Abstract
Nessler, Johnson, Bersick, and Friedman (D. Nessler, R. Johnson, Jr., M. Bersick, & D. Friedman, 2006, On why the elderly have normal semantic retrieval but deficient episodic encoding: A study of left inferior frontal ERP activity, NeuroImage, Vol. 30, pp. 299–312) found that, compared with young adults, older adults show decreased event-related brain potential (ERP) activity over posterior left inferior prefrontal cortex (pLIPFC) in a 400- to 1,400-ms interval during episodic encoding. This altered brain activity was associated with significantly decreased recognition performance and reduced recollection-related brain activity at retrieval (D. Nessler, D. Friedman, R. Johnson, Jr., & M. Bersick, 2007, Does repetition engender the same retrieval processes in young and older adults? NeuroReport, Vol. 18, pp. 1837–1840). To test the hypothesis that older adults’ well-documented episodic retrieval deficit is related to reduced pLIPFC activity at encoding, we used a novel divided attention task in healthy young adults that was specifically timed to disrupt encoding in either the 1st or 2nd half of a 300- to 1,400-ms interval. The results showed that diverting resources for 550 ms during either half of this interval reproduced the 4 characteristic aspects of the older participants’ retrieval performance: normal semantic retrieval during encoding, reduced subsequent episodic recognition and recall, reduced recollection-related ERP activity, and the presence of “compensatory” brain activity. We conclude that part of older adults’ episodic memory deficit is attributable to altered pLIPFC activity during encoding due to reduced levels of available processing resources. Moreover, the findings also provide insights into the nature and timing of the putative “compensatory” processes posited to be used by older adults in an attempt to compensate for age-related decline in cognitive function. These results support the scaffolding account of compensation, in which the recruitment of additional cognitive processes is an adaptive response across the life span.
Keywords: aging, elaborative episodic encoding, episodic memory, event-related potentials, divided attention
A cardinal feature of aging is the progressive loss of the ability to recollect personal event (episodic) memories, even as fact retrieval (semantic memory) remains intact. It is well established that these two memory systems interact in crucial ways during episodic encoding because semantic processing of events leads to more elaborately encoded memories (Craik & Lockhart, 1972). The elaborate memories produced by this interaction in turn lead to more distinctive, high-quality recollections at retrieval (e.g., Craik, 2002; Gallo, Meadow, Johnson, & Foster, 2008). Although difficult to tease apart behaviorally, studies employing both hemody-namic (i.e., positron emission tomography; functional MRI, fMRI) and event-related brain potential (ERP) techniques have been used successfully to parse the brain activity related to episodic encoding and retrieval processes. For example, researchers have supported Craik and Lockhart’s (1972) behavioral results by demonstrating that the selection processes supporting both semantic retrieval (e.g., Thompson-Schill, D’Esposito, Aguirre, & Farah, 1997) and elaborative episodic encoding appear to coexist within posterior left inferior prefrontal cortex (pLIPFC; e.g., Buckner, Kelley, & Petersen, 1999; Prince, Tsukiura, & Cabeza, 2007). In addition, the magnitude of pLIPFC activity during encoding has been shown to be directly related to both the number and quality of subsequently retrievable episodic memories (e.g., Paller & Wagner, 2002).
Consistent with the idea that age-related declines in episodic retrieval are due, at least in part, to altered encoding processes, hemodynamic studies of encoding have found significantly less pLIPFC activity in older compared with young adults (e.g., Cabeza et al., 1997; Grady et al., 1995). Furthermore, ERP studies have demonstrated that, compared with young adults, the memories that older adults do retrieve are typically of low quality, with reduced or no significant amounts of recollection-related brain activity (e.g., Nessler, Friedman, Johnson, & Bersick, 2007; Nessler, Johnson, Bersick, & Friedman, 2008). Taken together, the extant data suggest that the root of older adults’ episodic memory deficits lies in the failure of semantic and episodic systems to interact successfully to encode elaborate episodic memories. The aim of the present study, therefore, was to determine whether there is a causal link between reduced pLIPFC activity at encoding and deficient recollection during subsequent episodic retrieval.
One generally accepted explanation for the pattern of preserved semantic and deficient episodic memory functions in older adults is that the pool of available processing resources decreases with age (e.g., Craik & Byrd, 1982; Park et al., 1996). Craik and Byrd (1982) and Park et al. (1996) and others have posited that, whereas retrieval requires few resources and is relatively automatic, encoding elaborate episodic memories requires resource-demanding, controlled processes. Support for this hypothesis has come from studies using divided attention (DA) tasks, which reduce the amount of resources available for encoding by diverting them to a concurrently performed secondary task (Baddeley, Lewis, Eldridge, & Thomson, 1984; Craik, Govoni, Naveh-Benjamin, & Anderson, 1996). The results of such DA experiments on episodic encoding are striking because they show that subsequent retrieval performance in healthy young participants can be reduced to the levels typically seen when older participants retrieve memories encoded under full attention (FA) conditions (e.g., Anderson, Craik, & Naveh-Benjamin, 1998). Moreover, Jennings and Jacoby (1993) showed that DA manipulations produced the same pattern of preserved automatic and deficient controlled retrieval processes in young adults (i.e., intact familiarity and degraded recollection, respectively) as when their older adult group encoded the materials under FA conditions. Additional data supporting a link between these behavioral changes and alterations in underlying brain activity have come from fMRI studies. These studies have, for example, demonstrated that reducing the resources available for episodic encoding processes is associated with reductions in pLIPFC blood flow in young adults down to the low levels observed when older adults encode events under FA conditions (e.g., Anderson et al., 2000). Taken together, these behavioral and hemodynamic results support the hypothesis that processing resources decline with age, which affects the ability of older adults to encode episodic memories.
Temporal Dynamics of the Semantic–Episodic Interface During Elaborative Encoding
As reviewed above, the extant data indicate that the semantic– episodic interface, which promotes the formation of elaborately encoded episodic memories and is instantiated in pLIPFC, declines in function with age. Although the timing of the processes in this interface remains unknown, older adults’ pattern of spared semantic and deficient episodic memory functions provides some insights. For example, the fact that semantic memory retrieval is relatively unaffected in older adults suggests a serial process in which pLIPFC activity remains at or near normal levels until after semantic retrieval. The well-documented decrements in pLIPFC activity in older adults would thus presumably be due to reductions in brain activity following semantic retrieval but prior to the onset or completion of elaborative encoding processes. In this formulation, age-related reductions in available resources might prevent semantic and episodic processes from interacting successfully in a number of ways. To begin, episodic encoding may depend on specific processes being enabled in advance (i.e., “encoding mode”) in a manner analogous to the requirement that a person must be in the appropriate “retrieval mode” to retrieve episodic memories (Tulving, 1983). Furthermore, there are likely to be constraints on the timing of resource availability at a number of stages within the encoding process. For example, at the earliest stages, insufficient resource availability at or shortly after onset of the event to be encoded may result in a failure to initiate the semantic– episodic interface. Assuming successful initiation, additional resources would be required continuously to maintain retrieved semantic memories in an activated state for the duration of the elaboration process. Finally, other resources would be required for the processes responsible for integrating retrieved semantic information with the event being encoded. Hence, elaborative episodic encoding would require that resources be available continuously, with even temporary disruptions having deleterious effects on a person’s ability to subsequently recall these episodic memories.
Understanding the nature of the semantic–episodic interface and the moment-to-moment role of controlled processing resources in elaborative episodic encoding can only come from specifying the temporal dynamics of the processes involved. Unfortunately, the poor temporal resolution of hemodynamic techniques means that they cannot provide sufficiently detailed information about the timing of the blood flow reductions in pLIPFC associated with aging or DA manipulations. Specifically, it is not known whether older adults’ altered pLIPFC activity is due to long-duration reductions in brain activity or to intervals of normal activity preceded, followed, or interspersed with intervals of reduced or no activation. Therefore, Nessler, Johnson, Bersick, and Friedman (2006) used the high temporal resolution of ERPs to study episodic encoding processes within the pLIPFC in both young and older adults. Consistent with the idea that retrieval requires relatively few resources, both groups showed equivalent behavioral performance (i.e., same accuracy and decision times) in a semantic-selection task, regardless of the amount of processing required (i.e., low vs. high selection). Specifically, whereas the low-selection task in Nessler et al. (2006) required participants to decide whether a picture and word represented the same concept (i.e., a picture of a dog followed by the word dog), the high-selection task required participants to judge whether an adjective described a characteristic of the presented word (e.g., the adjective heavy followed by the word feather). Their ERP results revealed two temporally overlapping patterns of brain activity over left frontal cortex. One, a long-duration negativity, which began 300–400 ms after stimulus onset, was significantly larger for high-compared with low-selection judgments in both young and older adults. Most important, although there were no group differences in either the onset or amplitude of this “selection-related negativity,” there were significant group differences in its duration. Specifically, whereas this negativity continued well past the young’s responses signaling their semantic judgments, it decreased precipitously around 800 ms in the older adults and disappeared entirely by the time of their responses. A second briefer negativity (300–800 ms), which was entirely absent in the older adults, appeared in the young as a “pedestal” on which the nearly simultaneously starting selection-related negativity was riding. The results of two independent source analyses provided strong support for the idea that both their negativities reflected activity in pLIPFC (see Nessler et al., 2006, for complete details).
