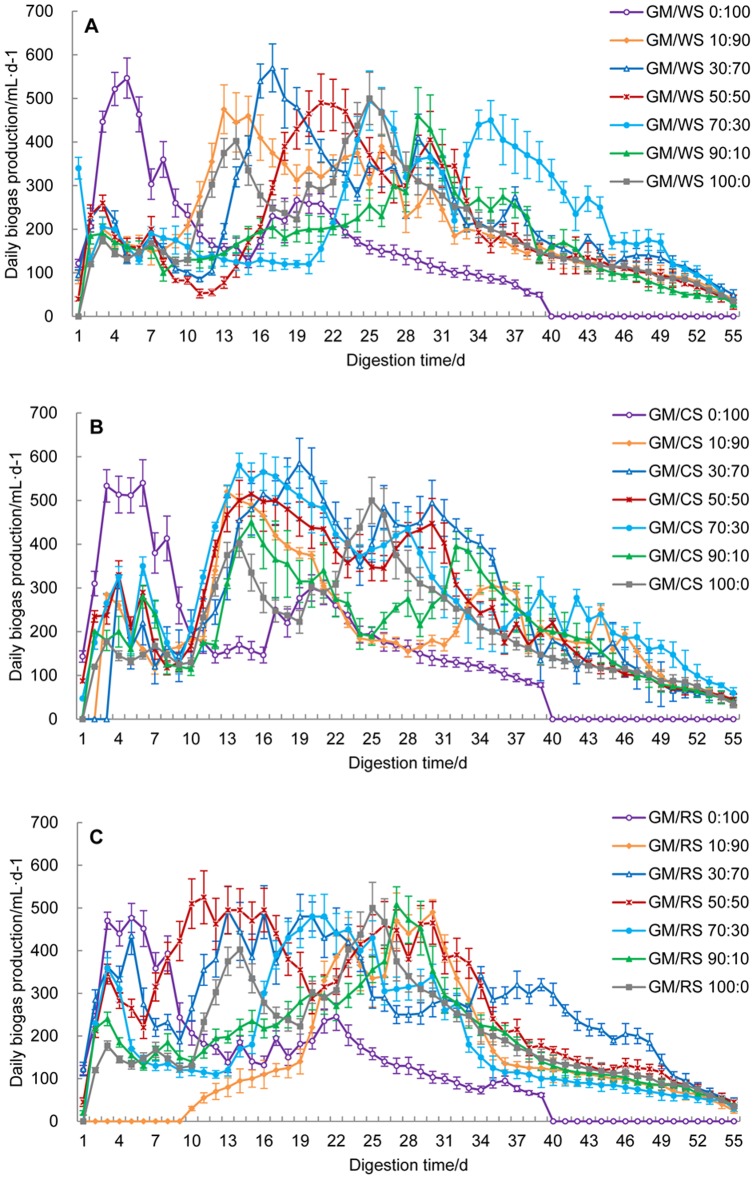
The daily biogas production by the co-digestion of GM and CRs during 55 d of digestion was calculated under different mixing ratios (Fig. 1). Samples from the mixing ratios of GM/WS 30:70, GM/CS 30:70 and GM/RS 50:50 were measured, and their peak yield values were 570, 585 and 525 mL/d on the 17th, 19th and 11th d, respectively (Fig. 1). The digestion of single GM substrate (100:0) produced biogas earlier than other combinations but had two relatively small peaks (402 and 500 mL/d) (Fig. 1). By contrast, the digestion of any single CR substrate (0:100) had only one peak (GM/WS 547, GM/CS 540 and GM/RS 477 mL/d) that occurred earlier than the other combinations (3rd d to 6th d) and decreased rapidly after the 16th d (Fig. 1). These results indicate that the co-digestion of GM and CRs could significantly delay the attainment of the highest gas production.
Figure 1. Daily biogas production from the co-digestion of GM and WS (A), CS (B) and RS (C) with different mixing ratios.
Mean values originated from three independent replications. Vertical bars represent standard deviations.
