Abstract
It is sometimes necessary for nonhuman primates to be restrained during biomedical and psychosocial research. Such restraint is often accomplished using a “primate chair.” The present paper details a method for training adult rhesus macaques to cooperate with a chair restraint procedure using positive and negative reinforcement. Successful training was accomplished rapidly in approximately 14 training days. The success of this training technique suggests that this method represents a refinement to traditional techniques despite the behavioral heterogeneity in the animal sample (which includes animals previously deemed unfit for traditional pole-and-collar training).
Keywords: chair training, nonhuman primate, positive reinforcement, negative reinforcement
Biomedical and psychosocial research with nonhuman primates often requires data to be collected from animals while they are awake and restrained. The training methods used in concert with those methods typically do not use positive reinforcement techniques or allow the animals to be voluntarily restrained. In this paper, we detail a method for training rhesus monkeys to be voluntarily restrained. We refer to this method as “cooperative training” because, while the training technique of choice is positive reinforcement, the method also uses desensitization and negative reinforcement (for other discussions of cooperative training see e.g., Perlman et al., 2012; Minier, Hannibal, Sharpe, & McCowan, 2012; Joint Working Group on Refinement, 2009; Reinhardt, 2003; Reinhardt, Liss, & Stevens, 1995). We first provide a brief review of previous restraint methods and then discuss the advantages of using cooperative training techniques. We then discuss our particular training needs and the protocol that was used to accomplish those goals, which we believe will be widely useful for other research groups.
NONHUMAN PRIMATE RESTRAINTS AND RESTRAINT TRAINING
Much of what is known about the biomedical and psychosocial lives of primates comes from laboratory-based studies of nonhuman primate physiological, cognitive, and social processing. Such studies often require animals to perform tasks while they are awake and aware (i.e., not sedated), necessitating some form of restraint. The number, construction, and function of nonhuman primate restraints are as diverse as the experiments utilizing them (for a review see Reinhardt, Liss, & Stevens, 1995). The backs of primate cages typically have a grate that can be moved forward (e.g., a “squeeze panel” or “squeeze back”) to restrain the animal at the front of the cage. If research goals require that animals be removed from their cages and restrained, a number of options, such as straps that position animals on their backs (Osborne, 1973), jackets and tethers used in open cages (Morten, Knitter, Smith, Susor, & Schmitt, 1987) are available.
The most common restraint device used with nonhuman primates outside of their cages is the primate “chair” in which an animal is trained to sit with his or her head or neck restrained. Prior to being trained for chair restraint, monkeys are typically fitted with aluminum collars to which a long metal pole can be attached (e.g., Anderson & Houghton, 1983). These poles are used to guide monkeys from their cages into the primate chair where their collars are subsequently attached to the chair. Primate chairs generally come in two different varieties; “open” and “closed”. Open primate chairs are typically constructed from metal or plastic bars and include a seat on which the monkey sits and a tether point at which the animal’s head or neck is restrained to the chair (see Figure 1a). Closed chairs are typically plastic or metal boxes with an internal seat on which the animal sits and a partially open top into which the animal’s head or neck can be secured (see Figure 1b).
FIGURE 1.
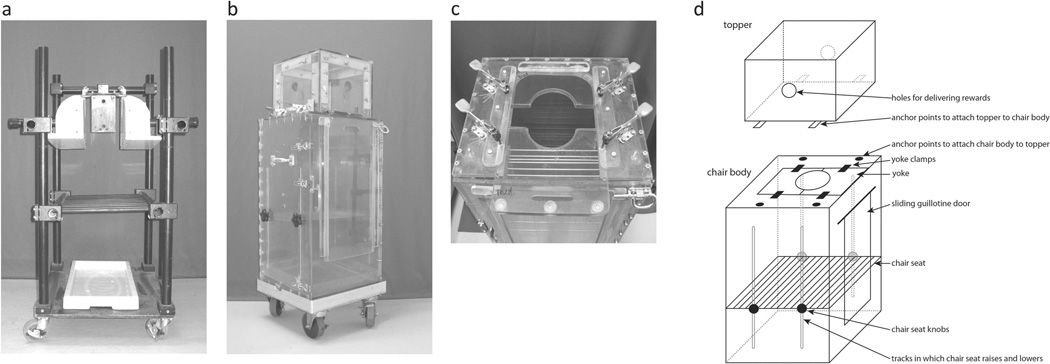
Photos of typical primate chairs. (a) Example of a commercially available open chair. (b) The closed chair used in the present study. The dimensions of the chair body were 37.5 cm × 34 cm × 70 cm, with 3-cm metal legs (obscured by the rolling cart in this photo). The front door of the chair can be opened allowing access to the monkey or for cleaning. Monkeys enter and exit the chair via a vertical sliding door (typically referred to as a guillotine door) on the front door that covers a 24-cm × 37-cm opening. A fitted cage pan sits at the bottom of the chair. The entire opening is covered by a topper that secures to the chair top via wing nuts and has circular openings on all 4 sides for delivering rewards. The chair seat is secured in the chair via screws to which external knobs attach. The knobs can be loosened, allowing the seat to slide up and down within the chair body. (c) The top of the chair body includes an opening into which 2 plastic yoke pieces slide and are affixed via clamps. A total of 3 different yoke sizes were used depending on the size of our animals—small (8.5-cm diameter), medium (10 cm, pictured here in the open position), and large (12 cm). (d) Chair schematic with labeled components.
A number of reports have detailed the construction of different types of chairs (e.g., Florence et al., 1995; Milhaud, Klein, & Merkel, 1980; Mason, 1958; Schmidt, Dold, & McIntosh, 1989; Henry & Bowman, 1971), some of which are similar to the one used in this study (e.g., Robbins, Zwick, Leedy, & Stearns, 1986; Barrow, Luschei, Nathan, & Saslow, 1966; Carlson, 1972; Sledkeski, 1969; Machado & Nelson, 2011). Some reports discussed training that has a reward component, insofar as the animals were given rewards during the chairing procedure (Barrow et al., 1966; Robbins et al., 1986) although none provide specific data about training length and outcomes and the most typical training methods (e.g., Anderson & Houghton, 1983) utilize no or essentially no positive reinforcement.