The differential timing of the two negativities in Nessler et al. (2006) suggests that they reflect different processes in the semantic–episodic interface. That is, the early onset and extended duration of the selection-related negativity is consistent with a long-duration process, such as the continuous activation of retrieved semantic memories, the elaborative encoding process, or a combination of both. The early brief negativity, by contrast, could reflect processes related to initiation of the semantic–episodic interface (i.e., enabling elaborative encoding). In this interpretation, the absence of the brief negativity in older adults would mean that their neural signal to initiate elaborative encoding was missing. If correct, this would explain why older adults’ selection-related negativity terminated after completion of the semantic-selection task rather than continuing past the response, as it did in the young. That is, with no “initiation negativity” to signal the start of elaborative encoding, there would be no need to maintain the retrieved semantic memories in an activated state after completion of the semantic-selection task.
The direct relation between the extent of elaborative processing and the amount of contextual detail incorporated into episodic memories (e.g., Craik, 2002; Gallo et al., 2008) means that the efficacy of elaborative encoding can be assessed by determining the extent of recollection during episodic retrieval. The ERP provides a well-established measure of recollection-related processing in the form of an enhanced left parietal positivity for correctly recognized items (old) compared with that elicited by correctly rejected items (new). Referred to as the left parietal episodic memory (EM) effect, this ERP component is maximal between 500 and 800 ms (see Friedman & Johnson, 2000; Johnson, 1995; Mecklinger, 2000; Rugg & Curran, 2007, for reviews) and there is ample evidence that its magnitude indexes the amount of recollected information (e.g., Vilberg, Moosavi, & Rugg, 2006; Wilding, 2000). Using this index, Nessler et al. (2007) showed that, compared with young adults, their older adults’ reduced encoding-related ERP activity was accompanied by greatly reduced recognition and no significant parietal EM effect. Thus, their results support the idea that there was a failure in older adults to produce or maintain elaborated episodic memories. Consistent with these results, Curran (2004) demonstrated that the presence of a DA task during encoding in young adults abolished the parietal EM effect during subsequent retrieval. Hence, when considered in their entirety, the data suggest that the root of older adults’ episodic memory deficits most likely lies in the failure of semantic and episodic systems to interact successfully to encode elaborate episodic memories.
As noted above, the magnitude of the parietal EM effect varies as a function of the amount of information recollected. Therefore, its magnitude varies widely across experiments depending on the specific encoding–retrieval conditions used, which in turn affects the young–old difference in the magnitude of the parietal EM effect. For example, item recognition paradigms generally produce large young–old differences in the magnitude of the parietal EM effect, with older adults showing anywhere from no significant amounts of recollection-related brain activity (e.g., Nessler et al., 2007, 2008; Wolk et al., 2009) to small but significantly reduced EM effects (e.g., Morcom & Rugg, 2004). By contrast, young–old differences in the parietal EM effect are typically much smaller in source memory studies (e.g., Duarte, Ranganath, Trujillo, & Knight, 2006; Mark & Rugg, 1998; Trott, Friedman, Ritter, Fabiani, & Snodgrass, 1999; Wegesin, Friedman, Varughese, & Stern, 2002). However, as meticulously detailed by Wolk et al. (2009, pp. 225–226), such discrepant results are readily explained by the large and important differences in the encoding and retrieval conditions in these two paradigms. Given the importance of maintaining the same encoding–retrieval conditions when comparing across studies, we used the same stimuli and experimental procedures in the present experiment as were used by Nessler et al. (2006).
Compensatory Brain Activity in Older Adults
A common co-occurrence of decreased cognitive function in older adults is the presence of additional brain activity where there is no comparable activity in the young (e.g., Cabeza, Anderson, Locantore, & McIntosh, 2002; Reuter-Lorenz & Cappell, 2008). This extra brain activity, which researchers have often labeled compensatory, is thought to occur when older adults attempt to compensate for age-related declines in cognitive abilities by using additional processes (e.g., Cabeza et al., 2002, 2004; Grady, McIntosh, Rajah, Beig, & Craik, 1999; Reuter-Lorenz et al., 2000). Because these additional processes depend on neural circuits other than those typically used in the young, this has been referred to as the compensation-related utilization of neural circuits hypothesis (see Reuter-Lorenz & Lustig, 2005, for a review). In an alternative, but similar, account known as the scaffolding theory of aging and cognition (STAC) model, older adults’ additional brain activity is explained using the concept of “scaffolding.” Scaffolding is the term used to describe the process in which additional (i.e., alternative) brain circuits are brought online in an adaptive response intended to enhance processing in a given task. A core concept of the STAC model is that this scaffolding process is a normal response, which can occur across the life span, when new or demanding processing conditions arise (e.g., when one learns a new task; see Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Park, 2010, for reviews). According to this view, the extra brain activity in older adults would reflect use of scaffolding processes to “protect” their performance against the cognitive declines that occur with aging. Although there is some evidence that this compensatory activity can reflect either task-specific or general-purpose (e.g., an upregulation of attention) cognitive processes in different tasks (e.g., Park & Reuter-Lorenz, 2009), relatively little basic information exists about the nature or role of these processes in older adults, including any specification of the necessary and sufficient conditions for its appearance and whether it is initiated consciously or unconsciously.
Despite the basic premise that older adults evoke additional brain activity to improve their task performance, the available data on its effectiveness are mixed. Although its presence has been correlated positively with retrieval performance, or found to be present selectively in high- compared with low-performing older adults in some hemodynamic studies (Anderson et al., 2000; Cabeza et al., 2002), this is not a universal finding. For example, other investigators have reported that the amount of compensatory activity is negatively correlated with performance (Colcombe, Kramer, Erickson, & Scalf, 2005; Nielson, Langenecker, & Garavan, 2002) or that its presence had no effect on behavior (Langenecker & Nielson, 2003), suggesting that attempts to compensate are not always successful. Similarly, Nessler et al. (2007) found a left anterior frontal negativity in their older adult group where there was no similar ERP activity in their young participants, which had no positive effect on retrieval. Such discrepant results could, however, be expected by the fact that the efficacy of compensatory processes necessarily depends on a variety of factors, ranging from the extent to which cognitive processes are compromised to the intactness of processing performed at earlier stages (e.g., perceptual or encoding processes). For example, whereas compensatory activity may fail to increase retrieval performance after severe failures of elaborative processing at encoding, it might have positive effects on retrieval performance when encoding processes are less impacted (e.g., high-performing people).
By their nature, aging studies are designed primarily to reveal which cognitive processes are compromised in older adults relative to the young and hence group (i.e., across-subjects) comparisons are used to define which brain activity is labeled “compensatory.” A related aspect of these studies is that the tasks employed are tailored to the reduced abilities of older adults and thus are frequently relatively easy for young participants to perform, thereby obviating the need for the latter group to engage compensatory processes (but see Smith et al., 2001, for a counterexample). By contrast, DA studies are designed to compromise the young’s ability to perform particular tasks, thereby creating the possibility that they will engage the same or similar compensatory processes as invoked by older adults. Hence, DA manipulations could shed light on the nature and workings of these processes in older adults by revealing how, and under what conditions, compensatory activity occurs in a healthy young brain.
The Present Study
Although older adults’ episodic retrieval deficit is associated with deficient levels of pLIPFC activity at encoding, the data are correlational. Thus, the extent to which altered processing at encoding can lead to subsequent episodic retrieval deficits remains unclear. Finding evidence for a causal link between these two phenomena would shed light on the nature of older adults’ episodic deficit, as well as on the related phenomenon of “compensatory” brain activity. To test the strength of this link, we adopted a strategy in which a DA task was imposed during a brief temporal interval while healthy young participants performed the semantic-selection episodic encoding task of Nessler et al. (2006). Specifically, the DA task was targeted to coincide with either the early (300–850 ms: DA1) or late (850 –1,400 ms: DA2) half of the interval when Nessler et al. found altered encoding-related ERP activity in older adults over pLIPFC. We reasoned that, if the presence of the DA task during episodic encoding produced the characteristic age-related retrieval deficit in young adults, it would provide causal evidence in favor of the hypothesis that older adults’ altered encoding-related ERP activity is responsible for their subsequent episodic retrieval deficits. Moreover, by restricting the timing of the DA task, we hoped to shed light on the temporal dynamics of the semantic–episodic interface by assessing the relative impact of diminished resources on encoding processes in the early and late intervals.
To test these hypotheses, we adopted an analysis strategy in which a series of planned comparisons was used to determine the relative impact of the FA and DA manipulations (FA vs. DA1; FA vs. DA2; DA1 vs. DA2) on both behavioral and ERP measures of subsequent episodic retrieval. In accord with previous results, we hypothesized that the presence of the DA task would not affect semantic recall but would disrupt elaborative episodic encoding (e.g., Anderson et al., 1998, 2000) and, therefore, subsequent behavioral and ERP measures of recognition and recall (e.g., reduced left-parietal EM effects).
Unlike the compensation account, the scaffolding account posits that extra brain activity can be summoned across the life span when altered strategies might enhance the ability to meet task goals or deal with increases in task load, such as when impoverished encoding conditions increase retrieval difficulty (Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Park, 2010). Implicit in the scaffolding account is the idea that compensatory activity should be present for abnormal, but not normal, processing conditions and should occur with the proper timing to assist task-related processing in a meaningful way (e.g., prior to when stimuli are categorized as old or new). Both these aspects of the scaffolding account were addressed in the present study. That is, in addition to the timing information provided by the ERP, the present experiment allowed us to determine whether individuals engage compensatory processes dynamically as retrieval difficulty increases, without having to resort to the between-groups comparisons (e.g., young vs. old, high performers vs. low performers) used in previous studies. Based on finding possible compensatory activity in older adults over anterior LIPFC (aLIPFC) during episodic retrieval in Nessler et al. (2007), we predicted that retrieval-related compensation would appear over left frontal scalp under DA conditions.