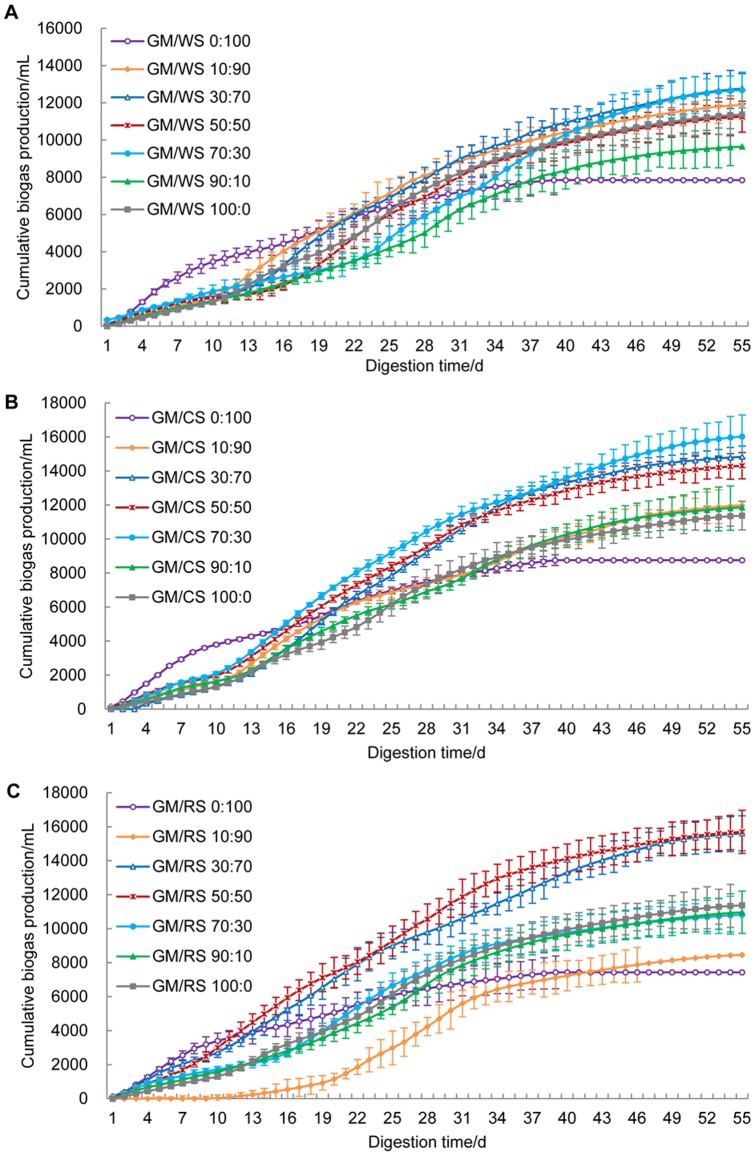
The final cumulative biogas productions by the co-digestion of GM and CRs at different mixing ratios are shown in Fig. 2. The cumulative biogas productions for GM/WS 10:90, 30:70, 50:50, 70:30 and 90:10 were 11890, 12765, 11253, 12685 and 9650 mL, respectively (Fig. 2A). These results showed an increase of 51.0%, 62.1%, 42.9%, 61.1% and 22.6% compared with single WS (7874 mL), and an increase of 51.0%, 62.1%, 42.9%, and 22.6% compared with single GM (10375 mL). However, the biogas production of GM/WS 90:10 (9650 mL) was lower than that of single GM (Fig. 2A). The same trends were observed for the GM/CS and GM/RS treatments, which had considerably higher increases (Fig. 2B and 2C). These data showed that the co-digestion of GM and CRs greatly improved biodegradability and biogas production at most mixing ratios compared with single substrate digestion. Our results supported those of Wu et al. [26], who found that co-digesting swine manure with CS, oat straw and WS significantly increase biogas production and net CH4 volume at all C/N ratios.
Figure 2. Cumulative biogas productions from co-digestion of GM and WS (A), CS (B) and RS (C) with different mixing ratios.
