Abstract
Background
Persistent atrial fibrillation (PAF) results in electromechanical and structural remodeling by mechanisms that are poorly understood. Myofibroblast proliferation and fibrosis is a major source of structural remodeling in PAF. Myofibroblasts also interact with atrial myocytes via direct physical contact and release of signaling molecules that may contribute to remodeling.
Objective
To determine whether myofibroblasts contribute to atrial myocyte electromechanical remodeling via direct physical contact and platelet-derived growth factor (PDGF) signaling.
Methods and Results
Myofibroblasts and myocytes from adult sheep atria were co-cultured for 24 hours. Myocytes making contact with myofibroblasts demonstrated significant reduction (p≤0.05) in L-type calcium (ICa,L) peak current density, shortening of action potential duration (APD)and reduction in calcium transients. These effects were blocked by pre-treatment with neutralizing PDGF-AB antibody (N-ab). Heterocellular contact also severely disturbed the localization of the L-type calcium channel. Exposure of adult sheep atrial myocytes to 1ng/ml recombinant PDGF-AB peptide for 24 hours reduced both APD50 and APD80 (p≤0.05). Peak ICa,L was reduced as well. Pretreatment with N-ab prevented these effects. Finally, while control atrial myocytes did not respond 1:1 to pacing frequencies >3 Hz, atrial myocytes from hearts that were tachypaced for 2 months and normal myocytes treated with PDGF-AB for 24 hrs could be paced at 10 Hz.
Conclusion
In addition to leading to fibrosis, atrial myofibroblasts contribute to electromechanical remodeling of myocytes via direct physical contact and release of PDGF-AB, which may be a factor in PAF induced remodeling.
Keywords: arrhythmia, electrophysiology, ion channels, atrial fibrillation, PDGF, calcium channel
INTRODUCTION
Atrial Fibrillation (AF) is the most common arrhythmia in adults, affecting 2.5 million people in the U.S.A alone,1 While atrial pathophysiology has been extensively studied, the mechanisms of initiation and perpetuation of the arrhythmia are still incompletely understood. This translates into limited therapeutic options, especially for patients with persistent AF.2 During AF the rapid electrical activation of the atria shortens the atrial effective refractory periods and AF cycle lengths, leading to progressively longer episodes of AF.3 AF-associated electrical remodeling paved the way for studies detailing changes in membrane ion channel conductance and pump currents, intracellular calcium dynamics and impulse propagation which occur as soon as the atria fibrillate.4 Structural changes also take place within the atrial parenchyma and are equally critical for AF onset and perpetuation.5 Foremost, the development of interstitial fibrosis and its impact on atrial contractile and electrical function was found to be pivotal for AF maintenance.6 In addition, the development of atrial fibrosis has been shown to be highly controlled by the renin-angiotensin-aldosterone system (Angiotensin-II, Ang-II, and aldosterone) and downstream activation of signaling pathways via soluble cytokines such as transforming growth factor-β1 (TGF-β1) and platelet-derived growth factor (PDGF). However, while it is known that fibroblasts occupy a very significant volume of the atrial mass,7 the effects of signaling molecules released by activated fibroblasts, (myofibroblasts) on atrial myocyte electrophysiology and contractility of the heart remain poorly understood.
Fibroblasts make up a significant proportion of the cells in the adult myocardium and are responsible for maintaining the extracellular matrix.8 Following myocardial injury, chemical mediators drive the transition of fibroblast into myofibroblast. Myofibroblasts can then migrate to the site of injury and participate in the repair process.9 Heterocellular culture experiments have shown that cardiac myofibroblasts can attach firmly to adult myocytes, causing anisotropic stretch and hypertrophic remodeling.10 We hypothesized that myofibroblasts within the fibrillating atria serve not only to induce fibrosis,8,9 but to disrupt normal electrical and mechanical function of atrial myocytes by the release of signaling molecules that contribute to electrical remodeling and subsequently AF perpetuation. Here we have examined the effects of direct atrial myocyte-to-myofibroblast heterocellular contact in 24 hr co-cultures, and of 24 hour exposure to PDGF-AB, with emphasis on the expression, subcellular localization and electromechanical function of the voltage gated L-type calcium channel. We demonstrate for the first time that heterocellular contact and PDGF, target atrial myocyte action potential duration (APD), L-type calcium current (ICa,L), CaV1.2 protein distribution and intracellular calcium handling, providing a substrate compatible with the electromechanical remodeling associated with PAF.
MATERIALS and METHODS
An expanded methods section is available in the Online Data Supplement
Cell Dissociation and Isolation
Atrial myocytes and fibroblasts were obtained from 20 anesthetized adult sheep (30–40 Kg) by enzymatic dissociation as previously published.11 Myofibroblasts were generated by passaging primary fibroblasts 3 times in culture as described previously.12
Fibrosis analysis in Persistent AF sheep
Persistent AF (PAF) was induced in 4 sheep (50 to 60 Kg) by right atrial burst pacing (20 sec) at 20 Hz for more than 20 days.13 PAF hearts were excised, atrial tissue was fixed in 10% formalin, embedded in paraffin, sectioned at a thickness of 4 µm sections for quantitative evaluations of fibrosis after staining with picrosirius red. Normal sheep (without pacemaker implantation and pacing) acted as controls.
Single Cell Electrophysiology
Action potentials were recorded at 37°C utilizing protocols previously described.19 Whole-cell cardiac ICa,L was recorded from cultured sheep atrial myocytes using standard methods (see Online Supplement).
Immunofluorescence
Immunofluorescence analysis was carried out on myocytes or myocyte-fibroblast co-cultures plated glass coverslips. Preparations were stained with antibodies against CaV1.2 (Alomone), sarcomeric α-actinin (Sigma) and DAPI.
Calcium Transients
Sheep atrial cells were co-cultured with atrial myofibroblasts in the absence or presence of a PDGF neutralizing antibody for 24 hours. Cells were loaded with Fluo-4AM, a Ca2+ indicator, and Ca2+ transients were measured using an IonOptix spectrophotometer and an inverted microscope. Only cells that followed 1:1 pacing were included in the analysis. Data were quantified using ion wizard software.
