Abstract
Objective
To evaluate the association of serum and synovial fluid cartilage oligomeric matrix protein (COMP) with systemic and local measures of osteoarthritis (OA) activity by bone scintigraphy.
Methods
Knee joint synovial fluid (total 275 knees) and serum were obtained from 159 participants with symptomatic OA of at least one knee. Technetium-99m-methylene diphosphonate (Tc-MDP) bone scintigraphy was performed and early phase knee scans and late phase whole body bone scans of 15 additional joint sites were scored semi-quantitatively. Correlations of bone scan scores with COMP utilized generalized linear modelling to control for within subject correlation of knee data. Principal components analysis was used to explore the contribution of each joint site to the variance in serum COMP.
Results
The correlation of synovial fluid and serum COMP was significant (r=0.206, p=0.006). Synovial fluid COMP correlated most strongly with early phase knee bone scan scores (p=0.0003), even after adjustment for OA severity by late phase bone scan (p=0.015), as well as with synovial fluid volume (p<0.0001). Serum COMP correlated with total body bone scan scores (r=0.188, p=0.018) and with a factor composed of bone scan scores of the shoulders, spine, lateral knees and sacroiliac joints (p=0.0004).
Conclusion
Synovial fluid COMP correlated strongly with two indicators of knee joint inflammation: early phase bone scintigraphy and synovial fluid volume. Serum COMP correlated with total body joint disease severity by late phase bone scintigraphy, supporting the hypothesis that whole body bone scintigraphy is a means of quantifying total body burden of OA for systemic biomarker validation.
Keywords: osteoarthritis, biomarkers, bone scintigraphy, principal components analysis, COMP
Introduction
At present, a variety of imaging modalities are used to assess OA including x-ray, magnetic resonance imaging, and bone scintigraphy, commonly referred to as a bone scan. These imaging modalities are generally applied to the study of one or only a few joint sites. However, OA is often generalized, affecting multiple joint sites simultaneously. One survey suggests that as much as 26% of OA (based on a participant pool of 809 patients with knee or hip replacements due to OA) presents as a generalized joint disease (1). Serum biochemical markers have the potential advantage of reflecting the total body burden of OA. The validation of this concept is nevertheless challenging and requires a method of imaging that encompasses the entire skeleton and that is reflective of OA disease state.
Bone scintigraphy has the unique capability of simultaneously demonstrating metabolically active joints throughout the body, not just localized joint disease (2). We have found a strong correlation between the localization and intensity of retention of technetium-99m methylene diphosphonate (99Tcm-MDP) on a late phase bone scintigram of the knee and localization and severity of radiographic OA (both osteophyte and joint space narrowing) as well as knee pain (3). A few studies have compared bone scintigraphy and knee magnetic resonance imaging (4, 5). In these studies, correlations have also been found between scintigraphic patterns and localization of 99Tcm-MDP retention and features of knee OA including osteophytes, subchondral bone alteration, patellofemoral disease, and cartilage defects of the medial and lateral tibia. Based on the relationship of bone scintigraphy and radiographic OA in the knee, we hypothesized that whole body bone scintigraphy would provide a measure of total body burden of OA and that this measure could be used for validation of a systemic biomarker of OA. A whole body bone scan entails no more radiation (440 millirems) than a joint specific scan (equal to half the radiation exposure of an abdominal computed tomogram); and it requires only 20 extra minutes of signal acquisition time with a gamma camera to acquire images of all joints.
To test this hypothesis, we chose to evaluate the relationship of skeletal activity by bone scintigraphy and a paradigmatic OA-related marker, cartilage oligomeric matrix protein (COMP). COMP, a biomarker of cartilage turnover, has been the focus (in serum (6–15), synovial fluid (16–18), or both (19, 20)), of many studies related to osteoarthritis (OA), lending credibility to the notion that COMP may be a reliable indicator of OA disease activity. Only two previous studies have evaluated the relationship of serum concentrations of COMP and bone scintigraphy; both of these studies focused on the knee. In one, a cross-sectional study (n=38), mean serum COMP was significantly higher in the 26 individuals with late phase knee bone scan abnormalities (p=0.02) (7) and correlated positively with the extent of bone scan abnormalities (r=0.56, p=0.002). In the second study (n=64), mean serum COMP was also significantly higher in the individuals (n=47) with late phase knee bone scan abnormalities (p < 0.001) (6). A total of 22 of the 23 individuals with radiographic knee OA progression at one year had late phase bone scan abnormalities at baseline. These studies demonstrate that serum levels of COMP differ between those with and without bone scan abnormalities in the knee joints. Our study represents the first, to our knowledge, to quantify OA by scintigraphy for the purpose of evaluating the relationship of total OA burden to serum concentrations of an OA-related biomarker. We also compared and contrasted the strength of the associations of serum versus synovial fluid COMP with measures of total OA burden versus knee OA.