Mean values originated from three independent replications. Vertical bars represent standard deviations.
To compare the effect of single substrate digestion and co-digestion with GM and CRs, the total biogas yield of each combination is shown in Fig. 3. The total biogas productions of most co-digestion systems were higher than the single digestion of either GM or CRs except those of GM/WS 90:10 and GM/RS 10:90. GM/CS 70:30 exhibited the highest total biogas yield of 16.02 L in all treatments, which was 83.02% and 54.44% higher than that of CS and GM alone, respectively. Among all the GM/RS treatments, the total biogas production of GM/RS 50:50 (15.70 L) was 111.28% and 51.31% higher than that of CS alone and GM alone, respectively. The co-digestion of GM/WS 30:70 was 62.12% and 23.04% higher than that of WS and GM alone, respectively. Compared with the TC contents of CS (28.82%) and RS (31.96%), the higher TC content of WS (35.83%) suppressed microbial growth and methanogenesis because of the ammonium nitrogen deficiency and low pH [22], [27], [28].
Figure 3. Total biogas productions from anaerobic co-digestion of GM with WS, CS and RS with different mixing ratios.
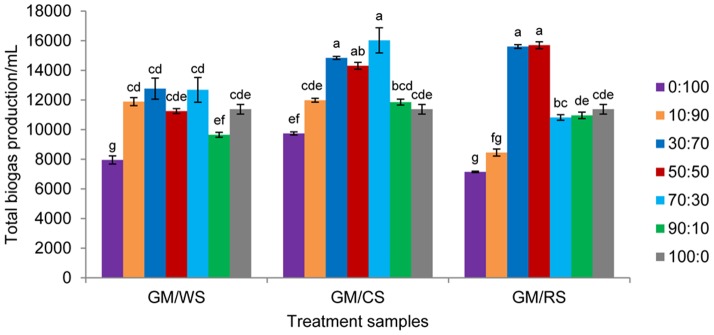
Mean values originated from three independent replications. Vertical bars represent standard deviations. The ANOVA test was conducted to determine the differences between each cultivar. Values with the same letters indicate no significant difference at P<0.01.
These results indicated that co-digestion with suitable GM and CRs mixtures is an effective way to prolong the period of the highest gas production and improve biogas yield. The ANOVA indicated that the total biogas production of co-digestions were significantly higher (P<0.01) than the single digestion of GM or CRs (Fig. 3).
Effect of C/N ratio on biogas production
The C/N ratio represents the relationship between the amount of carbon and nitrogen present in organic materials and is an important indicator for controlling biological treatment systems [23]. On one hand, a high C/N ratio indicates rapid nitrogen consumption by methanogens and leads to lower gas production. On the other hand, a low C/N ratio results in ammonia accumulation and an increase in pH values, which is toxic to methanogenic bacteria [29]. The mean value of C/N ratios for each co-digestion combinations and single digestion ranged from 92.79 to 17.97 (Table 2). The C/N ratios of co-digestions were significantly lower than those of CR materials (P<0.01, Table 1), thus indicating that co-digestion effectively reduced the C/N ratios of AD. Experimental data showed that the biogas yields of most co-digestions were higher than the corresponding single digestions. According to the cumulative biogas production (Fig. 3), the highest biogas yields (12765, 15698 and 16023 mL) at GM/WS 30:70 (C/N 35.64), GM/CS 70:30 (C/N 21.26) and GM/RS 50:50 (C/N 26.28) were 1.62, 2.11 and 1.83 times higher than that of CRs only, respectively. However, the total biogas yields of three GM/CR 10:90 treatments did not increase, and were even lower than that of single substrate. The reason for this result was that the C/N ratios of each GM/CR 10:90 treatment were less than 20 (Table 2). The results suggested that the ideal C/N ratio range is between 20 to 35 in the co-digestion of GM with CRs, which was consistent with the report of Verma [29], which revealed that the optimum C/N ratios in anaerobic digesters were between 20 to 30.