Western Blotting
Sheep left atrial tissue lysates (20 µg) were subjected to SDS-PAGE and blots were incubated with PDGF-AB, PDGF-AA, alpha smooth muscle actin (α-SMA), α-subunit of the L-type voltage gated calcium channel (CaV1.2), sodium – calcium exchanger (NCX1) or GAPDH antibodies.
PDGF-AB Enzyme-linked Immunosorbent Assay (ELISA)
Levels of PDGF-AB released in myocyte, fibroblast and myofibroblast culture media were analyzed using a commercially available ELISA kit (R & D systems, Minneapolis, MN
RESULTS
Persistent AF Induces Atrial Fibrosis and Increased α-SMA Expression
To examine whether myofibroblast proliferation occurs in vivo in the course of AF perpetuation, we utilized a sheep model of persistent atrial fibrillation (PAF) generated by implantation of an atrial pacemaker in the right atrium.13 Within 2 weeks of tachypacing, spontaneous AF episodes were commonplace. At 2 months most animals were in PAF. Utilizing this model we observe a significant increase in interstitial fibrosis accompanied by increased expression of α-SMA, a known marker of myofibroblasts9 (see Figure S1). Therefore to determine whether proliferating myofibroblasts within the fibrillating atria would contribute to atrial electrical remodeling by the release of signaling molecules we examined the effects of PDGF, a well-known cytokine released by myofibroblatsts12 on the electrical properties of isolated atrial myocytes.
PDGF-AB is the predominant isoform in the Sheep Atrium
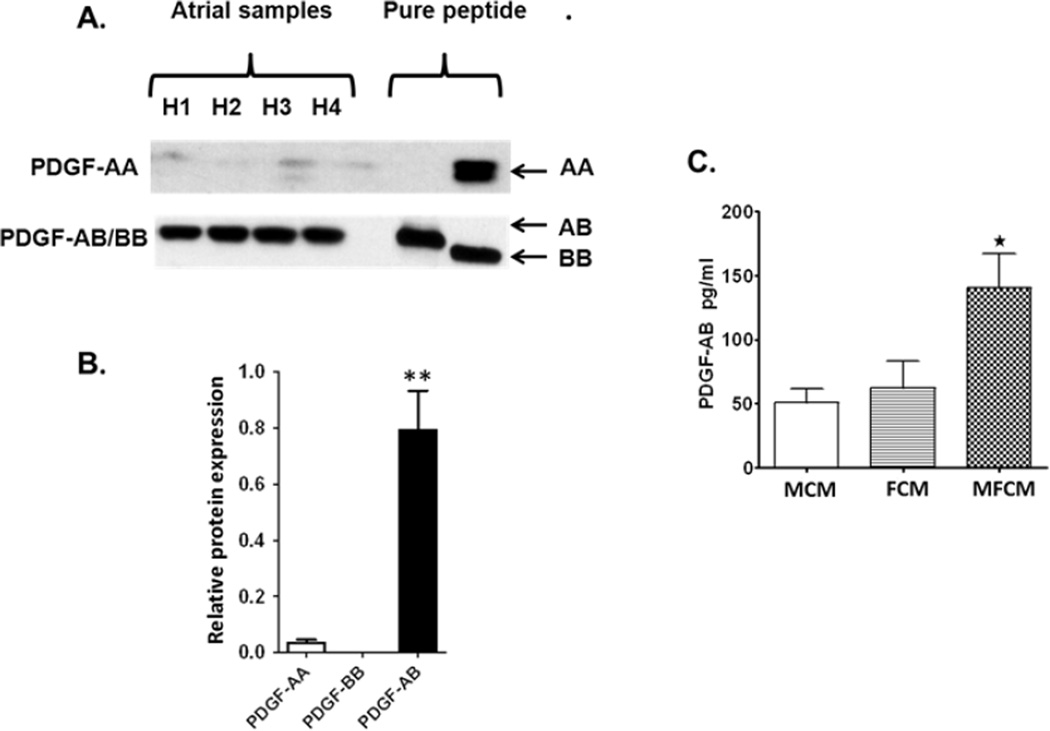
The PDGF family is composed of four different isoforms, PDGF –A, -B, -C, -D.12 These isotypes dimerize to form active proteins creating four homodimers and one heterodimer (PDGF-AB). To identify the predominant isoform of PDGF in unpaced ovine atria, we performed Western blot analysis utilizing primary antibodies directed to PDGF-AB, PDGF-BB and PDGF-AA. Isoforms – C and –D are primarily found in the ventricle. PDGF-AB was the predominant isoform detected in sheep atrial homogenates. PDGF isotype expression was normalized to GAPDH. (Figure 1A,B). To determine if atrial fibroblasts/myofibroblasts are a potential source of PDGF-AB, conditioned medium collected from primary fibroblast cultures (FCM), transformed myofibroblast cultures (MFCM) and myocytes (MCM) were collected and analyzed for the presence of PDGF-AB by ELISA. Atrial myofibroblasts secrete significantly higher amounts of PDGF-AB than atrial myocytes and trend higher compared to primary atrial fibroblasts (Figure 1C). These data support the idea that an increased presence of myofibroblasts in the atrial parenchyma would likely enhance the levels of PDGF-AB in the ovine atria.
Figure 1.
Western Blot analysis of PDGF isoforms in atrial homogenates. A. Tissue lysates from unpaced sheep atria probed with a PDGF antibody known to recognize PDGF-AB and PDGF-BB, and a separate antibody recognizing PDGF-AA. Lanes H1 thru H4, homogenate samples from four separate ovine atrial preparations, one empty lane and one/two lanes with recombinant PDGF dimers: PDGF-AA, PDGF-AB and PDGF-BB as positive controls. B. Expression levels of three PDGF isoforms relative to GAPDH (N= 4). C. Proteomic analysis of conditioned media demonstrates that ovine atrial myofibroblasts (MFCM) secrete significantly more PDGF-AB than atrial myocytes (MCM) and primary atrial fibroblasts (FCM) (**p ≤ 0.01).