A renewed international focus on the wellbeing of animals, and in particular, the wellbeing of nonhuman primates, has spurred interest in the use of positive reinforcement techniques to teach animals to cooperate with husbandry and laboratory procedures (for reviews and discussions see Reinhardt, 2004; Schapiro, Bloomsmith, & Laule, 2003; Laule, Bloomsmith, & Schapiro, 2003; Prescott & Buchanan-Smith, 2003). Positive reinforcement training can also be used for behavioral management challenges such as facilitating socialization (Schapiro, Perlman, & Boudreau, 2001), reducing abnormal stereotypic behaviors (Coleman & Maier, 2010), and reducing aggression (Minier et al., 2011).
Positive reinforcement training works by rewarding desired behaviors, which increases the likelihood that the desired behaviors will occur in the future. Complex behaviors can be trained by breaking down the desired behavior (e.g., move to the front of the cage and offer arm for venipuncture) into steps (e.g., 1: move to front of cage, 2: sit quietly at front of cage for a given duration, 3: allow trainer to touch arm, and so on) in which rewards are received. This process of rewarding small steps that will eventually lead to the desired behavior is called “successive approximation” or “shaping.” In addition to using shaping, the method outlined in this paper also used desensitization and negative reinforcement. Desensitization occurs when the animal is exposed to an unpleasant stimulus on multiple occasions and gradually becomes less reactive to it. It can be combined with positive reinforcement (providing rewards during the presentation of the unpleasant stimulus). Negative reinforcement occurs when the target behavior is generated by avoiding an unpleasant stimulus or when the unpleasant stimulus ceases when the target behavior is generated. Removing an aversive stimulus increases the likelihood that a desired behavior will occur in the future. It is important to note that negative reinforcement differs from punishment insofar as an aversive stimulus is presented during punishment in order to decrease the occurrence of a given behavior. No existing reports document procedures for using positive reinforcement or cooperative techniques (a blend of positive and negative reinforcement with desensitization) for chair training monkeys.
The present study was conducted because the animals needed to be to be chaired in a standard closed primate chair (see Figure 1b) in order to replicate a previously conducted experiment (Antoniadis, Winslow, Davis, & Amaral, 2009, 2007), but some of the animals were not able to be trained via pole-and-collar methods. The goal was to train the animals to lift their heads through the top of a closed chair, be yoked at the neck, and sit calmly and attentively. A pure positive reinforcement method for training macaques in a closed primate chair would successively approximate the final behavior (allowing neck to be restrained in the opening at the top of the chair) and reward desired behaviors (e.g., sitting quietly in box, lifting head into position, allowing neck to be restrained, etc.). Training was attempted in that manner and then the procedure was modified in order to accommodate a diverse group of animals and the speed with which they needed to be trained. The final procedure included mild negative reinforcement and desensitization. Our method allows the animals to actively participate in their training.
METHODS
Experimental procedures were developed in consultation with the research and veterinary staff at the California National Primate Research Center (CNPRC). All protocols were approved by the University of California Davis Institutional Animal Care and Use Committee.
Animals
Experimental subjects were 16 rhesus macaques (Macaca mulatta; 9 males, 7 females). Fourteen of the animals were part of a longitudinal developmental study from our laboratory (e.g., Bliss-Moreau, Bauman, & Amaral, 2011; Bliss-Moreau, Toscano, Bauman, Mason, & Amaral, 2010, 2011; Bauman, Lavenex, Mason, Capitanio, & Amaral, 2004a, 2004b; referred to as “experimental animals” henceforth). The other two animals were control animals for a previous project in our laboratory (Babineau, Bliss-Moreau, Toscano, Machado, & Amaral, 2011) and were included in the present study so that the subsequent experiment (not discussed here) could be pilot tested with them (referred to as “pilot animals” henceforth). All animals were born at the California National Primate Research Center and ranged from 9 to 10.5 years of age at the beginning of training.
Rearing and Experimental Histories
Experimental Animals
The 14 experimental animals were reared indoors as part of a longitudinal study on the impact of neonatal amygdala damage on social and emotional development. Details about the animals’ rearing history have been discussed in detail elsewhere (Bliss-Moreau et al., 2010, 2011a, 2011b; Bauman et al., 2004a, 2004b). Briefly, at approximately two weeks of age, the experimental animals underwent a surgery during which they received either bilateral ibotenic acid lesions to the amygdala or sham operations. After surgery, they were returned to their mothers. Mothers and infants participated in social groups (with other experimental animals) 5 days a week for 3 hr each day in large social cages (2.13 m wide × 3.35 m deep × 2.44 m high). After weaning at 6 months of age, experimental animals were socially housed in mixed sex groups in the large social cages that included an unrelated adult male and female. Group housing occurred both indoors and outdoors at various points in their lives. In adulthood, animals were pair-housed indoors in mixed-sex pairs. The present study included 6 amygdala-lesioned animals (3 males, 3 females) and 8 sham-operated controls (4 males, 4 females).
At the time of the present study, all animals were pair-housed in standard primate caging (112 cm × 68 cm × 92 cm or 87 cm × 66 cm × 83 cm) in male-female pairs and allowed access to their pair-mate (and his/her cage) either a minimum of 7 hr per day or continuously. Rooms were maintained on a 12 hr light-dark cycle at 26 °C. Animals were fed monkey chow twice daily, oat-rice-pea enrichment on forage boards once daily, produce 2 times per week, and they were offered supplemental enrichment (e.g., tubes or balls filled with fruit) at various times throughout the week. Water was provided ad libitum.