Table 2. Mean values for C/N ratios in the co-digestion of GM with three CRs.
| Treatment | Co-digestion mixing ratios | ||||||
| 0:100 | 10:90 | 30:70 | 50:50 | 70:30 | 90:10 | 100:0 | |
| GM/WS | 91.05±3.44, a | 58.24±0.48, b | 35.64±0.58, c | 29.71±1.22, d | 22.06±0.82, e | 19.12±0.83, f | 17.97±0.84, f |
| GM/CS | 88.51±4.65, a | 53.43±2.50, b | 32.64±1.46, c | 25.13±1.13, d | 21.26±0.97, e | 18.90±0.87, e | 17.97±0.84, e |
| GM/RS | 92.79±3.10, a | 57.46±0.30, b | 34.82±0.61, c | 26.28±0.77, d | 21.80±0.82, e | 19.05±0.83, f | 17.97±0.84, f |
The values are the mean ± standard deviation of triplicate measurements.
The ANOVA test was conducted to determine the differences between each cultivar. Values with the same letters indicate no significant difference at P<0.01.
CRs typically contain high lignocellulosic contents. Problems such as low gas yield during the AD of these materials were usually associated with a high C/N ratio or high lignin content [30]. Although the C/N ratio was reduced by most co-digestions, no apparent increasing trend was observed in the biogas production of GM/WS, which even decreased slightly (GM/WS 90:10) compared with GM only. This phenomenon possibly resulted from the significantly higher lignin content (24.34%) of WS substrate than those of CS and RS (15.38% and 9.47%, respectively) (P<0.01, Table 1). To overcome the low degradability of lignin, reducing the particle size of CR substrate can increase the degradation rate of lignocelluloses and further improve biogas production [31].
Effects of pH and VFA
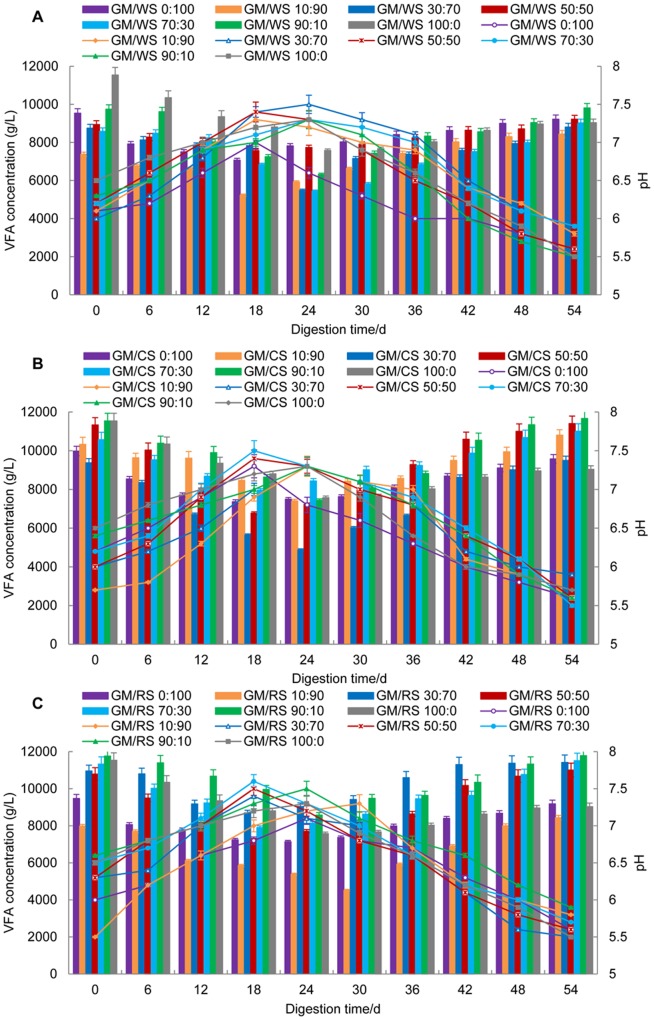
VFA and pH are the two key factors in AD [4]. The pH value and total VFA reflected the changing processes in the reactors (Fig. 4). The curves for the individual pH and total VFA of all mixtures and single substrates had similar trends. The growth of methanogens can be significantly influenced by the pH level [32]. The initial pH values of digesters gradually decreased from 6.5 to 6.0 with increasing CR percentage, and GM/RS 10:90 had the lowest pH value (5.5). The pH values increased from 6.5 as the percentage of GM increased in the 6th d, and then remained at approximately 6.8 until the 30th d. This stability confirmed that the daily biogas production of each mixture reached the methanogenesis stage, and that the pH value remained at approximately 6.8. Thereafter, the pH values dropped slightly to 6.0, thus indicating that the digestion changed in the later stages. However, the pH values of GM/CRs 0:100 decreased rapidly after the 18th d, thus showing the buffering capacity of GM. These results indicated that the best pH values for the co-digestion of GM and CRs ranged from 6.5 to 7.5.