PDGF Signaling Inhibition Prevents Remodeling of Myocytes in Contact with Myofibroblasts
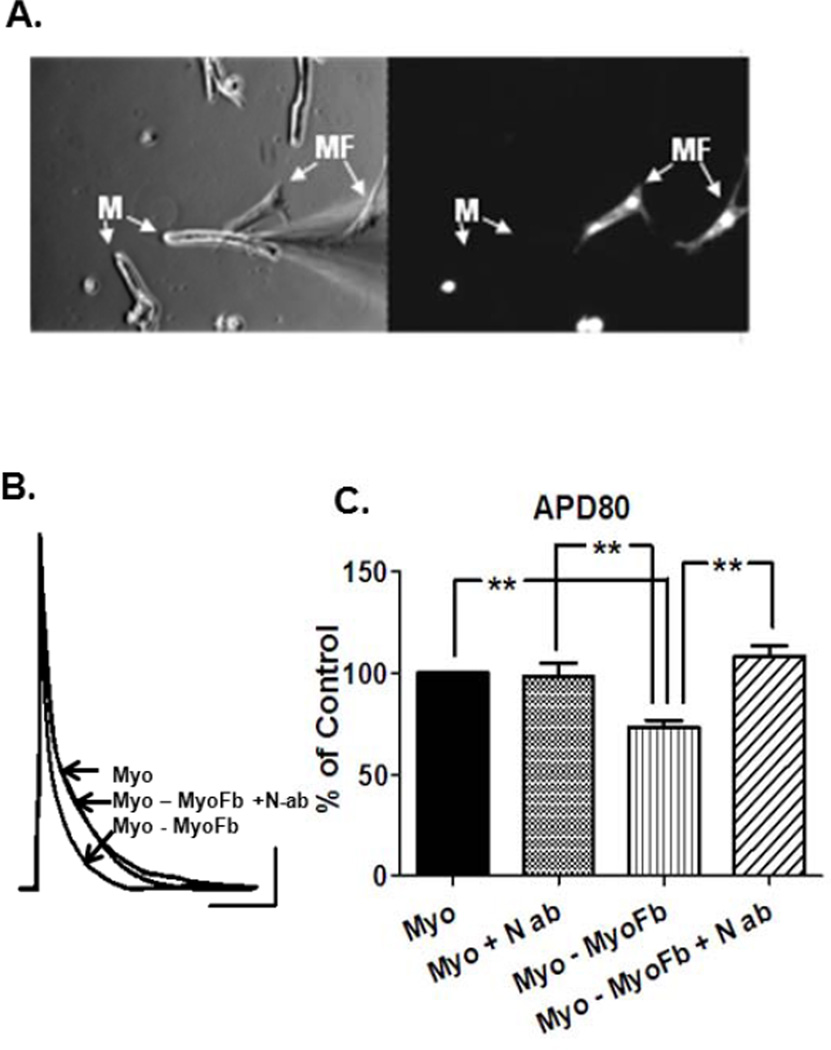
To determine the effects of myofibroblast contact on atrial cell electrophysiology, adult atrial myocytes from unpaced hearts were co-cultured with atrial myofibroblasts for 24 hours. In Figure 2A, digital photos of typical co-cultures used for patch-clamp analysis are shown. In the left panel an atrial myocyte (M) chosen for whole-cell recording is in direct contact with a myofibroblast (MF). Isolated atrial myocytes within the same culture dish were used as controls. The right panel shows the fluorescent image of the same field to identify myofibroblasts previously labeled with cell-tracker green. Additional characterization of these cell types was performed by utilizing cell-specific markers sarcomeric α-actinin (myocytes) and α-SMA (myofibroblasts). Primary antibodies directed at these markers were conjugated to fluorescent secondary antibodies and allowed us to clearly identify both cell types and ensure a homogenous culture of myofibroblasts (Figure S2). Dye transfer experiments using unlabeled myofibroblasts in contact with atrial myocytes were performed to rule out electrotonic effects resulting from gap junctional mediated coupling. Dye failed to transfer to myofibroblasts from donor atrial myocytes in all experiments (Figure.S3). In Figure 2B, representative current clamp recordings after 24 hours in co-culture demonstrated significantly abbreviated APDs in atrial myocytes directly contacting myofibroblasts when compared to isolated myocytes in the same dish. Importantly, this effect on myocyte electrophysiology was prevented by pre-incubating with a neutralizing PDGF-AB antibody (N-ab), which targets the PDGF domain that binds to the PDGF receptor. In Figure 2C, quantification of the data demonstrates a 27% reduction in APD80 that is both highly significant (p≤0.01) and preventable in the presence of N-ab. These data provide the first evidence that PDGF signaling can play a significant role in APD changes seen in adult atrial myocytes in a manner consistent with the electrophysiological remodeling that characterizes PAF.14
Figure 2.
Physical contact with myofibroblasts shortens ovine atrial myocyte APD. A. Left, bright field image of co-cultures used for patch-clamp analysis, Right, fluorescent image showing myofibroblasts. Myofibroblasts were labeled with fluorescent cell tracker green. B. Representative APs show that direct contact with MF results in atrial myocyte APD abbreviation (n=11) that is prevented by the presence of PDGF-AB neutralizing antibody (N-ab) (n=9); (non-contact, n=12). C. Quantification and statistical analysis demonstrates 37% reduction in APD80 in atrial myocytes in direct contact with myofibroblasts (** p ≤ 0.01). Scale bars 20mv, 100msec.