Methods
Participant Recruitment
A total of 159 participants were enrolled in the NIH-funded POP (Prediction of Osteoarthritis Progression) Study. Participants met the American College of Rheumatology criteria for symptomatic OA of at least one knee. In addition, all participants met the radiographic criteria for OA with a Kellgren Lawrence (KL) grade (21) 1–3 in at least one knee. Exclusion criteria included the following: bilateral knee replacement; bilateral knee KL4 scores; exposure to a corticosteroid (either parenteral or oral) within 3 months prior to the study evaluation; knee arthroscopic surgery within 6 months prior to the study evaluation; known history of avascular necrosis, inflammatory arthritis, Paget’s disease, joint infection, periarticular fracture, neuropathic arthropathy, reactive arthritis, or gout involving the knee, and current anticoagulation. This study was approved by the Duke University Medical Center institutional Review Board.
Sample Collection
Blood samples were obtained from all participants, centrifuged at 3500 rpm for 10 minutes, and serum frozen at −80°C. Synovial fluid aspiration was attempted for both knees of all participants, with the exception of replaced knees (a total of 10 knees). Arthrocentesis was performed via a lateral approach for 97% of knees with the participant supine and the knee in extension. Synovial fluid was aspirated directly and without lavage (neat) from 165 knees. When direct aspiration failed to yield synovial fluid, synovial fluid was obtained by lavage: the aspiration syringe was exchanged and 10 ml of sterile saline (without preservative) was injected through the same needle, thus requiring only a single skin perforation per arthrocentesis for each knee assessed. Synovial fluid samples were obtained in this manner for 110 knees. Bone scintigraphy was always completed prior to arthrocentesis; on average 5 hours intervened between injection of contrast and arthrocentesis, averting the possibility that trauma during the procedure would influence the scintigraphic results. Blood samples were obtained immediately prior to arthrocentesis. The synovial fluid samples were centrifuged at 3500 rpm for 10 minutes and the supernatant was frozen at −80°C.
COMP and Urea Measurements
Serum and synovial fluid samples were analyzed for serum COMP (sCOMP) and synovial fluid COMP (sfCOMP) in duplicate using a sandwich enzyme-linked immunosorbent assay (ELISA) with monoclonal antibodies 17C10 and 16F12 as previously described (22). The minimum detection limit is 120 ng/ml and intra-assay and inter-assay coefficients of variation were <10%. The synovial fluid COMP concentrations for samples obtained by lavage were corrected for dilution based on the serum/synovial fluid urea ratios as previously described (23). Concentrations of urea were determined by a CMA600 microdialysis analyzer (CMA Microdialysis, Solna, Sweden) on 5 μl samples of joint fluid (23). The total volume of synovial fluid was calculated based upon the urea method for those joints aspirated by lavage (110 knees in total) according to our previously published method (24). In brief, the volume of synovial fluid can be calculated based on the amount of dilution of synovial fluid urea that occurs when the joint is injected with a known volume of a non-urea containing fluid, such as saline. This is possible because urea is a small molecule that is neither metabolized nor produced by joint tissues and which diffuses from serum into the joint cavity and equilibrates rapidly, where a ~1:1 correlation of serum:synovial fluid urea is maintained. This allows us to use serum urea as a proxy for the pre-lavage synovial fluid urea concentration. The diminution in the synovial fluid concentration of urea upon addition of 10 ml saline (lavage procedure) enables us to estimate synovial fluid volume. The equation (derivation described in Kraus et al (24)) is as follows: SF volume in the entire joint=CD(VI)/(C-CD) with CD=concentration urea in lavage SF, VI=volume saline injected into joint, and C=0.897 x concentration urea in serum.
Radiographic Imaging
Posteroanterior fixed-flexion knee radiographs were obtained with the SynaFlexer™ lower limb positioning frame (Synarc, San Francisco) with a 10 degree caudal x-ray beam angle (25). Radiographs were graded by the consensus of two experienced graders (VBK, GM) blinded to the results of any other measures. The knee radiographs were scored for global knee OA severity by Kellgren Lawrence (KL) grade (21). Determination of compartmental OA severity was based on scoring of osteophyte and joint space narrowing (0–3) according to the OARSI standardized radiographic atlas (26). Blinded rescoring of 78 knee radiographs was performed to calculate the intrarater reliability of the x-ray readings by intraclass correlation coefficients, which were 0.69 for KL grade, 0.84 for JSN, and 0.81 for OST kappa 0.81.