To eliminate the need to run an older adult group for the purpose of replicating further their well-documented pattern of preserved and deficient memory, all relevant task, stimulus, and recording procedures used in the present study duplicated those used by Nessler et al. (2006, 2007). In this way, the effects of the DA manipulations on memory function in young adults are directly comparable to those of our previous studies employing both young and older adult groups. Hence, the behavioral and ERP results from those reports are included here for comparison with those obtained from the present young adult group.
Method
Participants
Eighteen young adults (12 women) with a mean age of 21.7 years (range 21–27 years) and a mean of 16 years of education (SD = 1.7) were paid to participate. All participants were native English speakers, with normal or corrected-to-normal vision, no history of neurological or psychiatric disorders, and free from medications known to affect the central nervous system. They had a mean score of 55.0 (SD = 1.3) on the modified Mini-Mental Status Exam (maximum = 57), a measure of global cognitive function (Mayeux, Stern, Rosen, & Leventhal, 1981). All signed informed consent according to New York State Psychiatric Institute’s Institutional Review Board criteria. Participants were paid $15/hr and, with the $10 bonus (see below), the approximate maximum amount participants received was $55/session.
Experimental Procedures and Stimuli
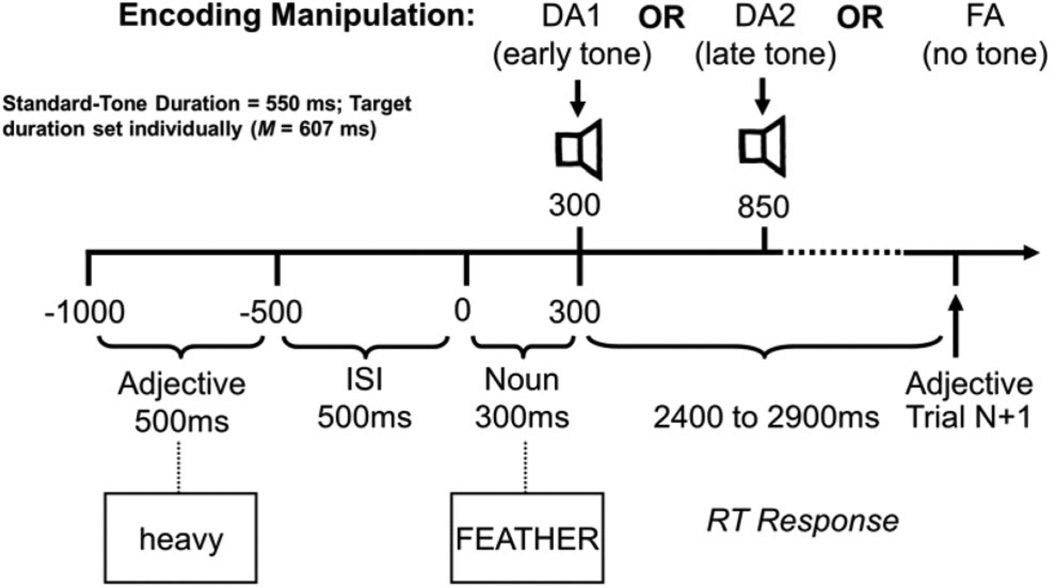
Participants made high-selection decisions (Nessler et al., 2006) on nouns in three encoding blocks. During encoding, an adjective (lowercase) was presented for 500 ms followed by a 500-ms blank presentation box (see Figure 1). The to-be-remembered noun (uppercase) was then presented for 300 ms. Participants were instructed to decide whether the adjective described a feature of the noun and make a choice reaction time (RT) response by pressing the appropriate button with their left or right thumb as quickly as possible. There was a variable intertrial interval (2,400 –2,900 ms) before the next adjective appeared. Nessler et al. (2006) used a variable intertrial interval (ITI) to reduce the effects of processing related to anticipation of the next trial, which can elicit overlapping negativities and thereby contaminate the ERP results. Randomly varying the ITI reduces the impact of such anticipatory activity on ERP averages by temporally decoupling it from task-related potentials. Each encoding block consisted of 60 trials and each was followed, after a 5-min delay, by a recognition test.
Figure 1.
Schematic diagram of an encoding trial. FA = full attention condition; DA1 = early divided attention condition; DA2 = late divided attention condition; ISI = interstimulus interval; RT = reaction time.
Two encoding blocks contained a secondary, tone-duration discrimination task. In these DA blocks, 85% of nouns were accompanied by standard tones (550 ms in duration) and 15% by target tones. The tone-duration task was designed to ensure that participants attended to the tones for the entire duration of the early or late interval to determine whether the longer duration tone had been presented. To ensure that the secondary task was difficult and drew resources away from elaborative encoding processes, we determined the duration of the target tone individually prior to the experiment on the basis of task performance. To set each individual’s target-tone duration, we created different blocks of trials to determine a target duration that approximated a tone-duration discrimination sensitivity (Pr) level of .40. Each of these blocks included 52 standard tones (550 ms) and 10 target tones of a specified duration (650, 630, 620, 610, 600, or 590 ms). The temporal parameters for the various events in these trials matched those used during the main experimental DA1 and DA2 encoding blocks.
To begin, all participants performed in two blocks, one with a target tone of 650 ms and one with a target tone of 620 ms. Following these two blocks, all participants performed four additional blocks in which target-tone duration was individually chosen on the basis of the participant’s performance in the first two blocks. This procedure led to the following target-tone durations: for two participants, 590 ms; for six participants, 600 ms; for seven participants, 610 ms; for one participant, 620 ms; for two participants, 630 ms. Across the 18 participants, mean duration of the target tone was 607 ms (SE = 2.6).
To motivate participants to perform well on the tone-discrimination task, prior to performing the DA conditions, we told them that they would receive an additional $10 if they achieved a tone-discrimination Pr level of .40. However, all participants, regardless of performance level, received the additional $10. Participants were instructed that they were to press an additional button with their right index finger whenever they detected a target tone. Auditory stimuli (1000 Hz, 80 dB SPL) were delivered either early (DA1; 300–850 ms) or late (DA2; 850–1,400 ms) after noun onset. The secondary task was indeed difficult, as mean Pr was .25 and .29 in the DA1 and DA2 conditions, respectively. Both mean Pr values differed reliably from zero via t test (ps < .0001), although they did not differ significantly from one another, t(17) = 0.92, p > .36. Moreover, as would be expected, performance on the tone-discrimination task when administered alone (Pr = .35) was significantly better than when it accompanied the semantic decision task (Pr = .27, averaged across the early and late tones), tone-tailed(17) = 2.0,p < .03. The third, FA, encoding block acted as a control condition and therefore had no secondary task.
The stimulus set for the high-selection task consisted of 396 concrete English nouns and 12 adjectives (cheap, expensive, big, small, man-made, natural, light, heavy, narrow, wide, short, tall), all taken from Nessler et al. (2006). Nouns appeared only once, whereas adjectives were used repeatedly. In each recognition test, all 60 nouns from the preceding encoding block were intermixed randomly with 60 previously unseen nouns. Studied and new nouns appeared for 300 ms followed by a variable ITI (1,900– 2,400 ms). Participants were instructed to make an old–new response as quickly and accurately as possible. Condition order was rotated across participants. Twenty minutes after the final recognition test, participants were given a surprise recall test in which they had 10 min to freely recall (i.e., write on a sheet of paper) as many words as possible from the three lists.
Electroencephalographic (EEG) Recording
EEG was recorded from 62 scalp sites (sintered Ag/AgCl) in accord with the extended 10–20 system (Sharbrough et al., 1990) using an Electrocap (Neuromedical Supplies) and an averaged-mastoid reference. Horizontal and vertical electrooculogram (EOG) were recorded bipolarly with electrodes placed, respectively, at the outer canthi of both eyes and above and below the left eye. EOG and EEG were recorded continuously (Synamp amplifiers; DC; 100-Hz low-pass filter; 500-Hz digitization rate). Eye movement artifacts were corrected offline (Semlitsch, Anderer, Schuster, & Presslich, 1986), and remaining artifacts were rejected manually.
Averaging Strategy
Processing on trials with target tones was fundamentally different from that on FA and standard-tone trials because of the addition of response-related (i.e., selection and execution) processing. To ensure that the averaged ERPs reflected more equivalent processing across conditions, we excluded trials with target tones from the averages. Consequently, the range of trials entering the six averages across participants was FA hits, 28–54; DA1 hits, 24–50; DA2 hits, 23–52; FA correct rejections (CRs), 18–53; DA1 CRs, 27–53; DA2 CRs, 28–52.
Data Analysis
In addition to the analysis of variance (ANOVA) strategy outlined above, the behavioral and ERP data were also analyzed using repeated measures ANOVAs with the within-subjects factor of condition (FA, DA1, DA2). The details about quantification of ERP amplitudes (i.e., temporal intervals, electrode sites) are provided with the presentation of the test results. Electrode sites were chosen based on the locations where surface potential or current source density (CSD) maps revealed maximal brain activity. In all tests involving ERP data, F ratios are reported with Greenhouse– Geisser-corrected p values and the epsilon value calculated to correct for nonsphericity (Jennings & Wood, 1976), along with uncorrected degrees of freedom. Partial η2 is presented as an estimate of main and interaction effect sizes. Tukey’s HSD post hoc tests were used to assess within-group main effects and, where appropriate, interaction effects.