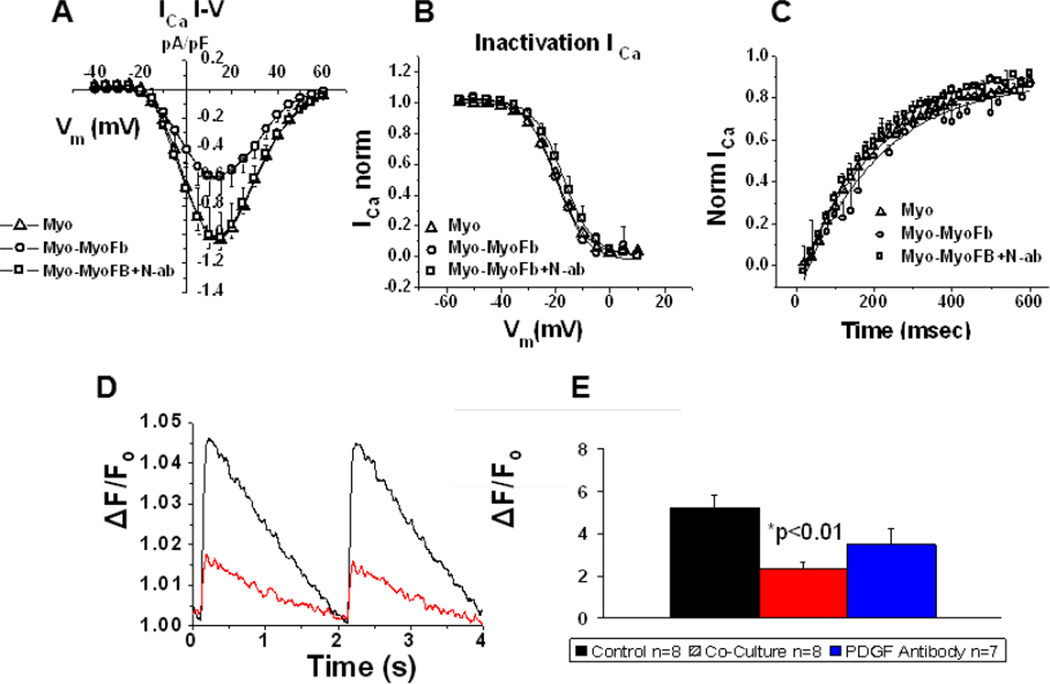
One hallmark of electrophysiological remodeling in both chronically paced atria in animals and PAF in humans is a reduction of ICa,L.15 Thus, to directly assess the effects of heterocellular contact on ICa,L we performed whole-cell patch clamp recordings in atrial myocytes isolated from unpaced ovine atria that were in direct contact with myofibroblasts and compared them to (ICa,L) recordings from isolated myocytes in the same dish (Figure 3A–C). The ICa,L current-voltage (I–V) profile of myocytes in direct contact with myofibroblasts demonstrates a significant attenuation of inward currents at several tested voltages. Furthermore, the I–V relationship of myocytes in contact with myofibroblasts but also in the presence of N-ab retained a profile similar to isolated myocytes. The voltage dependent inactivation and time dependent recovery of ICa,L were unaffected, as shown in Figures 3B,C respectively. In addition, representative traces of calcium transients elicited at 0.5 Hz pacing demonstrate reduced calcium transient amplitudes in atrial myocytes, isolated from unpaced hearts, in direct contact with myofibroblasts (Figure 3D). A quantitative analysis of the results is shown in Figure 3E. Peak calcium transient amplitudes were reduced from a control mean value of 5.20 ± 0.61% to a value of 2.34 ± 0.30% in the presence of heterocellular contact (p < 0.01). Preincubation with N-ab partially restored the transient with a value of 3.47 ± 0.74%. Values were calculated as a percent increase of fluorescence relative to baseline values. These data allow us to speculate that PDGF-AB released by myofibroblasts residing in close proximity to atrial myocytes can lead to calcium channel remodeling compromising the balance of inward currents and contributing to the observed heterogeneous shortening of APD previously demonstrated in atrial myocytes obtained from sheep and human atria undergoing PAF.16,17
Figure 3.
Contact between myofibroblasts and unpaced atrial myocytes remodels ICa-L. A. ICa,L was reduced at several voltages (p≤0.05) when myocytes were in direct contact with a myofibroblast (circles, n=4) relative to isolated myocytes (triangles n=5), and contacting myocytes pre-incubated with PDGF N-ab (squares, n=4) (N=2 for all subsets). B–C. ICa,L voltage-dependent inactivation and time-dependence of recovery remain unaltered. D. Representative calcium transients at 0.5 Hz from atrial cells in co-culture. E. Calcium transient amplitude (0.5 Hz) of atrial cells experiencing heterocellular contact (red) were reduced to 2.34 ± 0.30 % from a control value (black) of 5.20 ± 0.61% (p ≤ 0.01). Pre-incubation with PDGF-AB N-ab partially restored the transient amplitudes to 3.47 ± 0.74% (blue) (n= 8,8,7 respectively). All values reflect a % increase over baseline fluorescence set to a value of 1.
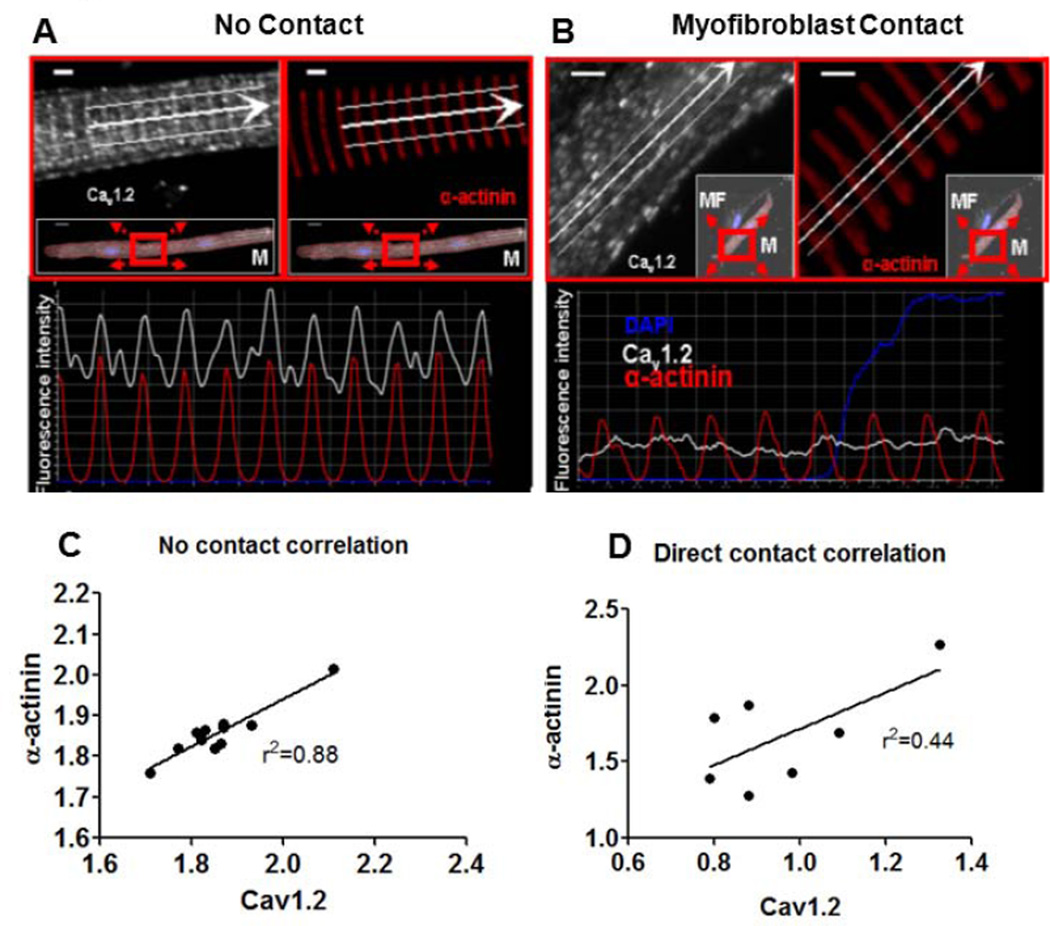
Myocyte Contact with Myofibroblasts Disrupts CaV1.2 Localization