Scintigraphic Imaging
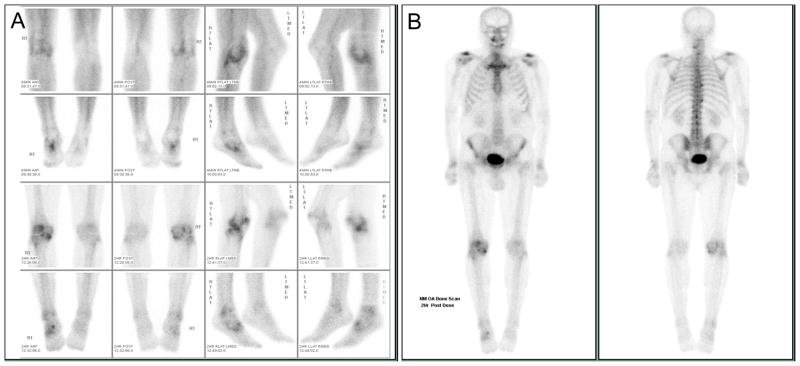
Scintigraphic images of the knee were obtained at 2 minutes (blood pool or early phase) and 2.5 hours (late phase) after intravenous administration of technetium-99m methylene diphosphonate. Four views of the knees (anterior, posterior, medial and lateral) were obtained at both time points after injection (Figure 1a). In addition, two views of the total body (anterior and posterior) were obtained after the late phase imaging of the knees was performed (Figure 1b). All bone scan images were scored semi-quantitatively by a consensus of two readers, an experienced nuclear medicine physician (REC) and a nuclear medicine resident who were blinded to the other data. The intensity of bone scintigraphic radiotracer uptake (0–3: 0=normal, 1=mild, 2=moderate, 3=intense) was scored at 16 sites. The 16 sites included the knees (medial, lateral and patellofemoral compartments) contributing a total possible score for both knees of 18, and 15 other joint sites with maximum possible scores contributed per site as follows: shoulders-6; elbows-6; wrists-6; hands-24; hips-6; sacroiliac-6; ankles-24; forefeet-6; 1st metatarsalphalangeal-6; sternoclavicular-6; acromioclavicular-6; sternomanubrial joints-3; and cervical-3; thoracic-3; and lumbar-3 spine. The knees, hands and ankles were scored in greater detail than the other joint sites resulting in higher overall possible scores for these sites. The hand included interphalangeal/metacarpalphalangeal (total score 18) and carpometacarpal (total score 6) joints. In addition, hand interphalangeal/metacarpal joint involvement was categorized and scored on the basis of the numbers of joints involved (1=a single joint, 2=2–4 joints, and 3=>5 joints). The overall intensity score was multiplied by the category to give the total score. Four regions of the ankle were scored: the medial talus, lateral talus, medial tibia, and lateral tibia. The maximum possible total body bone scan score was 132. A total of 30 of the knee scintigrams (20% of the total) were scored one year later by one of the same readers (REC) blinded to the original readings. The intra-class correlation coefficients (ICCs) and 95% confidence intervals were calculated for the knee bone scan scores. The proportion of agreement and 95% confidence intervals were calculated for the remainder of the joints to provide a more stable estimate of agreement in the face of many zero scores as was the case for several of the joint sites.
Figure 1. Representative bone scan images of a participant enrolled on the basis of symptomatic radiographic knee osteoarthritis.
A) Spot images of the knees (first and third rows) and ankles (second and fourth rows) obtained starting 4 minutes after injection of the radiopharmaceutical (upper 2 rows) and 2 hours after injection (bottom 2 rows). Abnormal activity is noted in the right knee (medial, lateral and patellofemoral compartments) and in the right ankle and midfoot. B) Whole body bone scan obtained starting 2 hours after injection of the radiopharmaceutical. Abnormal activity is noted in both acromioclavicular, shoulder, and wrist joints, the cervical and thoracic spine, and the right knee, ankle and foot joints as described in A.
Statistical Methods
Bivariate analyses were conducted to evaluate the association of bone scintigraphy with COMP values that were natural log transformed to achieve normality. Generalized linear models (Genmod procedure in SAS) and linear mixed models (Mixed procedure in SAS) were used to control the within subject correlation for parameters that were measured for each knee (e.g. knee bone scan and synovial fluid COMP). P-values were estimated by a two-sided t-test and the significance level (type 1 error rate) was controlled at 5%. We used Principal Components Analysis (PCA) to derive the optimal combination of site specific bone scan scores that accounted for the variation in serum COMP. Principal components analysis is a multivariate technique that derives linear combinations of variables (which are referred to as principal components) that reveal patterns in the data. It is used as a tool in exploratory data analysis and for making predictive models. The variables that contribute most to a given principal component are those that have the largest coefficients. Coefficients greater that 0.3 are generally considered significant. Therefore, those joint sites with the largest coefficients are the ones accounting most for the variance in sCOMP.
Results
The sample consisted of 159 participants with symptomatic knee OA. The participants (118 females, 41 males) ranged in age from 35 to 85 years old with a mean age of 64 years (SD 12 years). A total of 79 had bilateral moderate to severe radiographic knee OA (KL grades 2–4). Of the total 308 knees evaluated, 91 had KL grades of 0–1. Based on joint space narrowing, 53% had isolated medial compartment disease, 15% had isolated lateral compartment disease; and 22% had involvement of both compartments, while 10% had no joint space narrowing, just osteophyte. The overall correlation between sfCOMP and sCOMP was significant but modest (r=0.206, p=0.006). Figure 1 depicts representative images of an early and late phase knee bone scan and a whole body bone scan demonstrating metabolically active joints in this participant including abnormal activity in both acromioclavicular, shoulder, and wrist joints, the cervical and thoracic spine, the right knee (medial, lateral and patellofemoral compartments), and the right ankle and midfoot. The overall correlation of early and late phase knee bone scans was significant (r=0.687, p<0.001). The ICCs and 95% confidence intervals for the bone scan parameters were high and are listed in Table 2.