Results
Behavioral Data
Encoding
As predicted, overall performance on the semantic-selection task was unaffected by the presence of a DA task in either interval. In accord with our previous results, an overall Condition × Match ANOVA revealed that the percentage of no-match decisions was higher than the percentage of match decisions (59.2 ± 2.0 vs. 39.6 ± 1.9, respectively), F(1, 17) = 24.0, p < .0001, . Nevertheless, there were no differences in these percentages among the three conditions, F(2, 34) = 2.8, p > .09, and the Condition × Match interaction was not significant (F < 1). Based on this lack of differences across conditions, coupled with the fact that “match” and “no-match” decisions cannot be categorized unambiguously into correct or incorrect responses (see Nessler et al., 2006), we collapsed over match and no-match trials for the remaining analyses. The ANOVA on mean RT also revealed a lack of differences as a function of condition (MFA ± SD = 1,225 ± 148 ms; MDA1 = 1,205 ± 191 ms; MDA2 = 1,162 ± 178 ms), F(2, 34) = 1.75, p > .19, ε = .96.
Recognition
Presence of the DA task during encoding had the predicted effect of reducing performance on subsequent recognition tests. Table 1 presents mean uncorrected and corrected (i.e., Pr: hits – false alarms) recognition accuracy and response bias (Br) for each condition. For comparison, the young and older adult results from the Nessler et al. (2006) high-selection condition are also presented. These data reveal a dissociation in which the young’s subsequent recognition performance for FA-encoded words was at the same high level as that of the young controls in Nessler et al. (2006), whereas their recognition for DA1- and DA2-encoded words was at the same low level as the older adult group in Nessler et al. (2006). Planned comparisons performed on the Pr values indicated that subsequent recognition of FA-encoded words was significantly better than for either DA1-, F(1, 17) = 8.50,p < .01, , or DA2-encoded words, F(1, 17) = 17.40, p < .001, , although performance for DA1- and DA2-encoded words did not differ (p > .40). Most important, response bias (Br) was unaffected by the presence of the DA task (p > .24), indicating that Pr changes across conditions cannot be attributed in any simple way to changes in response bias.
Table 1.
Grand-Mean (±SE) Accuracy Rates, Memory Sensitivity (Pr), Response Bias (Br), and Number of Words Recalled for the Full Attention (FA), Divided Attention Early (DA1), and Divided Attention Late (DA2) Recognition Tests
| Encoding condition | %Olds | Pr* | Br* | Free recall |
|---|---|---|---|---|
| FA | 87.3 (1.8) | .73 (.03) | .46 (.06) | 12.2 (0.9) |
| DA1 (300–850 ms) | 76.3 (2.6) | .61 (.03) | .39 (.04) | 7.8 (0.9) |
| DA2 (850–1,400 ms) | 75.3 (2.8) | .58 (.03) | .41 (.05) | 8.2 (1.0) |
| Nessler et al. (2006) older adults | 70.8 (4.1) | .57 (.04) | .34 (.07) | |
| Nessler et al. (2006) young adults | 81.1 (2.6) | .73 (.03) | .29 (.04) |
Note. Older and young adult data from the high-selection condition of Nessler et al. (2006) are shown for comparison. %Olds = % old words correctly recognized; Pr = hits – false alarms; Br = false alarms/[1 – (hits – false alarms)]; (Snodgrass & Corwin, 1988).
All values significantly different from zero via t test (ps < .0001).
Free recall
Recollection-based processing is best assessed with tests of free recall because participants must self-generate the previously studied items. Planned comparisons on the number of items recalled at the end of the experiment revealed significantly greater recall for FA-encoded words (M ± SE = 12.2 ± 0.9) than for either DA1- (M = 7.8 ± 0.9), F(1, 17) = 22.8, p < .0001, , or DA2-encoded words (M = 8.2 ± 1.0), F(1, 17) = 18.6, p < .0001, . Again, the number of recalled DA1- and DA2-encoded words did not differ (F < 1).
To summarize, both DA conditions were associated with a pattern of results in young participants that is essentially identical to that reported previously for older participants under FA conditions (e.g., Anderson et al., 2000; Nessler et al., 2006), with intact semantic retrieval associated with significantly impaired episodic recognition and recall. Hence, the tone-duration discrimination tasks during both the early and late time periods successfully diverted resources away from episodic encoding.
ERP Data
Analyses of the ERP results were conducted in two stages. First, responses to old and new stimuli were compared within each condition to assess both the parietal EM effect and frontal compensatory activity. Second, the appearance of individual differences led us to divide participants into high- and low-performing groups to assess both within- and across-groups differences in frontal compensatory activity. These analyses were performed on correctly recognized items to not confound memory status with group or condition effects.
Recollection-related brain activity
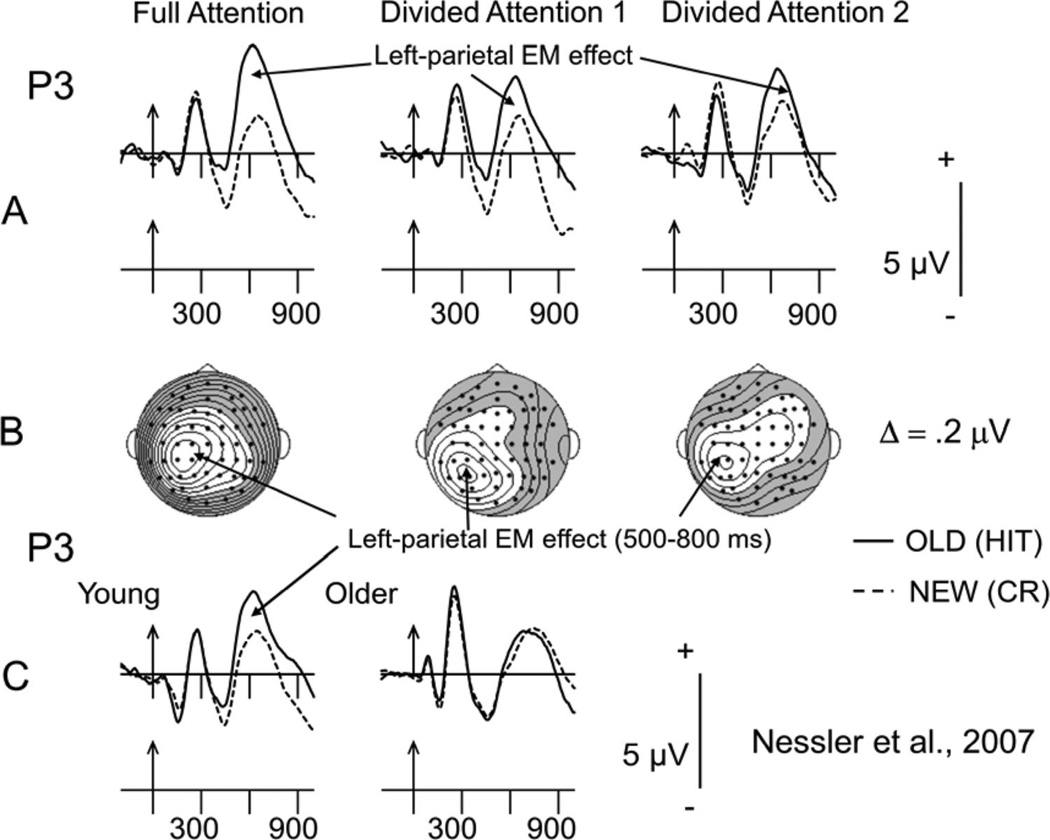
Recollection-related ERP activity elicited in all conditions at left parietal scalp is shown in Figure 2. Consistent with previous studies, items retrieved from episodic memory (i.e., hits) elicited greater positivity than items not in episodic memory (i.e., CRs) between 500 and 800 ms. As for the behavioral data described earlier, the ERP data from the left parietal (P3) scalp location in the 16 young and 16 older adults from the Nessler et al. (2007) high-selection condition are presented for comparison (see Figure 2C). Note that whereas the young adults produced a robust and reliable parietal EM effect that was similar in waveshape and magnitude to that for the FA condition in the current experiment, the older adults from the Nessler et al. (2007) study clearly did not.
Figure 2.
(A) Grand-averaged event-related potentials (ERPs) elicited at the left parietal electrode (P3) by old and new words in the recognition conditions that followed the full attention (FA), early divided attention (DA1), and late divided attention (DA2) conditions. Arrows mark stimulus onset with time markers every 300 ms. (B) Surface potential maps showing the scalp topographies of the parietal episodic memory (EM) effect (old – new difference) between 500 and 800 ms. The maps here and in subsequent figures were computed by calculating contours using the spherical spline method (Perrin, Pernier, Bertrand, & Echallier, 1989) and data from all 62 scalp electrodes (dots). Unshaded regions reflect positivity; shaded regions reflect negativity. (C) Grand-mean retrieval-related ERPs at the left parietal site, P3, for the 16 young (left column) and 16 older (right column) adults from the Nessler et al. (2007) high-selection encoding condition. Time lines and markers are the same as in A.