Pilot Animals
The 2 remaining male monkeys were chosen based on a successful pole-and-collar chair restraint training (Anderson & Houghton, 1983) history in order to pre-test the experimental procedure that the experimental animals would complete after chair training. The animals’ rearing and testing histories are described elsewhere (see Babineau et al., 2011). During the present study both animals were allowed full access to an adult male pair-mate and his cage 7 days a week for a minimum of 7 hr each day.
Chair Restraint History
Prior to the current study, 9 of the 16 monkeys (2 pilot animals, 7 experimental animals) underwent some chair restraint training with the pole-and-collar technique as described by Anderson and Houghton (1983). The two pilot males were successfully trained approximately 3.75 years prior to beginning the current training procedure. The other 7 animals underwent 4 to 11 days of training approximately 8 months prior to the current procedure (see Table 1). No experimental animals were deemed fully trained according the CNPRC standards in that time period. Training for these animals was stopped due to poor training outcomes after 11 days (see discussion of “Training Challenging Animals” in Results section).
TABLE 1.
Training Techniques Summary
| Previous training experience | Training techniques used in this study | ||||||
|---|---|---|---|---|---|---|---|
| Animal Name and Group |
Pole-and-collar training experience |
Successfully pole-and-collar chairing trained |
Positive reinforcement |
Move squeeze |
Move chair bottom |
Use pole |
Total number of training days required |
| Project Animals | |||||||
| Group 1 | |||||||
| Cecelia | x | x | 9 | 3 | 15 | ||
| Linus | x | 1 | 11 | 1 | 15 | ||
| Ike | x | x | 3 | 3 | 13 | ||
| Kermit | x | 5 | 9 | 3 | 20 | ||
| Max | x | 1 | 6 | 5 | 19 | ||
| Sandy | x | 5 | 3 | 15 | |||
| Group 2 | |||||||
| Britney | x | x | 4 | 12 | |||
| Darla | x | 7 | 1 | 14 | |||
| Isaiah | x | 3 | 4 | 10 | |||
| Zoe | x | x | 1 | 4 | 12 | ||
| Boca | x | x | 5 | 11 | |||
| Duke | x | x | 4 | 10 | |||
| Wanda | x | 8 | 10 | ||||
| Quebert | x | x | 1 | 8 | 15 | ||
| Pilot Animals | |||||||
| Halibut | x | x | x | 3 | |||
| Virgil | x | x | x | 9 | |||
Note. “x” indicates that a training experience or technique occurred for a given animal. Numbers in the “move squeeze,” “move chair bottom,” and “use pole” columns indicate the number of times that a given negative reinforcement technique was used with each animal. The techniques were never used with animals who do not have numbers in those columns.
Trainers
The trainers for this study were the authors, all of whom had experience with rhesus monkeys (3–8 years) and with basic training techniques (e.g., clicker training) used with nonhuman primates and other animals (e.g., dogs).
Training Room
Training occurred in a small laboratory test room (3.1 m × 3.1 m) at the CNPRC. Prior to each training day, 4 animals were transported from their home cage to a room adjacent to the training room with 4 cages (66 cm × 61 cm × 84 cm) to await their training. An additional primate cage (“transfer cage”) was positioned on the room floor on a metal frame (14 cm off of the ground) such that the door to the cage was at the same level as the door to the chair. Transferring animals to this cage via transfer box allowed for loading animals into the chair that were temporarily housed in upper holding cages. The transfer cage was equipped with a standard “squeeze” mechanism that could be moved forward to move the animal towards the cage door (a form of negative reinforcement). Animals entered the box chair in this room and were wheeled down a short hallway to the training room. The only visual stimuli in the training room were the experimental equipment and computer.
Box Chair
The shape of the box chair used for this experiment was similar to designs previously used in our laboratory (Machado & Nelson, 2011; Anatondis et al., 2007, 2009) and others (e.g., Robbins et al., 1986; Barrow et al., 1966; Carlson, 1972; Sledkeski, 1969; see Figure 1). The key features of the chair included a vertical sliding door on one side, a seat that could be moved up and down within the body of the chair, an opening at the top that allowed for different sized “yokes,” and a “topper” that allowed for animals to maneuver into the top of the chair without escaping. A wheeled cart allowed the chair to be easily moved from one room to another.
Food Rewards
Target behaviors (see specific training steps below) were rewarded with desired food rewards that were delivered to the animals with either forceps (25 cm long) or a curved-tip syringe (Kendall Monject 412 Curved Tip Syringes; Tyco Healthcare, Mansfield, MA). These rewards included a variety of dried fruits, cereals, marshmallows, and sugar-free juice. Rewards were chosen based on our subjective perception of animal preference and nutritional content.
Training Procedure
Prior to training, animals were sedated with 5 mg/kg of ketamine hydrochloride and fitted with aluminum collars (Primate Products, Immokalee, FL) as was standard practice at the CNPRC.
The training methods discussed evolved while training the first group of animals (Group 1 in Table 1). What is presented here is the final, refined procedure that represents what we believe to be the most effective method for training animals. The training procedure is discussed in terms of steps associated with achieving specific behavioral goals. Progression from one step to the next represented progress towards the ultimate goal: quick and voluntary presentation of neck for yoking (securing the animal in the chair), and, while yoked, calm behavior for extended periods of time. Often steps overlapped to expedite training, and determining when the animal should go from one step to the next was at the discretion of the trainer. Behavioral reactivity of each animal in response to the trainer, the chair, the yoke and the delivery of treats were monitored (see Table 2). In addition to rewarding target behaviors (as indicated in the training steps), animals were desensitized to the chair, yoke, and being yoked at the various steps of the training procedure.
TABLE 2.