Table 2.
Association of serum COMP and total body burden of OA assessed by late phase bone scintigraphy.
| Joint Site | Bone Scan Score Proportion of Agreement (95% CIs) | Bivariate Association with ln(sCOMP): r (p)† | Coefficients in Principal Components Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | Factor 7 | |||
| Shoulder | 0.641 (0.510, 0.754) | 0.140 (0.088) | 0.453 | −0.012 | 0.189 | −0.055 | −0.301 | 0.121 | −0.177 |
| Spine | 0.625–0.656* (0.438, 0.808) | 0.174 (0.028) | 0.396 | −0.002 | 0.003 | −0.291 | 0.149 | −0.182 | 0.211 |
| Lateral Knee | 0.864§ (0.661, 0.919) | 0.317 (<0.0001) | 0.330 | 0.286 | −0.364 | 0.104 | −0.227 | −0.043 | −0.029 |
| Sacroiliac | 0.844 (0.727, 0.919) | 0.062 (0.454) | 0.305 | −0.054 | 0.157 | 0.061 | 0.212 | −0.014 | 0.220 |
| Acromioclavicular | 0.531 0.403, 0.656) | 0.123 (0.135) | 0.298 | −0.260 | −0.155 | 0.097 | −0.077 | 0.325 | 0.249 |
| Ankle | 0.855–0.968** (0.737, 0.994) | 0.033 (0.686) | 0.252 | 0.258 | 0.023 | 0.297 | 0.225 | 0.353 | −0.254 |
| Wrist | 0.367 (0.249, 0.502) | 0.116 (0.161) | 0.251 | −0.033 | 0.374 | 0.057 | −0.398 | 0.192 | −0.106 |
| Hand | 0.565–0.903*** (0.433, 0.960) | 0.081 (0.311) | 0.246 | −0.358 | 0.131 | 0.197 | 0.001 | −0.353 | 0.013 |
| Hip | 0.656 (0.526, 0.768) | 0.015 (0.851) | 0.208 | −0.143 | −0.029 | −0.440 | 0.225 | −0.150 | −0.533 |
| Forefoot | 0.677 (0.545, 0.787) | 0.158 (0.054) | 0.174 | 0.344 | 0.041 | 0.022 | 0.325 | −0.381 | −0.241 |
| First Metatarsophalangeal | 0.774 (0.647, 0.869) | 0.027 (0.740) | 0.162 | 0.215 | 0.371 | 0.012 | 0.138 | −0.065 | 0.350 |
| Sternoclavicular | 0.688 (0.558, 0.794) | 0.028 (0.733) | 0.159 | −0.334 | −0.327 | 0.253 | 0.116 | −0.316 | 0.265 |
| Elbow | 0.969 (0.882, 0.995) | 0.043 (0.604) | 0.126 | −0.161 | −0.421 | 0.236 | 0.256 | 0.303 | −0.246 |
| Sternomanubrial | 0.813 (0.630, 0.921) | 0.021 (0.801) | 0.062 | −0.071 | −0.064 | −0.556 | 0.309 | 0.409 | 0.273 |
| Medial Knee | 0.887§ (0.775, 0.946) | 0.001 (0.992) | 0.061 | 0.545 | −0.153 | 0.111 | 0.093 | 0.017 | 0.253 |
| Patellofemoral Knee | 0.647§ (0.298, 0.832) | 0.083 (0.296) | −0.103 | −0.164 | 0.417 | 0.349 | 0.466 | 0.166 | −0.064 |
| Eigenvalue | 2.408 | 1.488 | 1.336 | 1.236 | 1.142 | 1.049 | 1.004 | ||
| Cumulative variance in sCOMP explained | 15% | 24% | 33% | 40% | 48% | 54% | 60% | ||
Range of scores for cervical, thoracic and lumbar spine
Range of scores for medial and lateral ankle tibiotalar and talonavicular joints
Range of scores for interphalangeal and carpometacarpal joints
Agreement based on Intraclass Correlation Coefficient per Table 1
Associations determined for sum scores of right and left joint sites for all except the spine
Synovial fluid COMP analyses
As shown in Table 1, in analyses controlling for within subject correlation between knees, sfCOMP was strongly associated with early phase knee bone scan scores (p=0.0003); sfCOMP was also associated, but to a much lesser extent, with late phase knee bone scan scores (p=0.091). Synovial fluid COMP was associated with early phase bone scan scores of the medial and lateral knee compartments but not the patellofemoral compartment. The association of sfCOMP with early phase bone scan scores of the medial and lateral knee compartments remained significant after controlling for results of the late phase knee bone scan (p=0.015). With respect to the late phase knee scan, the strongest association with sfCOMP was seen for the medial compartment (p=0.031); however, the significance level decreased after controlling for the results of the early phase knee bone scan (p=0.068). Interestingly, a significant association of the late phase knee scan with the patellofemoral compartment was revealed upon controlling for the early phase knee scan (p=0.01). There was also a strong positive correlation between sfCOMP and synovial fluid volume (r=0.60, p < 0.0001) based on the 110 knees for which synovial fluid volume results were available. Synovial fluid COMP concentrations in this cohort did not correlate significantly with the features of radiographic OA of joint space narrowing or osteophyte.