Figure 4. VFA concentrations and pH values from the co-digestion of GM and WS (A), CS (B) and RS (C) with different mixing ratios.
Mean values originated from three independent replications. Columns represent VFA, lines represent pH values, and vertical bars represent standard deviations. VFA, volatile fatty acid.
VFAs are intermediate organic acid products, and the total VFA concentration is considered an important indicator of metabolic status in addition to the pH value during AD [33], [34]. However, the VFA curves showed evidently contrasting trends with that of the pH values. VFA was initially approximately 7380 mg/L to 11767 mg/L for all treatments and then decreased to 4519 mg/L to 5484 mg/L at the 24th d. VFA increased again and finally decreased to 9812 mg/L to 11791 mg/L at the end of digestion (Fig. 3 and 4).
The ammonia produced by the biological degradation of proteins and urea often results in VFA accumulation. The accumulation of VFA leads to the decrease of pH value, thus affecting the growth of methanogens during the AD process [6], [24], [30]. Our results showed that pH and VFA were co-related with biogas yield in AD. Thus, the pH values were proportional to biogas yield, whereas VFAs were inversely proportional. These results further indicated that pH decreased with increasing VFA accumulation. High concentrations of VFA are toxic to methanogens and inhibits hydrolysis rates in reactors [35]. The interaction between pH and VFA may lead to an “inhibited steady state” with a lower methane yield [30], [36], [37]. The extended gas production peaks in each mixing treatment might be explained by the co-digestion of GM and CRs, which relieves the inhibited steady state caused by pH and VFA effectively. The co-digestion of GM and CRs improves the buffering capacity to VFA accumulation and inhibits the acidogenesis process, which is consistent with the previous study [38].
Conclusion
The anaerobic co-digestion of GM with CRs is a promising way for improving biogas production. This co-digestion not only resolves the environmental problems caused by straws burning, but also overcomes C/N ratio imbalances in single digestion substrates and enhances the AD process.
Our results showed that the anaerobic co-digestions of GM with CS and RS were efficient and produced more cumulative biogas by reducing the C/N ratios of substrates. The best ratios were GM/CS 30:70, GM/CS 70:30, GM/RS 30:70 and GM/RS 50:50. However, the co-digestion of GM with WS did not improve the biogas yield significantly, which is consistent with the result in previous research [26]. The higher TC content of WS suppressed microbial growth and methanogenesis because of the deficiency of ammonium nitrogen and low pH. For the pH and VFA ranges in this study, pH decreased with increasing VFA accumulation, thus leading to the inhibition of biowaste hydrolysis rates.
Acknowledgments
We thank Dr. Xiaodong Wang, Dr Furong Liu and Dr. Yuheng Yang for critical reading of this manuscript and editorial guidance.