Utilizing high resolution confocal laser scanning microscopy we determined if the subcellular localization of CaV1.2 in atrial myocytes was affected by myofibroblast contact. Figure 4A shows an atrial myocyte in co-culture that was not in direct contact with myofibroblasts. CaV1.2 staining is shown in white, sarcomeric α-actinin in red and the nuclei in blue (DAPI). Panel A shows that CaV1.2 localization in isolated myocytes has the same subcellular periodicity as α-actinin, as expected for a normal adult atrial myocyte.18 Panel B shows that direct contact of an atrial myocyte with a myofibroblast causes disorganization of CaV1.2 protein with preserved α-actinin structure. Linear regression fits of the data shows a significant correlation in α-actinin and CaV1.2 periodicity for isolated myocytes (Panel C, r2=0.88, n=11) that is lost when there is direct contact with one or more myofibroblasts (Panel D, r2=0.44, n=7). These results are compatible with previous co-culture experiments showing that direct contact of myocytes with myofibroblasts promotes morphological dedifferentiation of the myocytes.10 Together, the results presented in Figures 3 and 4 demonstrate for the first time that myocytes in direct contact with myofibroblasts also experience significant remodeling in the the structure and function of the CaV1.2 channel.
Figure 4.
Subcellular localization of CaV1.2 in atrial myocytes altered by myofibroblast contact. A. High-resolution confocal laser scan of an isolated atrial myocyte co-cultured with myofibroblasts. Inset scan at higher resolution. Fluorescence intensity profile along the white arrow demonstrates similar periodicity of fluorescence intensity for CaV1.2 and α-actinin (bottom panel). Scale bar=2µm. B, Heterocellular contact causes CaV1.2 redistribution with loss of t-tubular organization in atrial cardiac myocytes. Intensity profile (bottom panel) shows well-ordered sarcomeres (α-actinin, red), but disorganized CaV1.2 staining (white). Scale bar=2µm. Linear regression fits of the data show a higher degree of correlation in the periodicity of CaV1.2 and α-actinin in isolated atrial myocytes (5C, D; n=11, n=7 respectively).
Inhibition of PDGF Signaling Prevents PDGF-Induced Electromechanical Remodeling
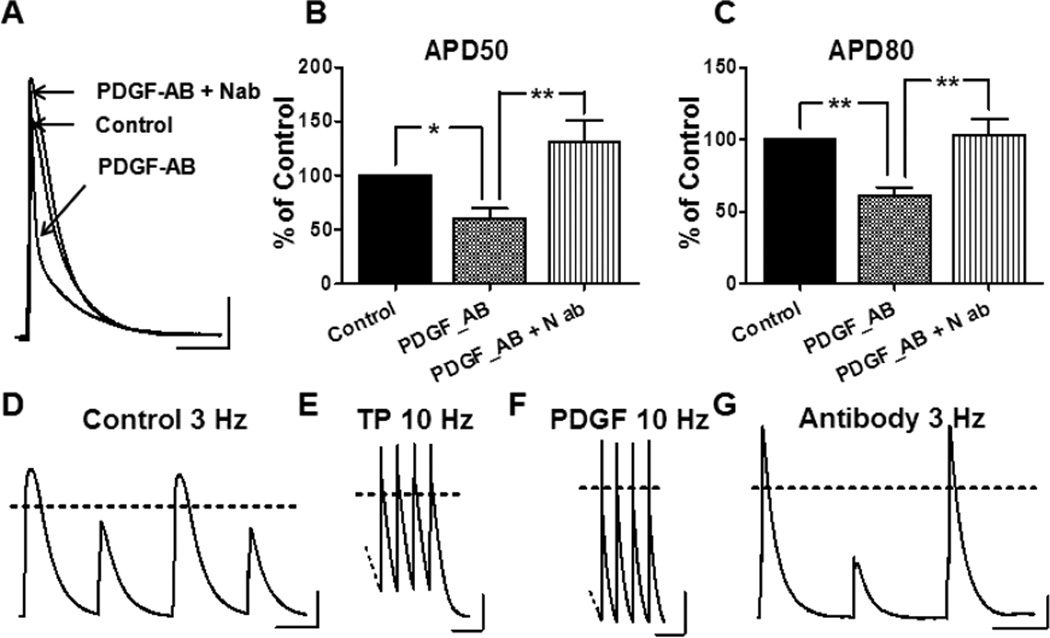
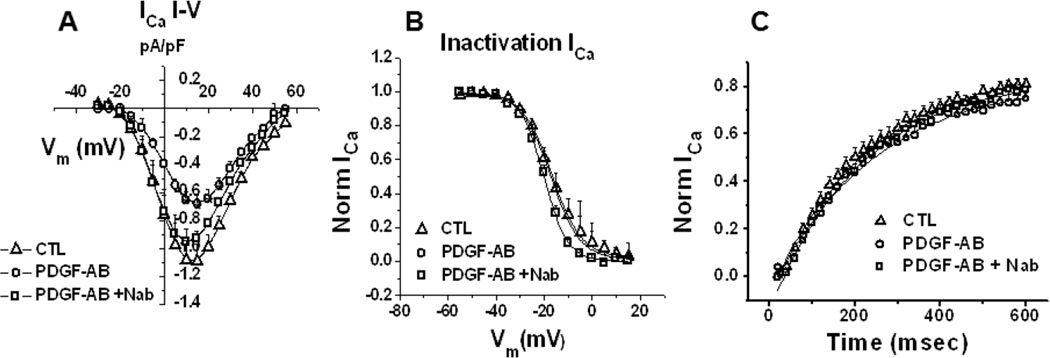
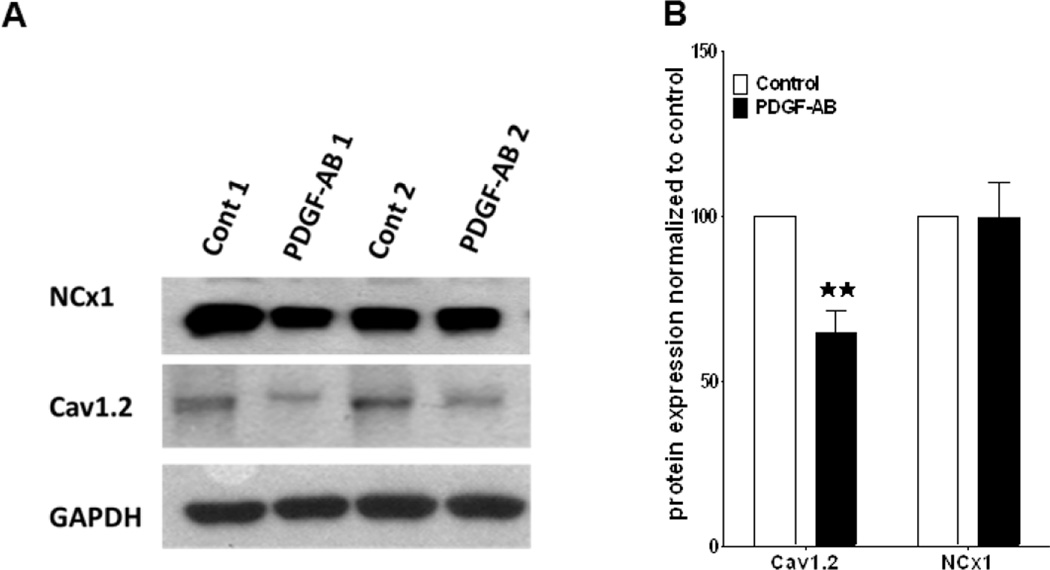
The co-culture studies demonstrate the effects of PDGF-AB released by myofibroblasts on atrial cell electrophysiology. Yet we must acknowledge that these cells are capable of secreting several other notable cytokines (e.g., TGF-β, TNF- α, etc.) that may complicate our analysis. Therefore, to address this limitation normal unpaced ovine atrial myocytes were incubated with 1ng/ml recombinant PDGF-AB peptide for 24 hours in culture. This PDGF-AB concentration is somewhat higher than that released by our myofibroblasts (see Figure 2B) but well within the range reported by other authors.19 As shown in Figure 5 A–C PDGF-AB causes significant reductions in both APD50 and APD80. Similar to what has been demonstrated in the co-cultures, PDGF N-ab prevented this effect. Figure 5 E,F illustrates that both atrial myocytes