Behavioral Indices Recorded During Training
| Index | Index Definition | Rating Scale |
Rating Scale Definition |
|---|---|---|---|
| Willingness to Participate |
The ease with which the animal raises head into place to be yoked. Only applicable when animal is yoked. |
0 |
Trainer had to lift collar with pole |
|
1 |
Trainer had to move chair bottom up |
||
|
2 |
Animal positions head willingly with prompt |
||
| Reactivity | The extent to which the animal generates behaviors in response to the chair, room, and/or trainer. |
0 | Not reactive |
| 1 | Mildly reactive: Mild threats, aggression, fear or nervousness. Minimal number of short bouts. |
||
| 2 | Moderately reactive: Either longer or more frequent bouts of mild threats, aggression, fear or nervousness. |
||
| 3 | Very reactive: Overt threats, aggression, fear, nervousness. Magnitude of reaction moderate. Sustained for most of day. |
||
| 4 | Extremely reactive: Overt threats, aggression, fear, nervousness. Magnitude of reaction great. Sustained for length of day. |
Trainers made every attempt to end each training day after the successful achievement of a step-related goal but before animals became too frustrated with the training. Because of this, training durations ranged from as short as 5 min to as long as 1 hr. Durations were recorded and rounded to the nearest 5 min. The time required to yoke each animal was recorded, and an average was computed for each animal using the data from the day on which the animal was deemed trained and a subsequent day. Times for one control male in the first group were only available for the days after he was deemed fully trained. The time from the day after being deemed trained was not available for one amygdala-lesioned male, so a time from the next day was used.
Steps for Cooperative Chair Training
See Figure 1 for a photo of the chair used in this training procedure (panels b and c) and a schematic diagram with labeled components (panel d).
Step 1: Entering the Chair
The goal of this step was to get the animals to willingly enter the chair without hesitation. The chair was assembled without the yoke, with the seat lowered to the level of the bottom of the door and with treats placed on the seat for the first 1 to 2 days of training. The chair was then wheeled into position in front of either the holding cage or transfer cage so that the door of the chair was directly in front of the cage door. The guillotine door of the chair was lifted, and animals were verbally instructed to jump into the chair. If the animal did not jump into the chair after approximately 1 to 3 min, the squeeze apparatus was moved forward slowly so that the animal would enter the chair. Use of the squeeze was not necessary for most animals (see Table 1). Animals were rewarded during the first few days for moving into the chair until they moved willingly into the chair on their own.
Step 2: Achieve Comfort in the Chair
The goal of this step was to have the animal readily take treats with their mouths from the trainer once their behavioral reactivity to the chair and the trainer had decreased. Once the animal was in the chair, the trainer offered treats to the animal slowly but continuously through the holes in the topper. Dry treats were delivered using a large pair of forceps, and juice was delivered using a curved-tipped syringe. Animals were required to take the treats with their mouths (not hands) by withdrawing the treat if the animal reached for it with his or her hands.
Step 3: Head Lifted for More Than 5 Seconds
The goal of this step was to have the animals hold their heads elevated for a treat for more than 5 s. Animals were rewarded for lifting their heads above the level of the yoke holder by being offered treats near the top of the topper and then outside of the topper holes. Animals were cued to lift their heads by the researcher saying “up” and gently tapping on the side of the chair.
Step 4: Exhibit Comfort With Yoke
The goal of this step was to get animals to hold their heads above the yoke holder for more 5 s while the yoke was moved in the holder. The yoke slid into the yoke holder such that the yoke opening was as large as it could be (“open position”). Animals were given treats while the yoke was moved in the yoke holder to desensitize them to the yoke.
Step 5: Neck Yoked
The goal of this step was to close the yoke, securing the animal’s head above the yoke. As the yoke was opened, animals were cued to lift their heads by the researcher saying “up” and briefly tapping on the chair. Animals were rewarded for allowing the yoke to be closed and secured after lifting their heads into place. Treats were offered immediately after yoking. The animal was released from the yoke once his or her behavioral reactivity had diminished, typically after 1 min. All animals’ behavioral reactivity diminished over time. In order to keep training progressing, it was important that once an animal was initially yoked, the animal was yoked again during each subsequent training day. The amount of time the animal spent yoked was extended with every day.
Animals typically attempted to evade yoking during the day(s) that followed their initial yoking. When animals evaded yoking, they were first baited by offering a single treat via the top of the topper to allow the animal to voluntarily move into position and once again cued to lift their heads (“up” plus tap). If the animal did not present his or her neck for yoking within 5 to 10 min, negative reinforcement was used to get the animal to present his or her neck for yoking. Using negative reinforcement was necessary because all 14 animals needed to be trained within a relatively short period of time. If the animal did not raise his or her head, the chair’s seat was then adjusted. The seat was raised slightly, decreasing the space the animals could occupy. This continued until the animal presented his or her head. For some animals, moving the seat so that the seat was slightly uneven (one side was higher than the other by ~ 4 cm) or moved up and down in short, rapid movements prompted them to lift their heads.
During early training days, and as a last resort, a bent metal pole (66 cm long and 4 mm in diameter) was attached to one side of the collar (via the hole in the ceiling of the topper) and the animal’s head was gently raised into place. The pole was used on isolated instances during training of the initial 7 animals, and our belief is that its use dramatically impeded training. Refinement of our training technique (e.g., beginning to use movement of the chair bottom to promote correct head positioning) precluded its use for any other animals (see Table 1).
Final Goal: Voluntary Yoking
Over time animals became accustomed to presenting their heads above the yoke holder for yoking when cued by the researcher saying “up” and tapping on the chair with little or no incentive (e.g., no showing or offering treats, no movement of the seat, etc.). Animals were deemed fully trained once they voluntarily presented their necks for yoking on 2 consecutive days in less than 5 min. Voluntary presentation was operationalized as “willingness to participate” scores of 2.
Typically, Steps 1 to 4 were accomplished during the first few days. All but 2 animals were at Step 5 (neck yoked) during the fourth training day. All additional training was related to reinforcing their head/neck presentation (as indexed by an increase in willingness to participate) and acclimation to the chair (as indexed by a decrease in reactivity).
Technique Refinement
After working with 4 animals (1 female control, 1 male amygdala-lesioned animal, and 2 control males) for 10, 11, 3, and 2 days, respectively, the chair was modified so that the seat could be raised higher within the chair body. This dramatically changed the training results. In the original position, the animals had to unnaturally stretch upwards in order to have their heads and necks positioned correctly. Given the major change, the number of training days that occurred prior to this modification was not included. Training for an additional control female (discussed as a case study below) was stopped after 10 days and restarted a week later. Data from her first 10 days were not included in the analyses.