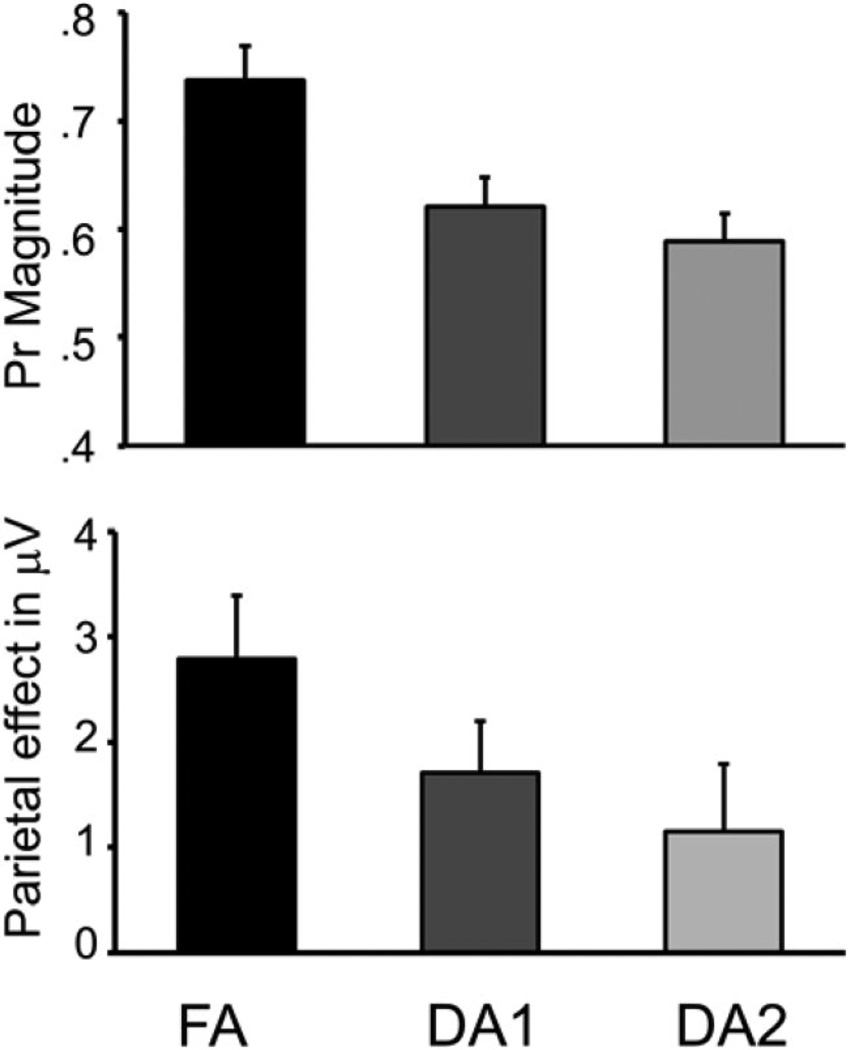
In the current data, the ERP difference waveforms revealed that this parietal EM effect had the typical left-parietal maximal scalp topography in all conditions (see the topographic maps below the waveforms in Figure 2B). To determine whether there was a significant parietal EM effect elicited after each encoding condition, we compared the ERP activity in the 500- to 800-ms interval at the left-parietal site (P3) for hits and CRs using paired-samples t tests. These tests revealed the presence of recollection-related brain activity for FA-encoded words, t(17) = 4.6, p = .001, and DA1-encoded words, t(17) = 3.4, p = .003, but not for DA2-encoded words, t(17) = 1.8, p = .09. Planned comparisons on the difference ERPs (i.e., hit – CR) from the P3 site confirmed that the parietal EM effect for FA-encoded words was significantly larger than that for either DA1-, F(1, 17) = 9.50, p < .007, , or DA2-encoded words, F(1, 17) = 8.50, p < .01, , which did not differ from one another, F(1, 17) = 1.34, p > .26. Hence, as shown in Figure 3, the magnitude of the parietal EM effect in the different conditions decreased with recognition performance.
Figure 3.
(Top) Grand-mean memory sensitivity (Pr ± SE) values for the full attention (FA), early divided attention (DA1), and late divided attention (DA2) conditions. (Bottom) Grand-mean hit – correct rejection (±SE) magnitudes for the FA, DA1, and DA2 conditions.
Additional left-frontal brain activity
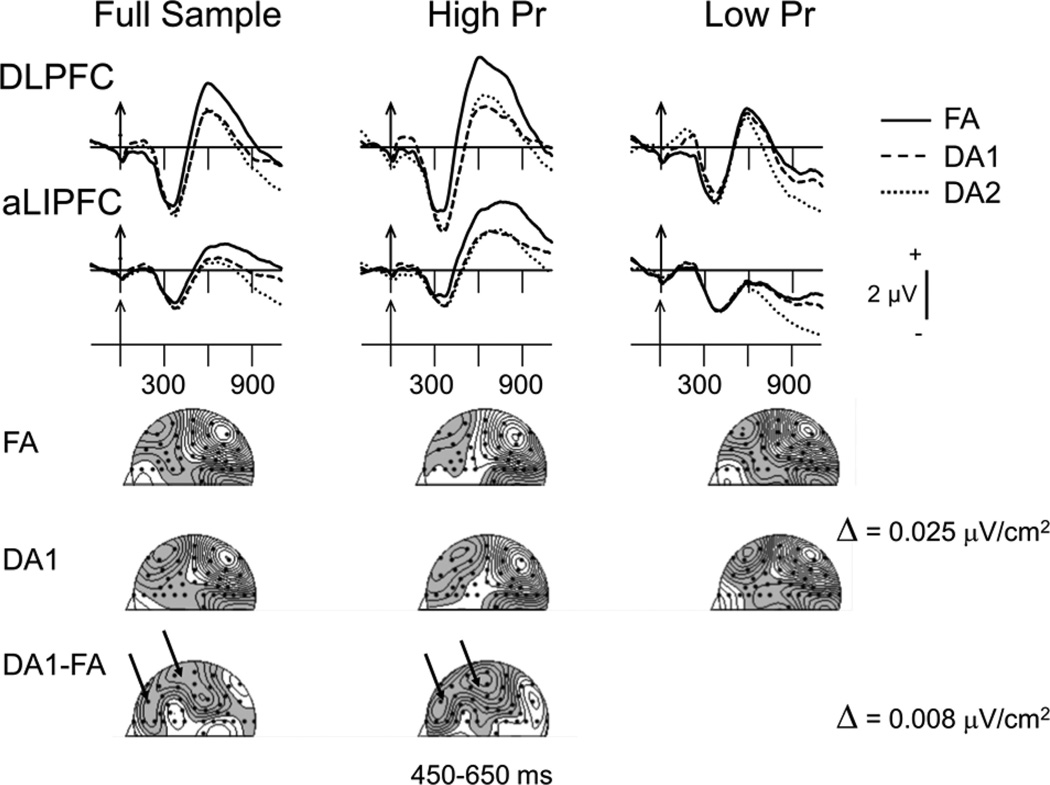
As shown in Figure 4 (left column), retrieval of DA-encoded words was characterized by ERPs with reduced amplitudes, relative to those elicited by retrieval of FA-encoded words, over two left frontal brain areas in the interval leading up to the old–new decision (i.e., 450–800 ms). Across-conditions amplitude differences such as these can be due either to reductions in a single pattern of neural generator activity or the presence of a second, temporally overlapping, pattern of generator activity. In all cases, determining which of these two possibilities is responsible for amplitude changes is accomplished by comparing the scalp distributions of difference ERPs, in which the altered brain activity is isolated, with those of unsubtracted ERPs (e.g., DA1 – FA compared with FA). That is, evidence of a new pattern of brain activity depends on finding that the scalp distribution of the difference ERP is different from that of the “parent” ERPs (see Johnson, 1993, for further details). As evident from Figure 4, comparing ERPs elicited in FA and DA1 conditions with the DA1 – FA difference ERPs clearly shows that the difference ERPs are characterized by a unique scalp distribution, which was localized over aLIPFC and dorsolateral prefrontal cortex (DLPFC; i.e., compare CSD maps in the top and middle rows with those in the bottom row). Consistent with the findings that DA1-and DA2-encoded words elicited roughly the same frontal ERP amplitudes during retrieval, the same two foci of activity were apparent in CSD maps of DA2 – FA difference ERPs (not shown). To confirm this visual impression, we vector-normalized ERP amplitudes over left frontal scalp (FP1, AF3, AF7, F7, F5, FT7, FC5, FC3, F3, FC1, F1) in the 450- to 800-ms interval (McCarthy & Wood, 1985) and subjected them to separate Waveform × Electrode ANOVAs, with the waveform factor including the DA1 – FA difference and either the FA or DA1 unsubtracted ERPs. These ANOVAs produced significant Waveform × Electrode interactions for both the DA1 – FA versus FA comparison, F(10, 170) = 4.16, p < .006, ε = .37, , and the DA1 – FA versus DA1 comparison, F(10, 170) = 4.92, p < .003, ε = .33, . Hence, these data confirm that the difference ERPs are characterized by a unique pattern of brain activity. Taken together, this combination of results indicates that retrieving words encoded under DA conditions recruited additional brain activity near these two left frontal foci.
Figure 4.
(Top) Grand-averaged event-related potentials (ERPs) elicited by successfully retrieved old words in all three conditions over dorsolateral prefrontal cortex (DLPFC; FC1) and anterior left inferior prefrontal cortex (aLIPFC; FT7). Averages for the full sample (N = 18) are shown in the left column, and those for the high-memory sensitivity (High Pr, n = 9) and low-memory sensitivity (Low Pr, n = 9) subgroups (based on a median split) are shown in the middle and right columns, respectively. Arrows mark stimulus onset with time markers every 300 ms. (Bottom) Current source density topographic maps showing activity in the full attention (FA) and early divided attention (DA1) ERP averages (first 2 rows) and the DA1 – FA difference averages (last row) in the 450- to 650-ms interval. DA2 = late divided attention condition.