isolated from a PAF animal and atrial myocytes isolated from an unpaced heart but incubated with PDGF-AB can be stimulated rapidly, up to 10 Hz. In contrast, myocytes from unpaced atria, and myocytes treated with PDGF in the presence of N-ab were unable to respond in a 1:1 manner to pacing frequencies of 3 Hz or higher (Figure 5 D,G). Furthermore, single cell electrophysiology in unpaced atrial cells incubated in PDGF-AB for 24 hours also recapitulates the effects seen in the co-cultures. ICa,L is reduced in PDGF-AB treated cells but retains a normal I–V profile when in the presence of N-ab (Figure 6 A–C). Other biophysical properties of ICa,L remain unaffected. Finally, we examined the effects of PDGF-AB on the expression of CaV1.2 protein in atrial cells. Cells were cultured in the presence or absence of PDGF-AB for a period of 24 hours. Cell lysates were collected and probed for the CaV1.2 protein. As shown in Figure 7A, exposure of atrial cells to PDGF-AB significantly reduced whole cell CaV1.2 immunoreactive protein when compared to untreated cells. The results of three separate experiments are presented in Figure 7B. Densitometry analysis revealed a 37% reduction in CaV1.2 protein in cells treated with PDGF (p≤0.05). The sodium calcium exchanger (NCX1) was used as a negative control. GAPDH was used as a loading control. Altogether, the results demonstrate the profound electrical, cellular and mechanical remodeling that can occur in the presence of PDGF-AB. Such results offer at least a partial mechanistic explanation for the manner in which myofibroblasts contribute to the electromechanical remodeling known to occur during rapid activation of the atria and to AF perpetuation.
Figure 5.
A–C: PDGF-AB (1 ng/ml) shortens sheep atrial myocyte APD50 and APD80 by 40% and 39% respectively (*p<0.05**p<0.01). N-ab blocks the effect (all conditions; n ≥9). D–G: After 24-hour incubation with PDGF-AB, atrial myocytes could be stimulated up to 10 Hz (panel F) similar to cells from a tachypaced animal (TP, panel E). In contrast atrial cells from an unpaced animal (panel D) as well as those exposed to PDGF-AB + Nab (panel G) could not be paced higher than 3 Hz. Scale bars 20mv, 100msec(A), 200msec(D–F).
Figure 6.
Recombinant PDGF-AB decreases ICa,L, calcium transients in unpaced atrial myocytes. A, whole-cell recordings of ICa,L from atrial myocytes treated with PDGF-AB for 24h (circles) demonstrated a significant (p<0.005) reduction in peak amplitudes relative to untreated myocytes (triangles) at several voltages. Pre-incubation with N-ab prevented these effects (squares). B–C, Voltage-dependent Inactivation and time-dependence of recovery remain unchanged (n=9,9,4 for CTL, PDGF-AB, PDGF-AB+N-ab respectively).
Figure 7.
A,B Western blot analyses demonstrate that 24hr. exposure to PDGF-AB down-regulates whole cell CaV1.2 protein. Results from 3 experiments, each performed in duplicate (Fig.7A), are shown in panel B. CaV1.2 protein was reduced by 35% (n=3, **p.0.01). Sodium-calcium exchanger (NCx1) unaffected; negative control. GAPDH; loading control.