RESULTS
Experimental Animals
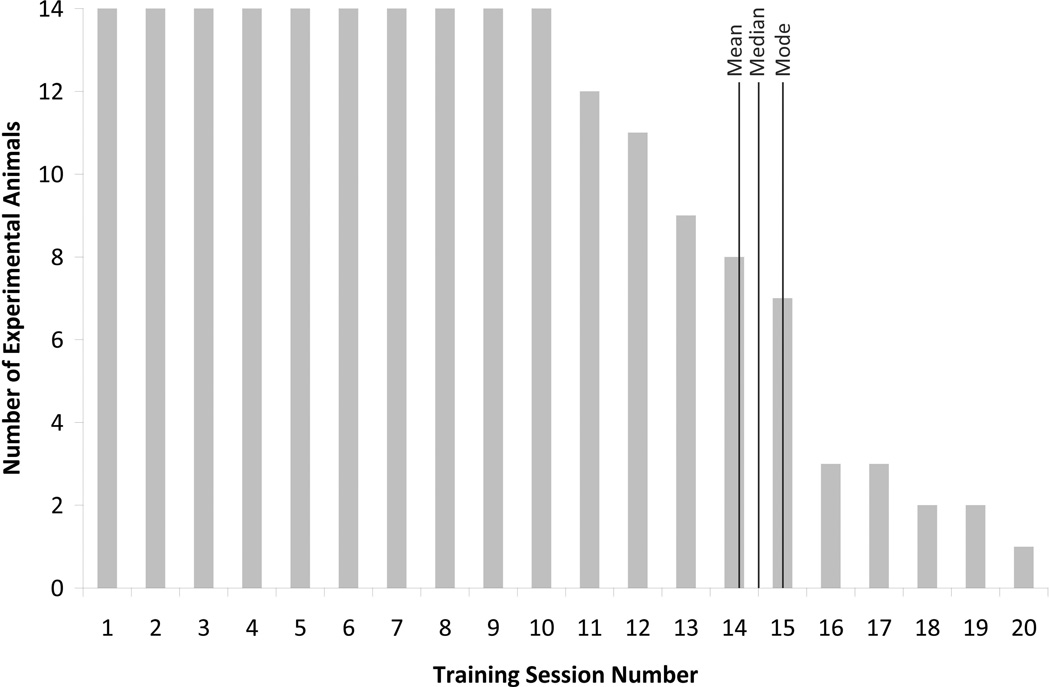
Experimental animals required an average of 14.14 training days (SD = 3.09) to reach training criterion. There were no differences between groups (control vs. amygdala-lesioned animals, F(1, 14) = 2.72, p < .13, ηp2 = .185; and males versus females, F(1, 14) = .26, p < .62, ηp2 = .021) in the number of days required for reaching criterion (see Figure 2).
FIGURE 2.
Number of training days required. Training day numbers are plotted on the x-axis. The number of animals requiring that number of training days is plotted on the y-axis. Note that the mean, median, and mode are all clustered around 14 through 15.
Animals were trained in groups because of space and staff limitations. To assess whether the number of training days to meet the criteria changed as the methods evolved, animals were assigned to 1 of 2 groups based on whether they were trained early (while we were still developing the methods, N = 6) or late (once the methods had been established, N = 8). Animals in the late group were trained in significantly fewer days than animals in the early group, F(1, 14) = 10.71, p < .01, ηp2 = .472, suggesting that refining the technique lead to a significantly reduced average number of days to reach criteria (Mearly = 16.50, SDearly = 2.67; Mlate = 12.38, SDlate = 2.07). The effect was the same when evaluating this interval-dependent variable with nonparametric statistical tests (Mann-Whitney U Test, p = .013).
Average length of training per day was 26.54 min although there was remarkable variance in the length of the training days across days (SD = 4.11). Duration of training was negatively correlated with training day, indicating that duration of training became significantly shorter as training progressed (r = −.20, p < .003) because animals presented their necks more quickly for yoking. At the end of training, animals were successfully yoked in an average of 1.23 min (SD = .84).
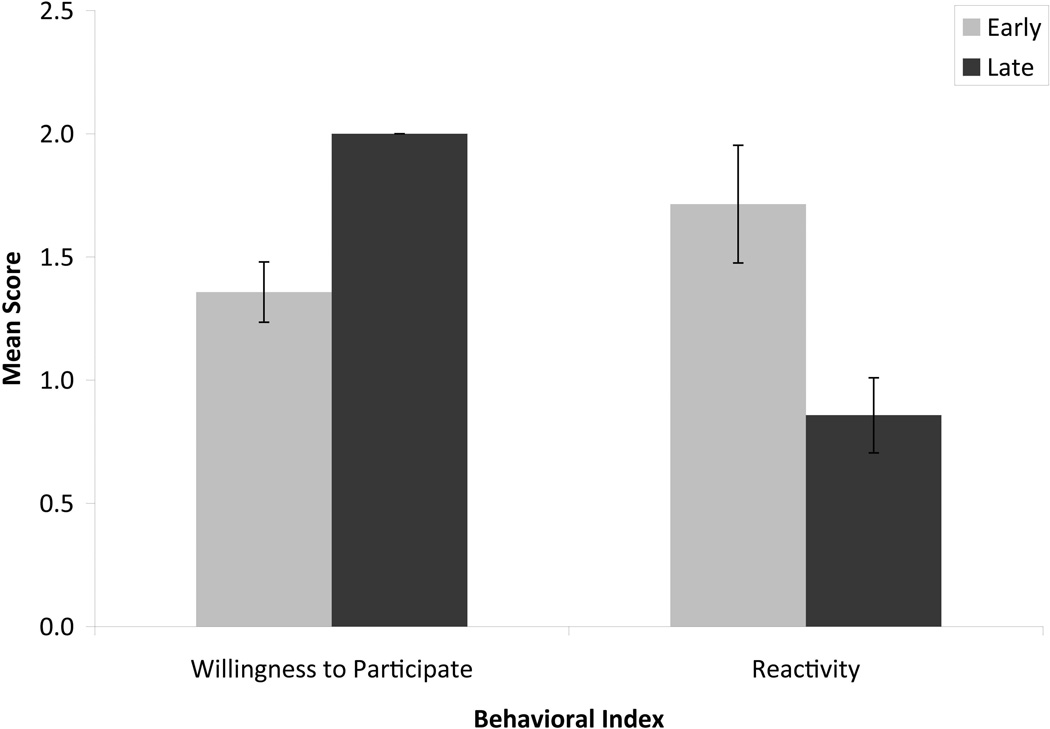
To assess behavioral changes from the first time the experimental animals were yoked to the end of the training procedure, a mean of each animal’s willingness to participate scores was computed from the first training day during which he or she was yoked and the subsequent training day (e.g., “early willingness to participate”) as well as a mean of each animal’s willingness to participate scores from the last two training days (e.g., “late willingness to participate”). “Early reactivity” and “late reactivity” were computed scores in the same manner. Animals were significantly more willing to participate at the end of training as compared to the beginning, t(13) = 5.26, p < .0001. Similarly, animals’ reactivity scores were significantly lower at the end of training as compared to the beginning, t(13) = 2.39, p < .033 (see Figure 3). Neither lesion condition nor sex significantly impacted either willingness to participate or reactivity.