To test the significance of the between-conditions ERP amplitude differences in the 450- to 800-ms interval, we performed planned comparisons using the same strategy as described earlier, separately for aLIPFC (FT7, FC5) and left DLPFC (FC3, FC1) sites. Over left DLPFC, whereas the amplitude difference between the FA and DA1 conditions (collapsed across FC1 and FC3: 1.64 µV ± 0.86 vs. 0.66 (µV ± 0.80, respectively) approached significance, F(1, 17) = 3.27, p < .08, , the difference between the FA and DA2 conditions was not significant (0.87 (µV ± 0.81), F(1, 17) = 1.42, p = .25. In this latter case, however, the Condition × Electrode interaction was marginally significant, F(1, 17) = 3.63, p < .07, , because of more reliable amplitude differences at FC1. Over aLIPFC, however, neither the FA versus DA1 nor FA versus DA2 amplitude difference was significant, F(1, 17) = 1.94, p > .18, , and F < 1, respectively.
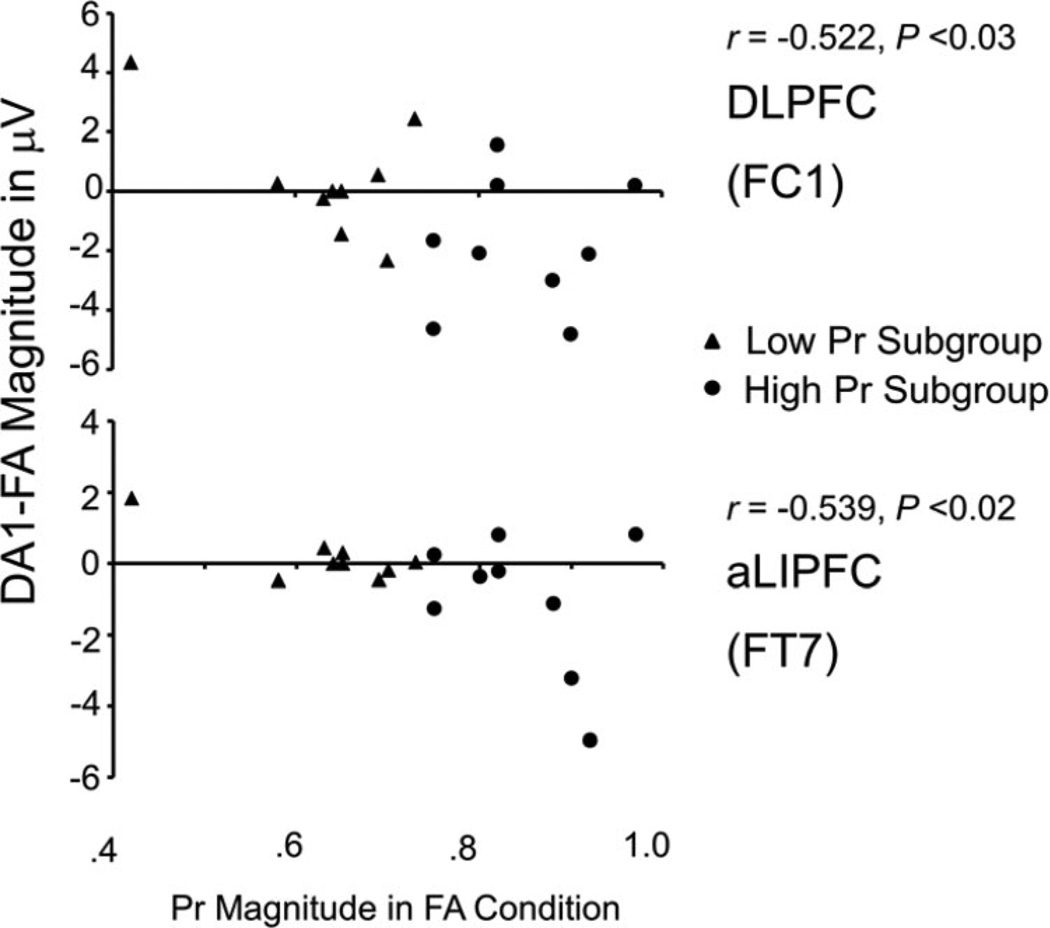
We noted that these frontal FA versus DA amplitude differences were highly variable across participants, suggesting that this might be the cause of the failure to find significant differences. To investigate this possibility, we correlated individuals’ FA Pr values with their DA1 – FA amplitude differences. To ensure that correlations were not influenced by the presence of outliers, we used the Robustfit linear regression function in MATLAB. This function uses an iteratively reweighted least-squares algorithm that is less susceptible to the influence of outliers than standard linear regression models. The results showed that better recognition performance was associated with significantly greater ERP amplitude decreases at both aLIPFC (r = −.539, p < .02) and left DLPFC (r = −.522, p < .03) sites (see Figure 5). However, although in the same direction, the equivalent Pr–ERP correlations with DA2 – FA differences were not significant at either aLIPFC (r = −.331, p = .17) or left DLPFC (r = −.264, p = .28) sites.
Figure 5.
Correlations between the DA1 – FA amplitude difference over dorsolateral prefrontal cortex (DLPFC; FC1, top) and anterior left inferior prefrontal cortex (aLIPFC; FT7, bottom) and memory sensitivity (Pr) magnitude during the FA condition. FA = full attention condition; DA1 = early divided attention condition.
Left frontal activity and recognition performance
To investigate these individual differences further, we created high- and low-performing groups by dividing the data from each condition on the basis of the median FA Pr value, when memory encoding– retrieval conditions were optimal for all participants. As would be expected from the data in Figure 5, this division produced large group differences in retrieval performance, and mean Pr in the high-performing group was significantly better than in the low-performing group (.84 vs. .63), t(16) = 5.31, p < .0001 (see Table 2). However, when we assessed potential group differences in RT (see Table 2) in a Group (low Pr, high Pr) × Condition (FA, DA1, DA2) × Memory Status (old, new) ANOVA, none of the main or interaction effects was significant (Fs < 3.00, ps > .10).
Table 2.
Grand-Mean Memory Sensitivity (Pr) and Reaction Times (±SE; in ms) for the Full Attention (FA), Divided Attention Early (DA1), and Divided Attention Late (DA2) Recognition Tests for the Two Pr Subgroups
| Pr subgroup |
||||||
|---|---|---|---|---|---|---|
| High (n= 9) |
Low (n= 9) |
|||||
| Encoding condition | Pr | Hit RT | CR RT | Pr | Hit RT | CR RT |
| FA | .84 (.03) | 829 (45) | 988 (74) | .63 (.03) | 921 (25) | 982 (64) |
| DA1 (300–850 ms) | .63 (.04) | 854 (39) | 1,011 (76) | .61 (.04) | 974 (33) | 986 (38) |
| DA2 (850–1,400 ms) | .63 (.04) | 895 (43) | 970 (56) | .55 (.04) | 945 (40) | 972 (43) |
Note. RT = reaction time; CR = correct rejections.
Nonetheless, the magnitude of the group differences in memory sensitivity is perhaps best illustrated by the finding that mean Pr for the low-performing group for retrieval of FA-encoded words dropped to the Pr level of the high-performing group for DA-encoded words and nearly down to the level of the older adult group in Nessler et al. (2007). Thus, for the high-performing group, FA-encoded words led to significantly better retrieval than either DA1-, F(1, 8) = 22.5, p < .001, , or DA2-encoded words, F(1, 8) = 25.2, p < .001, . By contrast and consistent with the lack of differences in their Pr values across conditions, neither of these differences was reliable for the low-performing group: DA1, F <1; DA2, F(1, 8) = 2.7, p > .13.
These group performance differences in Pr were associated with large differences in the frontal ERP activity across conditions for subsequently recognized old words (see Figure 4). In accord with the lack of a difference between their FA and DA Pr values, the low-performing group’s ERP amplitudes over left DLPFC and aLIPFC for FA-encoded words were not different from those elicited by either their DA-encoded words or those elicited by DA-encoded words in the high-performing group. Thus, both within and across groups, the magnitude of the amplitude reductions at both of these frontal sites closely tracked the magnitude of the changes in retrieval performance. As can be seen in the CSD maps of the difference ERPs, the negative foci over left DLPFC and aLIPFC areas observed in the full sample are again present in the high-performing group (see Figure 4, two left columns, bottom row of maps). The FA versus DA amplitude differences (450–800 ms) elicited in the high-performing group were tested in Condition (FA vs. DA1 and FA vs. DA2) × Electrode (FC1, FC3 for DLPFC and FT7, FC5 for aLIPFC) ANOVAs. Over DLPFC, compared with retrieval of FA-encoded words, retrieval of DA1-encoded words elicited significantly smaller positivities (2.9 vs. 1.1 µV), F(1, 8) = 7.5, p = .03, and the FA versus DA2 amplitude difference (2.9 vs. 1.6 µV) only approached significance, F(1, 8) = 4.0, p = .08. This pattern of results was repeated over aLIPFC, albeit with a less reliable FA versus DA1 difference, F(1, 8) = 3.5, p = .09, and no FA versus DA2 difference, F(1, 8) = 1.3, p = .3. Finally, separate Group (high, low) × Electrode ANOVAs on the difference means (DA1 – FA and DA2 – FA) revealed that, although the group amplitude difference over aLIPFC was borderline significant (FT7, FC5: 2.3 vs. −0.4 µV), F(1, 16) = 4.16, p < .06, the group difference over DLPFC was not (FC1, FC3: 2.9 vs. 0.6 µV), F(1, 16) = 1.66, p > .21.