Table 1.
Association of synovial fluid COMP and early and late scintigraphic uptake in the knee.
| Bivariate association with ln sfCOMP | ||||
|---|---|---|---|---|
|
| ||||
| Knee Bone Scan Phase | Knee Joint Site | Bone Scan ICC (95% CIs) | P value* | P value* (adjusted for late phase bone scan) |
| Early | Medial Knee | 0.724 (0.427, 0.863) | < 0.001 | 0.026 |
| Lateral Knee | 0.671 (−0.009, 0.76) | 0.002 | 0.047 | |
| Sum Medial and Lateral Knee | 0.848 (0.363, 0.759) | < 0.001 | 0.015 | |
| Patellofemoral Knee | 0.664 (0.319, 0.838) | 0.074 | 0.495 | |
| Total Knee (sum medial, lateral and patellofemoral) | 0.906 (0.607, 0.826) | < 0.001 | 0.087 | |
|
| ||||
| P value* (adjusted for early phase bone scan) | ||||
|
| ||||
| Late | Medial Knee | 0.887 (0.775, 0.946) | 0.031 | 0.067 |
| Lateral Knee | 0.864 (0.661, 0.919) | 0.122 | 0.696 | |
| Sum Medial and Lateral Knee | 0.902 (0.790, 0.950) | 0.012 | 0.069 | |
| Patellofemoral Knee | 0.647 (0.298, 0.832) | 0.681 | 0.010 | |
| Total Knee (sum medial, lateral and patellofemoral) | 0.884 (0.760, 0.943) | 0.091 | 0.507 | |
P values adjusted by GLM (generalized linear modelling to control for within subject correlation of knee data)
ICC=intra-class correlation coefficient
We also evaluated the association of sfCOMP and late phase total body bone scan. Although sfCOMP, summed over both knees, appeared to correlate with total body bone scan (r=0.170, p=0.005), the association was diminished (p=0.08) upon controlling for the knee early phase bone scan.
Serum COMP analyses
In bivariate analyses, serum COMP correlated with the sum score for total body late phase bone scan (r=0.188, p=0.018 based on the sum scores of all joints), and correlated with late phase bone scan score of the spine (r=0.174, p=0.03), as shown in Table 2. In addition, sCOMP correlated surprisingly well with knee bone scan uptake in the lateral knee compartment (r=0.317, p < 0.0001). These associations remained significant after controlling for age, gender, and BMI.
Principal components analysis of the bone scan scores yielded seven components with eigenvalues greater than or equal to 1. The component with the highest eigenvalue (2.408), accounted for 15% of the variation in sCOMP. The joints contributing the most to this principal component (i.e. having a coefficient greater than 0.300) were the shoulder, spine, lateral knee, and sacroiliac joints (Table 2). The factor with the second highest eigenvalue (1.488) included the medial knee and explained an additional 9% of variance in sCOMP. Interestingly, the medial knee had the highest loading coefficient (0.545) of any joint site. Using the coefficients derived from this analysis to weight the bone scan contributions of the joint sites of each participant yielded a strong association of the first principal component with sCOMP (p=0.0004).
Discussion
We hypothesized that COMP in the synovial fluid would correlate best with localized measures of knee OA (knee bone scan uptake) and that COMP in the serum would correlate best with total body burden of OA as reflected by scores derived from total body bone scan. This hypothesis has been confirmed by this study with additional interesting nuances. Synovial fluid COMP correlated remarkably well with intensity of early phase knee bone scans which reflect the blood pool of the joint, considered a surrogate for joint inflammation. Although sfCOMP, summed over both knees, appeared to correlate with total body bone scan, the association was diminished upon controlling for the early phase knee bone scan. Taken together, these data demonstrate that sfCOMP is a marker of localized inflammatory disease activity. Synovial fluid COMP also correlated remarkably well with the calculated total synovial fluid volume, another index of joint inflammation. Synovial fluid COMP did not correlate with radiographic features of OA. This is not unexpected given the finding that sfCOMP correlated strongly with signs of joint inflammation, and consistent with the finding that sfCOMP did not correlate with late phase bone scan. OA synovial fluid contains COMP fragments (27), and we previously noted a positive association of sCOMP and synovitis by clinical criteria (28) but until now, had not evaluated synovial fluid COMP and objective indices of joint inflammation. Another study noted a correlation between the severity of the joint capsular distention detected by ultrasound and serum COMP levels (29). Our results are compatible with the view that degradative products of cartilage extracellular matrix components are a proinflammatory stimulus and one etiology of joint inflammation and effusion in OA.