Funding Statement
This work was supported by science and technology support projects "the biological technology integration and demonstration of high yield biogas digestion from the mix ingredients" (2011BAD15B03) from Ministry of Science and Technology Department of the People’s Republic of China, and the Fundamental Research Funds for the Central Universities (QM2012002) from Ministry of Education of the People’s Republic of China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jiang D, Zhuang D, Fu J, Huang Y, Wen K (2012) Bioenergy potential from crop residues in China: Availability and distribution. Renew Sustain Energy Rev 16: 1377–1382. [Google Scholar]
- 2. Field CB, Campbell JE, Lobell DB (2008) Biomass energy: the scale of the potential resource. Trend Ecol Evolut 23: 65–72. [DOI] [PubMed] [Google Scholar]
- 3. Yang Z, Zhang H (2008) Strategies for development of clean energy in China. Petrol Sci 5: 183–188. [Google Scholar]
- 4. Madsen M, Holm-Nielsen JB, Esbensen KH (2011) Monitoring of anaerobic digestion processes: A review perspective. Renew Sustain Energy Rev 15: 3141–3155. [Google Scholar]
- 5. Song Z, Yang G, Guo Y, Zhang T (2012) Comparison of two chemical pretreatments of rice straw for biogas production by anaerobic digestion. BioResources 7: 3223–3236. [Google Scholar]
- 6. Weiland P (2010) Biogas production: current state and perspectives. Appl Microbiol Biotechnol 85: 849–860. [DOI] [PubMed] [Google Scholar]
- 7. El-Mashad HM, Zhang R (2010) Biogas production from co-digestion of dairy manure and food waste. Bioresour Technol 101: 4021–4028. [DOI] [PubMed] [Google Scholar]
- 8.Zhu D (2010) Co-digestion of Different Wastes for Enhanced Methane Production: The Ohio State University.
- 9. Zhang P, Yang Y, Tian Y, Yang X, Zhang Y, et al. (2009) Bioenergy industries development in China: Dilemma and solution. Renew Sustain Energy Rev 13: 2571–2579. [Google Scholar]
- 10. Wang FH, Ma WQ, Dou ZX, Ma L, Liu XL, et al. (2006) The estimation of the production amount of animal manure and its environmental effect in China. China Environ Sci 26: 614–617. [Google Scholar]
- 11. Jain M, Singh R, Tauro P (1981) Anaerobic digestion of cattle and sheep wastes. Agr Wastes 3: 65–73. [Google Scholar]
- 12. Kanwar S, Kalia A (1993) Anaerobic fermentation of sheep droppings for biogas production. World J Microbiol Biotechnol 9: 174–175. [DOI] [PubMed] [Google Scholar]
- 13.Dai X, Duan N, Dong B, Dai L (2012) High-solids anaerobic co-digestion of sewage sludge and food waste in comparison with mono digestions: Stability and performance. Waste Management. [DOI] [PubMed]
- 14. Creamer K, Chen Y, Williams C, Cheng J (2010) Stable thermophilic anaerobic digestion of dissolved air flotation (DAF) sludge by co-digestion with swine manure. Bioresour Technol 101: 3020–3024. [DOI] [PubMed] [Google Scholar]
- 15. Luostarinen S, Luste S, Sillanpää M (2009) Increased biogas production at wastewater treatment plants through co-digestion of sewage sludge with grease trap sludge from a meat processing plant. Bioresour Technol 100: 79–85. [DOI] [PubMed] [Google Scholar]
- 16. Bouallagui H, Lahdheb H, Ben Romdan E, Rachdi B, Hamdi M (2009) Improvement of fruit and vegetable waste anaerobic digestion performance and stability with co-substrates addition. J Environ Manage 90: 1844–1849. [DOI] [PubMed] [Google Scholar]
- 17. Álvarez J, Otero L, Lema J (2010) A methodology for optimising feed composition for anaerobic co-digestion of agro-industrial wastes. Bioresour Technol 101: 1153–1158. [DOI] [PubMed] [Google Scholar]
- 18. Macias-Corral M, Samani Z, Hanson A, Smith G, Funk P, et al. (2008) Anaerobic digestion of municipal solid waste and agricultural waste and the effect of co-digestion with dairy cow manure. Bioresour Technol 99: 8288–8293. [DOI] [PubMed] [Google Scholar]
- 19. Xie S, Lawlor P, Frost J, Hu Z, Zhan X (2011) Effect of pig manure to grass silage ratio on methane production in batch anaerobic co-digestion of concentrated pig manure and grass silage. Bioresour Technol 102: 5728–5733. [DOI] [PubMed] [Google Scholar]
- 20. Nguyen VCN, Fricke K (2012) Energy recovery from anaerobic co-digestion with pig manure and spent mushroom compost in the Mekong Delta. J Vietnamese Environ 3: 4–9. [Google Scholar]
- 21.APHA (1995) Standard methods for the examination of water and wastewater: Washington. DC, American Public Health Association.