Discussion
A DA paradigm was used to study the effects of diverting resources during episodic encoding on young adults’ ability to subsequently retrieve these items. In accord with our hypothesis, compared with normal recognition of FA-encoded words, retrieval of words encoded in both the early and late DA conditions was reduced in healthy young adults to levels typically seen in older adults under FA conditions. Consistent with the idea that elaborative encoding processes were disrupted, DA conditions produced significantly reduced free recall and recollection-related brain activity. By contrast, concomitant semantic recall and semantic-selection performance during the encoding task were unaffected by either DA condition. In addition, compared with retrieval of FA-encoded words, retrieval of DA-encoded words elicited additional brain activity over left DLPFC and aLIPFC that fits the definition of compensatory activity. Furthermore, the magnitudes of these frontal amplitude reductions, which occurred in the interval preceding the old–new decision, were positively correlated with retrieval performance. Taken together, the findings indicate that the DA tasks interfered with elaborative episodic encoding processes and thereby produced an “age-related episodic-memory deficit” in the young adults, complete with the appearance of compensatory brain activity.
Effect of the DA Manipulations on Subsequent Episodic Retrieval
The purpose of this experiment was to provide evidence of a causal link between older adults’ well-documented reductions in pLIPFC activity during encoding and their subsequent episodic retrieval deficit (e.g., Anderson et al., 1998, 2000; Cabeza et al., 1997; Grady et al., 1995). To do this, we introduced a brief tone-duration discrimination task in a temporally specific manner. The occurrence of the DA tasks was targeted, in different blocks, to disrupt encoding processes during either the first or second half of the 300- to 1,400-ms interval when Nessler et al. (2006) found altered encoding-related ERP activity over pLIPFC in their older adult group. In accord with our hypotheses, compared with retrieval of FA-encoded words, the presence of the DA task during both temporal windows produced the same pattern of preserved semantic and deficient episodic memory in healthy young controls that is the hallmark of performance in older adults (cf. Anderson et al., 1998, 2000). Thus, recognition of FA-encoded words was at the same level as that of the young group in Nessler et al. (2007), whereas retrieval of words encoded during the two DA conditions was reduced to levels that were indistinguishable from those obtained from their older adult group under FA conditions. Indeed, the young’s DA2 memory sensitivity score (Pr) here (.58) was virtually identical to that found when their older adult participants performed the same encoding–retrieval task (.57). This result also replicates the magnitude of the DA-induced recognition deficits found in other studies (e.g., Anderson et al., 1998, 2000). Our results thus confirm that, whereas semantic retrieval requires few if any resources, elaborative encoding of episodic memories is a controlled process requiring considerable resources (e.g., Craik & Byrd, 1982; Park et al., 1996).
The fact that the 300- to 1,400-ms poststimulus interval was crucial for elaborative encoding processes in particular was established by assessing recollection with both behavioral and brain measures. Behaviorally, free recall here was significantly reduced for DA- compared with FA-encoded words in accord with the DA-related decreases in recollection demonstrated previously in young adults (Curran, 2004; Jennings & Jacoby, 1993). Moreover, using the well-established ERP measure of recollection (Friedman & Johnson, 2000; Johnson, 1995; Mecklinger, 2000; Rugg & Curran, 2007), we demonstrated that retrieval of DA-encoded words elicited either greatly reduced (i.e., DA1) or no (i.e., DA2) parietal EM effects compared with those elicited during retrieval of FA-encoded words. Because recollection is based on the success of elaborative encoding processes (e.g., Buckner et al., 1999; Craik & Lockhart, 1972; Prince et al., 2007), the present results indicate that even brief reductions in the availability of resources greatly affect the encoding processes that lead to successful recollection. Given that our DA tasks were targeted to coincide with the occurrence of young–older differences in ERP activity over pLIPFC, the reductions in recall and recollection-related processing suggest that elaborately encoded episodic memories are dependent at least to some extent on processing in pLIPFC. Taken together, the present results provide causal support for the hypothesis that a major determinant of older adults’ episodic memory retrieval deficit is an age-related decline in the pool of available processing resources (e.g., Craik & Byrd, 1982; Craik & McDowd, 1987; Kramer & Madden, 2008; Luo, Hendriks, & Craik, 2007; Park et al., 1996), which leads to inefficient or impoverished processing during episodic encoding. Moreover, the close similarity between the deficits revealed by the behavioral and ERP results for DA-encoded words here and older adults’ recollection deficits for FA-encoded words argues against the idea that older adults’ memory deficits can be attributed to changes in non–memory-related processes (e.g., age-related declines in general-purpose and/or attentional processes). Perhaps more important, this similarity means that older adults’ failure to engage elaborative encoding processes is unlikely to be due to any age-related alterations in brain structure and function, raising the possibility that this deficiency might be ameliorated with remedial strategies.
Unlike previous studies employing DA tasks (e.g., Anderson et al., 2000; Curran, 2004), new insights into the functioning of the semantic– episodic interface and the timing of elaborative episodic encoding processes were obtained by controlling precisely when the participants’ resources were diverted. For example, the large decrements in recollection-related ERP activity indicate that processing vital to elaborative episodic encoding appears to occur in a 300- to 1,400-ms interval. Overall, the results argue that even healthy young adults, with intact cognitive processes and full resources both before and after each of the 550-ms DA intervals, were unable to adjust the timing of their elaborative processing to counter the effects of the brief secondary task (e.g., delay their onset by several hundred milliseconds or suspend and restart them after the tone ended). Although not conclusive, there were trend-level differences as a function of when the DA task occurred, with slightly worse recognition performance with DA2-encoded words and no significant parietal EM effect. The reasons for these potential differential effects, however, will require further study. Together, these results suggest that elaborative encoding processes performed in the semantic–episodic interface are temporally constrained within a 300- to 1,400-ms interval, largely uninterruptible and require the continuous availability of sufficient resources.
Several previous attempts to provide support for a causal link between episodic encoding and pLIPFC activity have used transcranial magnetic stimulation (TMS) to study how disruption of activity in this brain region during encoding affects subsequent episodic retrieval. These studies, however, have produced quite minor retrieval deficits, with none even approaching those found here or in other DA studies. In fact, some TMS studies actually found no subsequent retrieval deficits (e.g., Machizawa, Kalla, Walsh, & Otten, 2010) and one even found enhanced episodic retrieval (Köhler, Paus, Buckner, & Milner, 2004). Hence, it is interesting that the only encoding TMS study that did report subsequent episodic retrieval deficits like those in DA studies or studies of older adults did so only for stimulation of left DLPFC (Rossi et al., 2011). In addition to demonstrating these localized effects, Rossi and colleagues (2011) determined that, despite trying a wide variety of stimulation intervals, retrieval deficits occurred only when stimulation was delivered in a 500- to 1,400-ms poststimulus interval during encoding. The similarity of their crucial interval to our combined DA1/DA2 intervals is intriguing and raises the possibility that pLIPFC and left DLPFC work together during episodic encoding. In this scheme, pLIPFC would be responsible for operating on and maintaining the retrieved semantic memories in an activated state, whereas left DLPFC would be responsible for the elaboration processes performed in verbal working memory. Hence, older adults’ early termination of the selection-related negativity found by Nessler et al. (2006) could signal a failure to maintain retrieved representations in LIPFC, which in turn deprives working memory processes of the basis for elaborative encoding. Finally, it is important to note that, whereas the timing of our DA task interval was determined by that of the young–older encoding-related ERP differences over pLIPFC, the presence of our DA tasks would also disrupt any other resource-dependent processes occurring in parallel in other brain areas (e.g., DLPFC).
Although the young’s retrieval performance for DA1-encoded words (Pr = .61) was essentially the same as that for the Nessler et al. (2006) older adult group (Pr = .57) under FA conditions, it highlights a dissociation between the behavioral and brain measures of retrieval. That is, although older adults frequently show no significant parietal EM effect after a single encoding episode in item retrieval tasks (e.g., Nessler et al., 2007, 2008; Wolk et al., 2009), other studies have found small but significantly reduced effects (e.g., Morcom & Rugg, 2004). As in the Morcom and Rugg study (2004), a significantly reduced but small parietal EM effect remained here for DA1-encoded words. This small amount of parietal EM effect can be explained by the fact that young adults typically recollect more episodic details about each individual item than older adults (e.g., St Jacques & Levine, 2007). Alternatively, this parietal EM effect may simply reflect the slightly higher performance in the young. Consistent with this idea, words encoded in the DA2 condition, which produced retrieval performance almost identical to the Nessler et al. (2007) older adult group (Pr = .58), also matched the Nessler et al. (2007) results in that they failed to elicit a significant parietal EM effect. It is possible, then, that once retrieval drops below a certain level, there is no opportunity to elicit recollection-related processes in either older adult or healthy young participants.
Taken together, the present results provide strong support for the idea that pLIPFC plays a central role in elaborative episodic encoding and that older adults’ altered brain activity in this region, both ERP and hemodynamic, is responsible for at least part of their episodic memory deficit (e.g., Anderson et al., 1998; Cabeza et al., 1997; Grady et al., 1995; Nessler et al., 2007).