As expected, sCOMP correlated with several parameters indicative of the total body burden of OA. The results of the principal components analysis showed that sCOMP in this cohort most strongly reflected bone turnover in the shoulder, spine, lateral knee and sacroiliac joints. These results are compatible with those of the genetic cohort of the GARP study, wherein sCOMP (as part of a factor within a principal components analysis) correlated most strongly with degenerative disc disease and facet disease of the spine (30). It was surprising to note that sCOMP also correlated quite well with lateral knee bone scan uptake. We do not know the reason for this. Subtle differences in medial and lateral meniscal composition, as demonstrated by Stephan et al (31), might contribute to this if it resulted in differential COMP production with lateral versus medial compartmental knee OA involvement. Blood was drawn immediately prior to synovial fluid, and although the majority of arthrocenteses were performed by a lateral approach, this should have had no bearing on the serum COMP results.
Localized bone turnover and bone remodeling are hallmarks of OA (32) and progressive OA (33, 34). Cortical and trabecular bone rapidly alter skeletal architecture and shape via cell-mediated modeling and remodeling in response to load (35), which in turn is altered by cartilage degeneration and loss, explaining the association of bone remodeling and joint space narrowing or cartilage loss. Scintigraphy reflects bone remodeling and like bone, scintigraphic retention has been shown to correlate with cartilage lesions (5). In this way a biomarker could plausibly relate to a marker of bone turnover, as seen here for sCOMP and bone scintigraphy.
There are several limitations of this study. It has been established that scintigraphy reflects features of radiographic OA at both the knee (3, 36, 37), and hands (38–42), however, we do not know how well scintigraphy reflects radiographic OA at other joints because there are currently no data, to our knowledge, exploring this issue. In this report we only analyzed one biomarker to test the paradigm stated earlier, that is, to evaluate the association of a biomarker in the serum and synovial fluid with systemic and local measures of OA activity by bone scintigraphy. We presume that other biomarkers may behave differently depending upon the aspect of disease best represented by the particular biomarker and this will require further exploration.
A critical need for OA clinical trials is the availability of robust and cost effective surrogate outcomes. The only truly appropriate means of validating a systemic biomarker is through the use of a measure of total body burden of OA. Although scintigraphy involves a radioactive tracer, it is nevertheless a procedure that is used routinely for clinical musculoskeletal imaging and provides one feasible means of quantifying total body burden of OA. As demonstrated here, the correlations of a systemic biomarker (sCOMP) with total body bone scintigraphy, and a local biomarker (sfCOMP) with knee bone scintigraphy, provide a proof of concept for the underlying principle that bone scintigraphy can reflect OA burden of disease. In summary, the use of this approach has the potential to facilitate the process of validation of OA-related biomarkers.
Acknowledgments
Supported by NIH/NIAMS grant RO1 AR48769, and by the National Center for Research Resources NIH MO1-RR-30, supporting the Duke General Clinical Research Unit where this study was conducted.
We wish to thank Dr. Gabor Varju (Department of Medicine, East Carolina University, Greenville, NC) for assistance with radiographic scoring; Dr. Vladimir Vilim (Institute of Rheumatology, Prague, Czech Republic) for the kind gift of anti-COMP monoclonal antibodies; and the following individuals from Duke University: Dr. T Parker Vail for assistance with participant recruitment; Dr. David Williams for assistance with the bone scan interpretations; and So Yeon Kong for assistance with COMP measurements.