FIGURE 3.
Average willingness to participate and reactivity scores for the first two days during which animals were yoked (early) as compared to the last 2 training days (late). Error bars represent standard errors. Willingness to participate was scored on a scale of 0 to 2; reactivity was scored on a scale of 0 to 4.
Special Cases
Retraining After Pole-and-Collar Chairing
We retrained 2 animals who had been previously pole-and-collar trained so that we could test an experimental task. Neither pilot animal was collared in order to assess whether this training technique could be accomplished without collars. Both pilot animals learned extremely quickly to voluntarily raise their heads and be yoked. The first pilot animal required 4 days to reach criteria. No attempt was made to yoke the first animal during his first 2 training days, but he was willingly yoked (willingness to participate score of 2) on the subsequent 2 days. He received a reactivity score of 1 on 1 day and a score of 0 on the remaining 3 days. The second pilot animal required 9 days to reach criteria, but no attempt to yoke him was made during his first 4 days (during which he was mildly reactive, average score 1.5). His reactivity decreased substantially during the final 5 days (average score .6). Taken together these data suggest that animals with previous chairing experience can be quickly retrained, even after a long delay (up to 3.75 years), and that collars are not necessary for training using this method.
Training “Challenging” Animals
One hallmark of our method is that it is particularly suitable for use with challenging animals. Pole-and-collar training was attempted with a group of the experimental animals prior to this study. That training was stopped when 2 of the amygdala-lesioned animals (1 male and 1 female) who were known to engage in self-directed behaviors increased the intensity of these behaviors and when 2 of the male control animals had not made any progress in training after 10 and 11 days, respectively. Both of the amygdala-lesioned animals were easily and readily trained using the chair-training method discussed here with no increase in evidence or intensity of self-directed behaviors. The male was trained in 13 days and was one of the least reactive animals on the project (average reactivity score = .76). The female was trained in 15 days and was also lowly reactive (average reactivity score = 1.07).
Two of the control animals refused to participate in pole-and-collar training. The first control male had not accomplished the first step of training (positioning body and presenting arm in order to allow pole to be attached to collar) after 11 days of training. He was reported as “combative” during training days. Using the method outline in this paper, he was fully trained in 10 days. His reactivity scores at the beginning of training (3 s and 2 s) decreased to stable scores of 1 by the end of training (average reactivity score = 1.6). Similarly, a second control male had not accomplished the first level of training after 11 days, and was reported as “difficult,” “resistant,” and “very aggressive.” Using the method outlined in this paper, he was completely trained in 14 days (average reactivity score = 1.5).
Pole-and-collar training was not initially attempted with our final challenging animal. This control female is the only animal on the project who is primarily Chinese (¾ Chinese origin, ¼ Indian origin). Her mixed heritage may have been the source of her heightened behavioral reactivity, as macaques of Chinese origin and Chinese-Indian hybrid animals are significantly more behaviorally reactive than macaques of pure Indian origin (Campoux, Higley, & Suomi, 1997; Champoux, Suomi, & Schneider, 1994). During the initial days of chair training, the animal was reactive, fixated on her collar, refused to take any treats offered to her, and spun rapidly in the chair. Her level of reactivity was not seen in any other animal. The trainer noted that her collar obstructed the yoke on many attempts to close it and she became even more reactive when this occurred. The animal would also physically force the yoke out of the chair even when it was clamped into place, necessitating that the clamps be reconstructed. After 10 training days, the veterinary staff asked to temporarily stop training her due to an unrelated health issue. She was returned to chair training approximately 1 week later.
We elected to remove her collar before resuming training. When training resumed we also tried a variety of other treats and finally found one she liked (sugar-free strawberry kiwi juice). Once training resumed without the collar and with her preferred juice, she progressed along a trajectory similar to the other animals (trained in 15 days with declining reactivity) although no attempt to close the yoke was made until the 6th training day.
DISCUSSION
The training procedure outlined in the present report, as well as the data collected during training days, clearly indicate that rhesus macaques can be trained to cooperate in restraint training. Training for all animals proceeded relatively quickly. Our methods, therefore, are a good fit for fast-paced research environments where financial and personnel resources are limited and in which animals need to be quickly prepared for research participation. What’s more, challenging animals deemed unfit for traditional methods were easily trained via cooperative methods. During the training period, none of the animals were found to have poor appetites or to be withdrawn, or demonstrated any other behavioral characteristics that were not normal for them. Behavioral reactivity diminished across training days, indicating that animals became accustomed to the procedure and being chaired. Taken together, these findings suggest that this protocol can be easily adjusted to accommodate variations in animals’ behavioral patterns.