DISCUSSION
This is the first study demonstrating a role of PDGF signaling in the electromechanical remodeling of atrial cardiac myocytes and the potential role they play in the evolution of AF. Our data demonstrate that myofibroblasts in culture release PDGF-AB and that elevated concentrations of this cytokine can shorten APD, reduce ICa,L and cause alterations in calcium handling of cultured atrial cardiac myocytes. These alterations are consistent with the electrical remodeling that has been shown to occur in myocytes during AF.4 Our data also demonstrate that two months of atrial tachypacing in sheep results in increased interstitial fibrosis characterized by increased α-SMA expression.
AF induced atrial fibrosis is characterized by myocyte loss and increased collagen deposition leading to localized regions of conduction slowing and heterogeneity.20,21 These conditions create a substrate for unidirectional block and reentry. A strong temporal correlation exists between AF stabilization and structural remodeling. It is well established that these changes take place on a time scale of weeks to months. In contrast, several animal models of AF demonstrated a sustained increase in AF duration and stability achieved within a few days of beginning the experimental protocol, indicative of a second more rapid remodeling process These alterations were characterized by a shortening of APD and effective refractory period both hallmarks of electrical remodeling.22
The molecular mechanisms governing electrical remodeling are mediated by ion channel remodeling, the primary targets being ICa,L, the transient outward potassium current (Ito) and the inward rectifier potassium current (IK1).23–26 ICa,L, the focus of this study, has been shown to be downregulated in both human patients with AF and rapid atrial pacing in animal models.27,28 This reduction in ICa,L has been attributed to several possible mechanisms including reduced protein expression, hyperphosphorylation of the channel and increased degradation of the protein due to increased calpain activity.29,30 The data presented here provide compelling evidence that intracellular mislocalization and reduced expression of CaV1.2 protein are possible mechanisms for alterations in L-type calcium current and calcium handling.
The mechanisms underlying the progression of short-term to PAF are unclear. Recently, the focus has turned to profibrotic molecules and their associated signaling pathways. The best known among these is Ang-II and its potential downstream mediators, TGF-β, and of most recent interest, PDGF.31,32 Increased levels of Ang-II have been correlated to increased levels of atrial PDGF and its role in cardiac fibrosis is just beginning to be investigated. A recent study demonstrated that mast cells co-cultured with cardiac myocytes or fibroblasts led to increased production of PDGF-AA, and promoted cell proliferation and collagen expression in cardiac fibroblasts.31 In addition, they demonstrated that PDGF neutralizing antibody could attenuate atrial fibrosis and AF inducibility in pressure-overloaded hearts. In another study, administration of PDGF-AA for 10 days to non-operated mice induced atrial fibrosis and enhanced the susceptibility to AF.31
The effects of profibrotic signals on the electro-mechanical properties of cardiac myocytes, are key to understanding their contribution to the initiation, progression and persistence of AF. Here we demonstrate that in addition to leading to fibrosis, myofibroblasts contribute to the electromechanical remodeling of atrial myocytes via direct physical contact and release of signaling molecules such as PDGF. Our data show that two months of atrial tachypacing in sheep results in increased interstitial fibrosis associated with increased α-SMA expression. This elevation of α-SMA is likely due to increased activation and proliferation of atrial myofibroblasts. Furthermore, we demonstrate that cultured sheep atrial myofibroblasts produce significant levels of PDGF-AB. Heterocellular contact between myofibroblasts and atrial myocytes lead to a shortening of the atrial APD mediated by a reduction in L-type calcium current density. These effects were prevented by the presence of a neutralizing antibody specific to PDGF-AB. Myofibroblast contact also severely disturbed CaV1.2 localization, normally shadowing sarcomeric α-actinin. Therefore, we anticipate that myocytes in direct contact with myofibroblasts will experience significant cellular and electrophysiological remodeling, specifically affecting the structure and function of CaV1.2 and possibly other channels. Furthermore, we can conclude that PDGF plays a significant role in mediating these alterations.
Clearly, myofibroblasts release of numerous bioactive effectors, other than PDGF.20,32 Here we demonstrate the effects of applying recombinant PDGF-AB peptide directly to healthy sheep atrial myocytes. PDGF (1ng/ml) caused a reduction of both APD50 and APD80, ICa,L density and calcium transients. Similar to the co-cultures these effects were preventable in the presence of N-ab. Furthermore, we demonstrate that both myocytes isolated from sheep atria that underwent tachypaced induced remodeling and normal myocytes treated with PDGF-AB can be paced rapidly, up to 10 Hz. In contrast, non-treated myocytes are unable to respond in a 1:1 manner to a pacing frequency of 3 Hz. Nevertheless, we must acknowledge here the limitation of AP analysis in sheep atria as the nature of this waveform is variable in both morphology and duration from cell to cell.33
Unlike the co-cultures, incubation of myocytes with PDGF-AB peptide for 24 hours did not disrupt CaV1.2 localization (See Fig. S4). In extension of previous studies, showing myofibroblast induced anisotropic stretch in the myocytes,10 we demonstrate that CaV1.2 mislocalization is an important marker of de-differentiation induced by actual physical contact, unrelated to the release of PDGF-AB. Such data are consistent with the possibility that the electromechanical remodeling of atrial myocytes in persistent AF involves significantly direct contact with the activated myofibroblasts that proliferate in the fibrillating atrial myocardium. PDGF released from this cell population should in addition alter the expression, and function of CaV1.2, altogether disrupting normal calcium homeostasis.
Both structural and electromechanical remodeling of the atria play defining roles in the generation and progression of persistent AF. Arguably, a better understanding of the role of myofibroblasts and of individual profibrotic cytokines such as PDGF will be invaluable in identifying downstream mediators of AF induced remodeling and open the door for new more effective therapies for the treatment and prevention of AF.