References
- 1.Sturmer T, Sun Y, Sauerland S, Zeissig I, Gunther KP, Puhl W, et al. Serum cholesterol and osteoarthritis. The baseline examination of the Ulm Osteoarthritis Study. J Rheumatol. 1998;25(9):1827–32. [PubMed] [Google Scholar]
- 2.Jonsson H, Eliasson GJ, Petursson E. Scintigraphic hand osteoarthritis (OA)--prevalence, joint distribution, and association with OA at other sites. J Rheumatol. 1999;26(7):1550–6. [PubMed] [Google Scholar]
- 3.Kraus V, McDaniel G, Worrell T, Feng S, Vail T, Varju G, et al. Association of bone scintigraphic abnormalities with knee malalighment and pain. Annals Rheum Dis. 2008 Nov 3; doi: 10.1136/ard.2008.094722. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAlindon TE, Watt I, McCrae F, Goddard P, Dieppe PA. Magnetic resonance imaging in osteoarthritis of the knee: correlation with radiographic and scintigraphic findings. Ann Rheum Dis. 1991;50(1):14–9. doi: 10.1136/ard.50.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boegard T, Rudling O, Dahlstrom J, Dirksen H, Petersson IF, Jonsson K. Bone scintigraphy in chronic knee pain: comparison with magnetic resonance imaging. Annals of the Rheumatic Diseases. 1999;58(1):20–6. doi: 10.1136/ard.58.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharif M, Saxne T, Shepstone L, Kirwan JR, Elson CJ, Heinegard D, et al. Relationship between serum cartilage oligomeric matrix protein levels and disease progression in osteoarthritis of the knee joint. Br J Rheumatol. 1995;34(4):306–10. doi: 10.1093/rheumatology/34.4.306. [DOI] [PubMed] [Google Scholar]
- 7.Petersson I, Boegard T, Dahlstrom J, Svensson B, Heinegard D, Saxne T. Bone scan and serum markers of bone and cartilage in patients with knee pain and osteoarthritis. Osteo & Cartilage. 1998;6:33–39. doi: 10.1053/joca.1997.0090. [DOI] [PubMed] [Google Scholar]
- 8.Conrozier T, Saxne T, Fan CS, Mathieu P, Tron AM, Heinegard D, et al. Serum concentrations of cartilage oligomeric matrix protein and bone sialoprotein in hip osteoarthritis: a one year prospective study. Ann Rheum Dis. 1998;57(9):527–32. doi: 10.1136/ard.57.9.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark AG, Jordan JM, Vilim V, Renner JB, Dragomir AD, Luta G, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42(11):2356–64. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 10.Dragomir A, Jordan J, Kraus V, Renner J, Luta G, Clarke A, et al. Serum cartilage oligomeric matrix protein (COMP) is associated with joint pain, independent of knee and hip osteoarthritis (OA) Arthritis Rheum. 2000;43(9 Suppl):S136, 418. [Google Scholar]
- 11.Sharif M, Kirwan JR, Elson CJ, Granell R, Clarke S. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum. 2004;50(8):2479–88. doi: 10.1002/art.20365. [DOI] [PubMed] [Google Scholar]
- 12.Bruyere O, Collette J, Kothari M, Zaim S, White D, Genant H, et al. Osteoarthritis, magnetic resonance imaging, and biochemical markers: a one year prospective study. Ann Rheum Dis. 2006;65(8):1050–4. doi: 10.1136/ard.2005.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharif M, Granell R, Johansen J, Clarke S, Elson C, Kirwan JR. Serum cartilage oligomeric matrix protein and other biomarker profiles in tibiofemoral and patellofemoral osteoarthritis of the knee. Rheumatology (Oxford) 2006;45(5):522–6. doi: 10.1093/rheumatology/kei216. [DOI] [PubMed] [Google Scholar]
- 14.Hunter DJ, Li J, LaValley M, Bauer DC, Nevitt M, DeGroot J, et al. Cartilage markers and their association with cartilage loss on magnetic resonance imaging in knee osteoarthritis: the Boston Osteoarthritis Knee Study. Arthritis Res Ther. 2007;9(5):R108. doi: 10.1186/ar2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaganti RK, Kelman A, Lui L, Yao W, Javaid MK, Bauer D, et al. Change in serum measurements of cartilage oligomeric matrix protein and association with the development and worsening of radiographic hip osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):566–71. doi: 10.1016/j.joca.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lohmander LS, Saxne T, Heinegard DK. Release of cartilage oligomeric matrix protein (COMP) into joint fluid after knee injury and in osteoarthritis. Ann Rheum Dis. 1994;53(1):8–13. doi: 10.1136/ard.53.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersson IF, Sandqvist L, Svensson B, Saxne T. Cartilage markers in synovial fluid in symptomatic knee osteoarthritis. Ann Rheum Dis. 1997;56(1):64–67. doi: 10.1136/ard.56.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Momohara S, Okada N, Ikari K, Mizuno S, Okamoto H. Dermatan sulfate in the synovial fluid of patients with knee osteoarthritis. Mod Rheumatol. 2007;17(4):301–5. doi: 10.1007/s10165-007-0594-7. [DOI] [PubMed] [Google Scholar]
- 19.Saxne T, Heinegard D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992;31(9):583–91. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- 20.Senolt L, Braun M, Olejarova M, Forejtova S, Gatterova J, Pavelka K. Increased pentosidine, an advanced glycation end product, in serum and synovial fluid from patients with knee osteoarthritis and its relation with cartilage oligomeric matrix protein. Ann Rheum Dis. 2005;64(6):886–90. doi: 10.1136/ard.2004.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilim V, Voburka Z, Vytasek R, Senolt L, Tchetverikov I, Kraus VB, et al. Monoclonal antibodies to human cartilage oligomeric matrix protein: epitope mapping and characterization of sandwich ELISA. Clin Chim Acta. 2003;328(1–2):59–69. doi: 10.1016/s0009-8981(02)00375-3. [DOI] [PubMed] [Google Scholar]