Animals were trained in phases and did not immediately begin the next experiment, so periodic training was continued with the animals (~ 1 day per animal per week) to ensure that training was retained. Animals were positively reinforced during these additional days when they moved their necks into position and allowed yoking. Use of negative reinforcement became unnecessary over time for most animals. Only 2 of the 14 experimental animals required moving the chair seat in order to get them into position during maintenance days. All animals continue to cooperate, and the speed with which they present their necks for yoking has been maintained. Furthermore, all animals are currently being chaired twice a week for an experiment, and there have been no changes in indices of wellbeing (e.g., abnormal behaviors, poor appetite, social withdrawal, etc.), which indicates that the chairing procedure does not cause them undue stress.
We will make a few modifications to the procedure in the future that are worth noting here. Early in training, a pole was used to move animals’ heads into position. It is our belief that using the pole impeded training progression. Thus, we do not advocate the use of a pole. Additionally, our experience with the 2 pilot animals and our 1 challenging control female suggest to us that it is not necessary to use primate collars either. For most animals, the collar hit the yoke at least a few times during training which was, at least initially, startling. It is possible that training might have proceeded more swiftly if animals were not collared. We are currently training a new cohort of animals and have elected not to use collars or a pole. To date, 8 adult male macaques have been trained in an average of 8.63 days (SD = 3.5) using this method.
CONCLUSION
Restraint is widely recognized to be stressful to animals (for a review see Reinhardt, 2004; Reinhardt, Liss, & Stevens, 1995). Further, behavioral indices of stress do not map perfectly onto physiological markers of stress (blood cortisol concentrations) during restraint when animals are chair-trained via traditional methods described by Anderson and Houghton (1983; Ruys, Mendoza, Capitanio, & Mason, 2004). Given that monkeys are typically restrained, at least initially, without their cooperation (Reinhardt, Liss, & Stevens, 1995), it is not clear whether stress responses are a result of restraint per se, the method used to teach the animal to be restrained, or some combination of the two. One possibility is that the context itself (the chair) becomes negative (and able to generate a stress response) when animals learn to be restrained via traditional means.
Contextual conditioning of this sort occurs when negative experiences in a given context imbue the environment itself with negativity (for reviews see Bouton, 2004, 2002). In this view, cooperatively training animals should serve to imbue the environment with positive value, thus reducing (or precluding) stress. Evidence from training studies with chimpanzees provides preliminary evidence in support of the view that positive reinforcement training reduces stress levels associated with laboratory procedures (Lambeth, Hau, Perlman, Martino, & Schapiro, 2006; Videan, Fritz, Murphy, Howell, & Heward, 2005). Whether or not this chair training procedure would reduce animals’ overall stress levels while being restrained is a testable hypothesis to which the present data does not speak. Investigating whether or not restraint after cooperative chair training has stress-related physiological consequences is a potentially fruitful avenue for future research.
ACKNOWLEDGMENTS
This research was supported by a grant from the National Institute of Mental Health (R37MH57502) and by the base grant of the California National Primate Research Center (RR00169). E. B. M. is supported by a National Service Research Award from the National Institute of Mental Health (F32MH087067). We thank Bradley Lane and Anthony Santistevan for their assistance with training, and the veterinary, Behavioral Management, husbandry, and research staff of the California National Primate Research Center for excellent care of the animal subjects, and three anonymous reviewers for their helpful comments on a previous draft. In particular, we would like to thank Darren E. Minier and Dr. Brenda McCowan for their help with this project and their comments on this manuscript.
REFERENCES
- Anderson JH, Houghton P. The pole and collar system A technique for handling and training nonhuman primates. Lab Animal. 1983;12:47–49. [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. Role of the primate amygdala in fear-potentiated startle Effects of chronic lesions in the rhesus monkey. Journal of Neuroscience. 2007;27:7386–7396. doi: 10.1523/JNEUROSCI.5643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biological Psychiatry. 2009;65:241–248. doi: 10.1016/j.biopsych.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babineau B, Bliss-Moreau E, Toscano JE, Machado CJ, Amaral DG. Context specific social behavior is altered by oribitofrontal cortex lesions in adult rhesus macaques. Neuroscience. 2011;179:80–93. doi: 10.1016/j.neuroscience.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow S, Luschei E, Nathan M, Saslow C. A training technique for the daily chair of monkeys. Journal of the Experimental Analysis of behavior. 1966;9:680. doi: 10.1901/jeab.1966.9-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. Journal of Neuroscience. 2004a;24:711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of social behavior following neonatal amygdala lesions in rhesus monkeys. Journal of Cognitive Neuroscience. 2004b;16:1388–1411. doi: 10.1162/0898929042304741. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Bauman MD, Amaral DG. Neonatal amygdala lesions result in globally blunted adult affect in adult rhesus macaques. Behavioral Neuroscience. 2011;125:848–858. doi: 10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala or hippocampus lesions influence responsiveness to objects. Developmental Psychobiology. 2010;52:487–503. doi: 10.1002/dev.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss-Moreau E, Toscano JE, Bauman MD, Mason WA, Amaral DG. Neonatal amygdala lesions alter responsiveness to objects in juvenile rhesus macaques. Neuroscience. 2011;178:123–132. doi: 10.1016/j.neuroscience.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context ambiguity and unlearning Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Carlson KR. A temporary restraint chair for monkeys. Physiology and behavior. 1972;9:493–494. doi: 10.1016/0031-9384(72)90181-3. [DOI] [PubMed] [Google Scholar]