Supplementary Material
Acknowledgments
Funding Sources: NHLBI grants P01-HL039707 and P01-HL087226 and the Leducq Foundation (JJ), RO1HL087055 (JK), RO1GM076648 (JMBA)
Abbreviations
- M
myocyte
- MF
myofibroblast
- PAF
Persistent Atrial Fibrillation
- PDGF
Platelet-Derived Growth Factor
- ICa,L
L-type Ca2+ current
- APD
Action Potential Duration
- TGF-β1
Transforming Growth Factor Beta-1
- α-SMA
alpha smooth muscle actin
- N-ab
neutralizing PDGF antibody
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES:
None
REFERENCES
- 1.Kannel WB, Benjamin EJ. Current perceptions of the epidemiology of atrial fibrillation. Cardiol Clin. 2009;27:13–24. doi: 10.1016/j.ccl.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpert JS, Petersen P, Godtfredsen J. Atrial fibrillation: natural history, complications, and management. Annu Rev Med. 1988;39:41–52. doi: 10.1146/annurev.me.39.020188.000353. [DOI] [PubMed] [Google Scholar]
- 3.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 4.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 5.Eiras S, Narolska NA, van Loon RB, et al. Alterations in contractile protein composition and function in human atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2006;41:467–477. doi: 10.1016/j.yjmcc.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 6.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 7.Hinescu ME, Gherghiceanu M, Mandache E, et al. Interstitial Cajal-like cells (ICLC) in atrial myocardium: ultrastructural and immunohistochemical characterization. J Cell Mol Med. 2006;10:243–257. doi: 10.1111/j.1582-4934.2006.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souders CA, Bowers SLK, Baudino TA. Cardiac Fibroblast: The Renaissance Cell. Circulation Research. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santiago JJ, Dangerfield AL, Rattan SG, et al. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Dev Dyn. 2010;239:1573–1584. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- 10.Driesen RB, Verheyen FK, Dispersyn GD, et al. M. Structural adaptation in adult rabbit ventricular myocytes influence of dynamic physical interaction with fibroblasts. Cell Biochemistry and Biophysics. 2006;44:119–128. doi: 10.1385/CBB:44:1:119. [DOI] [PubMed] [Google Scholar]
- 11.Dhamoon AS, Pandit SV, Sarmast F, et al. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004;94:1332–1339. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- 12.Zhao W, Zhao T, Huang V, et al. Platelet-derived growth factor involvement in myocardial remodeling following infarction. J Mol Cell Cardiol. 2011;51:830–838. doi: 10.1016/j.yjmcc.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filgueiras-Rama D, Price NF, Martins RP, et al. Long-term frequency gradients during persistent atrial fibrillation in sheep are associated with stable sources in the left atrium. Circ Arrhythm Electrophysiol. 2012;5:1160–1167. doi: 10.1161/CIRCEP.111.969519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 15.Van Wagoner DR, Pond AL, Lamorgese M, et al. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–436. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- 16.Gaborit N, Steenman M, Lamirault G, et al. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112:471–481. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
- 17.Lau DH, Psaltis PJ, Mackenzie L, et al. Atrial remodeling in an ovine model of anthracycline-induced nonischemic cardiomyopathy: remodeling of the same sort. J Cardiovasc Electrophysiol. 2011;22:175–182. doi: 10.1111/j.1540-8167.2010.01851.x. [DOI] [PubMed] [Google Scholar]
- 18.Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA, Chiamvimonvat N. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res. 2007;100:112–120. doi: 10.1161/01.RES.0000253095.44186.72. [DOI] [PubMed] [Google Scholar]
- 19.Ivarsson M, McWhirter A, Borg TK, Rubin K. Type I collagen synthesis in cultured human fibroblasts: regulation by cell spreading, platelet derived growth factor and interactions with collagen fibers. Matrix Biol. 1998;16:409–425. doi: 10.1016/s0945-053x(98)90014-2. [DOI] [PubMed] [Google Scholar]
- 20.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 21.Li D, Fareh S, Leung TK, et al. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 22.Yue L, Feng J, Gaspo R, et al. Ionic remodelingunderlying action potential changes in a canine model ofatrial fibrillation. Circ Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]
- 23.Grammer JB, Bosch RF, Kuhlkamp V, et al. Molecular remodeling of Kv4.3 potassium channels in human atrial fibrillation. J Cardiovasc Electrophysiol. 2000;11:626–633. doi: 10.1111/j.1540-8167.2000.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 24.Christ T, Wettwer E, Voigt N, et al. Pathology-specific effects of the IKur/Ito/IK,ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br J Pharmacol. 2008;154:1619–1630. doi: 10.1038/bjp.2008.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaborit N, Steenman M, Lamirault G, et al. Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation. 2005;112:471–481. doi: 10.1161/CIRCULATIONAHA.104.506857. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Garratt CJ, Zhu J, et al. Role of up-regulation of IK1 in action potential shortening associated with atrial fibrillation in humans. Cardiovasc Res. 2005;66:493–502. doi: 10.1016/j.cardiores.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Bosch RF, Scherer CR, Rub N, et al. Molecular mechanisms of early electrical remodeling: transcriptional downregulation of ion channel subunits reduces I(Ca,L) and I(to) in rapid atrial pacing in rabbits. J Am Coll Cardiol. 2003;41:858–869. doi: 10.1016/s0735-1097(02)02922-4. [DOI] [PubMed] [Google Scholar]
- 28.Grammer JB, Bosch RF, Kühlkamp V, et al. Molecular and electrophysiological evidence for "remodeling" of the L-type Ca2+ channel in persistent atrial fibrillation in humans. Z Kardiol. 2000;89(Suppl 4):IV23–IV29. doi: 10.1007/s003920070060. [DOI] [PubMed] [Google Scholar]
- 29.Christ T, Boknik P, Wöhrl S, et al. L-type Ca2 current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–2657. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- 30.Goette A, Arndt M, Rocken C, et al. Calpains and cytokines in fibrillating human atria. Am J Physiol. 2002;283:H264–H272. doi: 10.1152/ajpheart.00505.2001. [DOI] [PubMed] [Google Scholar]
- 31.Liao CH, Akazawa H, Tamagawa M, et al. Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest. 2010;120:242–253. doi: 10.1172/JCI39942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saba S, Janczewski AM, Baker LC, et al . Atrial contractile dysfunction, fibrosis, and arrhythmias in a mouse model of cardiomyopathy secondary to cardiac-specific overexpression of tumor necrosis factor-{alpha} Am J Physiol Heart Circ Physiol. 2005;289:H1456–H1467. doi: 10.1152/ajpheart.00733.2004. [DOI] [PubMed] [Google Scholar]
- 33.Deroubaix E, Folliguet T, Rücker-Martin C, et al. Moderate and chronic hemodynamic overload of sheep atria induces reversible cellular electrophysiologic abnormalities and atrial vulnerability. J Am Coll Cardiol. 2004 Nov 2;44:1918–1926. doi: 10.1016/j.jacc.2004.07.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.