- 23.Kraus VB, Huebner JL, Fink C, King JB, Brown S, Vail TP, et al. Urea as a passive transport marker for arthritis biomarker studies. Arthritis Rheum. 2002;46(2):420–7. doi: 10.1002/art.10124. [DOI] [PubMed] [Google Scholar]
- 24.Kraus V, Stabler T, Kong S, Varju G, McDaniel G. Measurement of synovial fluid volume using urea. Osteoarthritis Cartilage. 2007;15(10):1217–20. doi: 10.1016/j.joca.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterfy C, Li J, Saim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–132. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 26.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15 (Suppl A):A1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Vilim V, Lenz ME, Vytasek R, Masuda K, Pavelka K, Kuettner KE, et al. Characterization of monoclonal antibodies recognizing different fragments of cartilage oligomeric matrix protein in human body fluids. Archives of Biochemistry & Biophysics. 1997;341(1):8–16. doi: 10.1006/abbi.1997.9941. [DOI] [PubMed] [Google Scholar]
- 28.Vilim V, Vytasek R, Olejarova M, Machacek S, Gatterova J, Prochazka B, et al. Serum cartilage oligomeric matrix protein reflects the presence of clinically diagnosed synovitis in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2001;9(7):612–8. doi: 10.1053/joca.2001.0434. [DOI] [PubMed] [Google Scholar]
- 29.Jung YO, Do JH, Kang HJ, Yoo SA, Yoon CH, Kim HA, et al. Correlation of sonographic severity with biochemical markers of synovium and cartilage in knee osteoarthritis patients. Clin Exp Rheumatol. 2006;24(3):253–9. [PubMed] [Google Scholar]
- 30.Meulenbelt I, Kloppenburg M, Kroon HM, Houwing-Duistermaat JJ, Garnero P, Hellio-Le Graverand MP, et al. Clusters of biochemical markers are associated with radiographic subtypes of osteoarthritis (OA) in subject with familial OA at multiple sites. The GARP study Osteoarthritis Cartilage. 2007;15(4):379–85. doi: 10.1016/j.joca.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Stephan JS, McLaughlin RM, Jr, Griffith G. Water content and glycosaminoglycan disaccharide concentration of the canine meniscus. Am J Vet Res. 1998;59(2):213–6. [PubMed] [Google Scholar]
- 32.Messent EA, Ward RJ, Tonkin CJ, Buckland-Wright C. Tibial cancellous bone changes in patients with knee osteoarthritis. A short-term longitudinal study using Fractal Signature Analysis. Osteoarthritis Cartilage. 2005;13(6):463–70. doi: 10.1016/j.joca.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Bettica P, Cline G, Hart DJ, Meyer J, Spector TD. Evidence for increased bone resorption in patients with progressive knee osteoarthritis: longitudinal results from the Chingford study. Arthritis Rheum. 2002;46(12):3178–84. doi: 10.1002/art.10630. [DOI] [PubMed] [Google Scholar]
- 34.Buckland-Wright C, Messent E, Papaloucas C, GAC, Beary J, Meyer K. Tibial cancellous bone changes in OA knee patients grouped into those with slow or detectable joint space narrowing (JSN) Arthritis Rheum. 2004;50(S9):S145. [Google Scholar]
- 35.Goldring SR. Role of bone in osteoarthritis pathogenesis. Med Clin North Am. 2009;93(1):25–35. doi: 10.1016/j.mcna.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52(8):557–63. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazzuca SA, Brandt K, Shauwecker D, Buckwalter KA, Katz BP, Meyer J, et al. Bone Scintigraphy is not a better predictor of progression of knee osteoarthritis than Kellgren and Lawrence grade. J Rheumatology. 2004;31:329–32. [PubMed] [Google Scholar]
- 38.Hutton CW, Higgs ER, Jackson PC, Watt I, Dieppe PA. 99mTc HMDP bone scanning in generalised nodal osteoarthritis. II. The four hour bone scan image predicts radiographic change. Ann Rheum Dis. 1986;45(8):622–6. doi: 10.1136/ard.45.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macfarlane D, Buckland-Wright J, Emery P, Fogelman I, Clark B, Lynch J. Comparison of clinical, radionuclide, and radiographic features of osteoarthritis of the hands. J Rheum Dis. 1991;50:623–626. doi: 10.1136/ard.50.9.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy C, Cushnaghan J, Dieppe P. The predictive role of scintigraphy in radiographic osteoarthritis of the hand. Osteoarthritis Cartilage. 1994;2(1):25–8. doi: 10.1016/s1063-4584(05)80003-2. [DOI] [PubMed] [Google Scholar]
- 41.Buckland-Wright C. Current status of imaging procedures in the diagnosis, prognosis and monitoring of osteoarthritis. Baillieres Clin Rheumatol. 1997;11(4):727–48. doi: 10.1016/s0950-3579(97)80007-6. [DOI] [PubMed] [Google Scholar]
- 42.Olejarova M, Kupka K, Pavelka K, Gatterova J, Stolfa J. Comparison of clinical, laboratory, radiographic, and scintigraphic findings in erosive and nonerosive hand osteoarthritis. Results of a two-year study. Joint, Bone, Spine: Revue du Rhumatisme. 2000;67(2):107–12. [PubMed] [Google Scholar]