- Champoux M, Higley JD, Suomi SJ. Behavioral and physiological characteristics if Indian and Chinese-Indian hybrid rhesus macaque infants. Developmental Psychobiology. 1997;31:49–63. [PubMed] [Google Scholar]
- Champoux M, Suomi SJ, Schneider ML. Temperament differences between captive Indian and Chinese-Indian hybrid rhesus macaque neonates. Laboratory Animal Science. 1994;44:351–357. [PubMed] [Google Scholar]
- Coleman K, Maier A. The use of positive reinforcement training to reduce stereotypic behavior in rhesus macaques. Applied Animal Behaviour Science. 2010;124:142–148. doi: 10.1016/j.applanim.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence G, Riondet L, Malecki Hl, Blanquie J-P, Martin F, Viso M, Milhaud CL. A restraining system for rhesus monkeys used in space research. Journal of Medical Primatology. 1995;24:61–67. doi: 10.1111/j.1600-0684.1995.tb00147.x. [DOI] [PubMed] [Google Scholar]
- Henry KR, Bowman RE. A long term restraint device for primates. Physiology and behavior. 1971;7:271–272. doi: 10.1016/0031-9384(71)90298-8. [DOI] [PubMed] [Google Scholar]
- Joint Working Group on Refinement. Refinements in husbandry, care and common procedures for non-human primates Ninth report of the BWAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab Animal. 2009;S1:1–47. doi: 10.1258/la.2008.007143. [DOI] [PubMed] [Google Scholar]
- Lambeth SP, Hau J, Perlman JE, Martino M, Schapiro SJ. Positive reinforcement training affects hematologic and serum chemistry values in captive chimpanzees (Pan troglodytes) American Journal of Primatology. 2006;68:245–256. doi: 10.1002/ajp.20148. [DOI] [PubMed] [Google Scholar]
- Laule GE, Bloomsmith MA, Schapiro SJ. The use of positive reinforcement training techniques to enhance the care management and welfare of primates in the laboratory. Journal of Applied Animal Welfare Science. 2003;6:163–173. doi: 10.1207/S15327604JAWS0603_02. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Nelson EE. Eye-tracking with nonhuman primates is now more accessible than ever before. American Journal of Primatology. 2011;73:562–569. doi: 10.1002/ajp.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JW. Restraining chair for the experimental study of primates. Journal of Applied Physiology. 1958;12:130–133. doi: 10.1152/jappl.1958.12.1.130. [DOI] [PubMed] [Google Scholar]
- Milhaud CL, Klein MJ, Merkel MC. A new restraining chair for rhesus monkeys (Macaca mulatta) Journal of Medical Primatology. 1980;9:62–70. doi: 10.1159/000460122. [DOI] [PubMed] [Google Scholar]
- Minier DE, Hannibal D, Sharpe N, McCowan B. Manuscript submitted for publication By the carrot or by the stick Practical differences between the animal training strategies of reinforcement and punishment. 2012 [Google Scholar]
- Minier DE, Tatum L, Gottlieb DH, Cameron A, Snarr J, Elliot R, McCowan B. Human-directed contra-aggression training using positive reinforcement with single and multiple trainers for indoor-housed rhesus macaques. Journal of Applied Animal behavior. 2011;132:178–186. [Google Scholar]
- Morton WR, Knitter GH, Smith PM, Susor TG, Schmitt K. Alternatives to chronic restraint of nonhuman primates. Journal of the American Veterinary Medical Association. 1987;191:1282–1286. [PubMed] [Google Scholar]
- Osborne BE. A restraining device for use when recording electrocardiograms in monkeys. Laboratory Animals. 1973;7:289–292. doi: 10.1258/002367773780944030. [DOI] [PubMed] [Google Scholar]
- Perlman JE, Bloomsmith MA, Whittaker MA, McMillan JL, Minier DE, McCowan B. Implementing positive reinforcement animal training programs at primate laboratories. Applied Animal Behavior Science. 2012;137:114–126. [Google Scholar]
- Prescott MJ, Buchanan-Smith HM. Training nonhuman primates using positive reinforcement techniques. Journal of Applied Animal Welfare Science. 2003;6:157–161. doi: 10.1207/S15327604JAWS0603_01. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Working with rather than against macaques during blood collection. Journal of Applied Animal Welfare Science. 2003;6:189–197. doi: 10.1207/S15327604JAWS0603_04. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Common husbandry-related variables in biomedical research with animals. Laboratory Animals. 2004;38:213–235. doi: 10.1258/002367704323133600. [DOI] [PubMed] [Google Scholar]
- Reinhardt V, Liss C, Stevens C. Restraint methods of laboratory non-human primates A critical review. Animal Welfare. 1995;4:221–238. [Google Scholar]
- Robbins DO, Zwick H, Leedy M, Stearns G. Acute restraint device for rhesus monkeys. Laboratory Animal Science. 1986;36:68–70. [PubMed] [Google Scholar]
- Ruys JD, Mendoza SP, Capitanio JP, Mason WA. Behavioral and physiological adaptation to repeated chair restraint in rhesus macaques. Physiology & behavior. 2004;82:205–213. doi: 10.1016/j.physbeh.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Laule GE. Positive reinforcement training as a technique to alter nonhuman primate behavior Quantitative assessments of effectiveness. Journal of Applied Animal Welfare Science. 2003;6:175–187. doi: 10.1207/S15327604JAWS0603_03. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Perlman JE, Boudreau BA. Manipulating the affiliative interactions of group-housed rhesus macaques using positive reinforcement training techniques. American Journal of Primatology. 2001;55:137–149. doi: 10.1002/ajp.1047. [DOI] [PubMed] [Google Scholar]
- Schmidt EM, Dold GM, McIntosh JS. A simple transfer and chairing technique for nonhuman primates. Laboratory Animal Science. 1989;39:258–260. [PubMed] [Google Scholar]
- Sledjeski M. A monkey chair for temporary restraint with minimal human contact. Physiology and behavior. 1969;4:273–276. [Google Scholar]
- Videan EN, Frizt J, Murphy J, Howell S, Heward CB. Does training chimpanzees to present for injection lead to reduced stress. Laboratory Primate Newsletter. 2005;44:1–2. [Google Scholar]