- 22. Cuetos MJ, Fernández C, Gómez X, Morán A (2011) Anaerobic co-digestion of swine manure with energy crop residues. Biotechnol Bioprocess Eng 16: 1044–1052. [Google Scholar]
- 23. Wang X, Yang G, Feng Y, Ren G, Han X (2012) Optimizing feeding composition and carbon-nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour Technol 120: 78–83. [DOI] [PubMed] [Google Scholar]
- 24. Kayhanian M (1999) Ammonia inhibition in high-solids biogasification: an overview and practical solutions. Environ Technol 20: 355–365. [Google Scholar]
- 25.Siddiqui Z, Horan N, Anaman K (2011) Optimisation of C: N ratio for co-digested processed industrial food waste and sewage sludge using the BMP test. Int J Chem React Eng 9.. [Google Scholar]
- 26. Wu X, Yao W, Zhu J, Miller C (2010) Biogas and CH4 productivity by co-digesting swine manure with three crop residues as an external carbon source. Bioresour Technol 101: 4042–4047. [DOI] [PubMed] [Google Scholar]
- 27. Panichnumsin P, Nopharatana A, Ahring B, Chaiprasert P (2010) Production of methane by co-digestion of cassava pulp with various concentrations of pig manure. Biomass Bioenerg 34: 1117–1124. [Google Scholar]
- 28. Carucci G, Carrasco F, Trifoni K, Majone M, Beccari M (2005) Anaerobic digestion of food Industry Wastes: Effect of codigestion on methane yield. J Environ Eng 131: 1037–1045. [Google Scholar]
- 29.Verma S (2002) Anaerobic digestion of biodegradable organics in municipal solid wastes: Columbia University.
- 30. Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: A review. Bioresour Technol 99: 4044–4064. [DOI] [PubMed] [Google Scholar]
- 31.Palmowskl L, Müller J (2000) Influence of the size reduction of organic waste on their anaerobic digestion. Water Sci Technol: 155–162. [PubMed]
- 32. Duarte A, Anderson G (1982) Inhibition modelling in anaerobic digestion. Water Sci Technol 14: 749–763. [Google Scholar]
- 33. Fernández A, Sanchez A, Font X (2005) Anaerobic co-digestion of a simulated organic fraction of municipal solid wastes and fats of animal and vegetable origin. Biochem Eng J 26: 22–28. [Google Scholar]
- 34. Habiba L, Hassib B, Moktar H (2009) Improvement of activated sludge stabilisation and filterability during anaerobic digestion by fruit and vegetable waste addition. Bioresour Technol 100: 1555–1560. [DOI] [PubMed] [Google Scholar]
- 35. Veeken A, Hamelers B (1999) Effect of temperature on hydrolysis rates of selected biowaste components. Bioresour Technol 69: 249–254. [Google Scholar]
- 36. Angelidaki I, Ahring B (1992) Effects of free long-chain fatty acids on thermophilic anaerobic digestion. Appl Microbiol Biotechnol 37: 808–812. [DOI] [PubMed] [Google Scholar]
- 37. Angelidaki I, Ahring B (1993) Thermophilic anaerobic digestion of livestock waste: the effect of ammonia. Appl Microbiol Biotechnol 38: 560–564. [DOI] [PubMed] [Google Scholar]
- 38.Angelidaki I (1997) Anaerobic digestion in Denmark. Past, present and future. Servicio de Publicaciones. pp. 335–342.