Left Frontal Brain Activity and Compensation
Two additional foci of ERP activity appeared over aLIPFC and left DLPFC when young adults attempted to retrieve DA-encoded words in the interval preceding the old–new decision. Because neither pattern was present during retrieval of FA-encoded words, both fit the definition of “compensatory” activity (e.g., Cabeza et al., 2002). Unlike all previous studies, however, in which compensatory activity was revealed only in group comparisons (i.e., young vs. older adults), the extra brain activity here was obtained from within-subject, across-conditions comparisons (i.e., FA vs. DA) in both the full sample and high-performing groups. However, in accord with previous reports of compensatory activity in older adults (cf. Anderson et al., 2000; Cabeza et al., 2002), the magnitude of ERP amplitude decrements at both aLIPFC and left DLPFC during retrieval of DA1-encoded words (DA1–FA) were positively correlated with retrieval performance. This result suggests that the compensatory processes used here reflect individual differences in retrieval ability and retrieval strategies, and therefore are presumably voluntary. This result fits with the suggestion above that the use and efficacy of compensatory processes here would be related to the quality of the antecedent encoding operations, which determine whether there is a sufficient basis on which compensatory processes can operate. Although in the same direction, the equivalent DA2–FA correlations were not significant, reinforcing the idea that activity in the early and late encoding intervals is not equal. Taken together, the results suggest that, rather than being a function of either aging or reduced brain function, compensatory processes can be recruited in the young when deficiencies in prior elaborative encoding processes reduce their ability to retrieve episodic memories. This interpretation fits well with the scaffolding view in which compensatory activity is a normal cognitive response, across the life span, when a person is faced with increased task (e.g., episodic retrieval) difficulty (e.g., Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Park, 2010).
The timing information inherent in the ERP provides new information on the nature and possible roles of this putative compensatory activity. That is, the activity at both frontal foci began early and continued over the 450- to 800-ms interval, with peak activity in both locations occurring at roughly the same time. Given that activity in both locations began well before the old–new categorizations were completed indicates that it was brought online to assist in episodic retrieval. Its early onset also suggests that participants had to decide quickly whether to invoke these extra frontal processes. Given that familiarity judgments have been found to remain intact in older adults under FA encoding conditions (e.g., Jennings & Jacoby, 1993) and in the young under DA conditions (Curran, 2004; Jennings & Jacoby, 1993), such decisions might rely on some type of rapid judgment based on item familiarity or “oldness.” The need to make such rapid, preliminary determinations of possible “oldness” could explain why the majority of the retrieval–ERP relations were larger in high-performing individuals (see Figure 5). That is, less elaborately encoded memories would result in weaker traces and fewer items with a familiarity value exceeding the threshold necessary to elicit compensatory processes. Thus, although the overall positive relation between memory performance and increased compensatory activity suggests that it can improve performance, it is also the case that the range of trace strengths over which improvement can occur appears to be limited.
Further investigation of the individual subject differences for FA-encoded words revealed surprisingly large group differences in both retrieval performance and the magnitude of the left frontal ERP effects. Despite being a sample of healthy young people, retrieval performance in the low-performing group for FA-encoded words was as low as that for the high-performing group for DA-encoded words. More unexpected was the fact that FA retrieval performance in the low-performing group closely approximated that of the Nessler et al. (2007) older adult group under the same encoding–retrieval conditions. This finding raises the possibility that low-performing participants may have invoked compensatory processes under FA, as well as DA, conditions. This speculation, however, cannot be confirmed because of the lack of FA–DA differences in the low-performing group’s ERP amplitudes and the resulting inability to identify the source of their reduced ERP amplitudes.
The nature and role of the cognitive processes underlying compensatory activity in older adults remain unknown. Part of the difficulty in specifying which particular processes are involved lies in the large disparities in cognitive ability between young and old and the use of young–older adult group comparisons to define or study compensatory activity. Thus, our use of within-group comparisons of young adults with intact cognitive abilities can shed light on the nature of the underlying processes. For example, aspects of the present study, including the tasks and locations of the extra left frontal foci for DA-encoded words, closely resemble those in a recent study on the role of aLIPFC in episodic retrieval. Given that aLIPFC has been linked to the controlled activation of semantic memories (e.g., Badre, Poldrack, Pare-Blagoev, Insler, & Wagner, 2005), Raposo, Han, and Dobbins (2009) sought to test the hypothesis that aLIPFC is also activated as a retrieval aid when episodic memories are difficult to recover (e.g., due to suboptimal encoding). This hypothesis posits that a top-down guided retrieval of semantic memories can be used to create a “semantic elaboration” framework by which episodic retrieval can be facilitated through emphasizing the semantic memories that were activated at the time of encoding. Thus, Raposo et al. created conditions with differing amounts of available semantic information to incorporate in elaborative episodic encoding, which can be seen as roughly analogous to our FA/DA encoding manipulations. Their results supported the interpretation that aLIPFC activation was self-initiated in a proactive manner to aid in selecting between competing episodic representations. It is noteworthy that left DLPFC also showed greater activation during retrieval of nondistinctive compared with distinctive episodic memories. The locations where Raposo et al. found increased activations for difficult-to-retrieve episodic memories were thus roughly equivalent to our two left frontal scalp foci present during DA retrievals. Moreover, in another result strikingly similar to ours, they reported that the magnitudes of the blood flow increases in both left frontal areas were highly variable across individuals and correlated significantly and positively with episodic retrieval performance. In sum, we think that this type of retrieval aid is a good candidate for both compensatory and scaffolding accounts of the extra brain activity seen in studies of both young and older adult participants during episodic retrieval.
Conclusions
We reproduced all features of older adults’ well-documented episodic memory deficit in healthy young adults using a temporally targeted DA paradigm to disrupt processing in the two halves of the 300- to 1,400-ms interval when ERP evidence suggests that there are elaborative episodic encoding failures in older adults. These results thus provide causal support for the idea that older adults’ episodic memory deficit has its roots in their failure to engage elaborative encoding processes successfully, which can be attributed to an age-related reduction in controlled processing resources (e.g., Craik & Byrd, 1982). The finding of extra left frontal ERP activity in young participants when the difficulty of episodic retrieval increased provides new information on the nature of putative “compensatory” processes. Specifically, the fact that altered ERP activity was seen over aLIPFC and left DLPFC for DA-encoded words supports the scaffolding account of compensation, in which the recruitment of additional cognitive processes in the face of momentary increases in task difficulty is an adaptive response across the life span. Finally, the present results validate the use of temporally targeted DA tasks to noninvasively produce cognitive failures to investigate the temporal dynamics of altered processing in older adults and other groups. This technique is likely to be particularly useful given its ability to separate changes in cognition from those due to age-related alterations in brain structure and function.
Acknowledgments
This work is supported by Grant AG005213 and the New York State Department of Mental Hygiene. We are grateful to Charles L. Brown, III, for computer programming and technical assistance, and Efrat Schori, Rebecca Edelblum, and Brenda Malcolm for their assistance in the recruiting and screening of participants. Thanks to Julianna Kulik for providing statistical advice and analysis. We also thank all volunteers for participating in this experiment.
Contributor Information
Ray Johnson, Jr., Brain and Cognition Laboratory, Department of Psychology, Queens College of CUNY
Doreen Nessler, Cognitive Electrophysiology Laboratory, Division of Cognitive Neuroscience, New York State Psychiatric Institute.
David Friedman, Cognitive Electrophysiology Laboratory, Division of Cognitive Neuroscience, New York State Psychiatric Institute.
References
- Anderson ND, Craik FI, Naveh-Benjamin M. The attentional demands of encoding and retrieval in younger and older adults: 1. Evidence from divided attention costs. Psychology and Aging. 1998;13:405–423. doi: 10.1037//0882-7974.13.3.405. [DOI] [PubMed] [Google Scholar]
- Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FI. The effects of divided attention on encoding- and retrieval-related brain activity: A PET study of younger and older adults. Journal of Cognitive Neuroscience. 2000;12:775–792. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Lewis V, Eldridge M, Thomson N. Attention and retrieval from long-term memory. Journal of Experimental Psychology: General. 1984;113:518–540. [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Kelley WM, Petersen SE. Frontal cortex contributes to human memory formation. Nature Neuroscience. 1999;2:311–314. doi: 10.1038/7221. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Craik FIM. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. The Journal of Neuroscience. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychology and Aging. 2005;20:363–375. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- Craik FIM. Levels of processing: Past, present, and future? Memory. 2002;10:305–318. doi: 10.1080/09658210244000135. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits: The role of attentional resources. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. New York, NY: Plenum Press; 1982. pp. 191–211. [Google Scholar]
- Craik FIM, Govoni R, Naveh-Benjamin M, Anderson ND. The effects of divided attention on encoding and retrieval processes in human memory. Journal of Experimental Psychology: General. 1996;125:159–180. doi: 10.1037//0096-3445.125.2.159. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart S. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Craik FIM, McDowd JM. Age differences in recall and recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:474–479. [Google Scholar]
- Curran T. Effects of attention and confidence on the hypothesized ERP correlates of recollection and familiarity. Neuropsychologia. 2004;42:1088–1106. doi: 10.1016/j.neuropsychologia.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. Journal of Cognitive Neuroscience. 2006;18:33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson R. Event-related potential (ERP) studies of memory encoding and retrieval: A selective review. Microscopy Research and Technique. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Meadow NG, Johnson EL, Foster KT. Deep levels of processing elicit a distinctiveness heuristic: Evidence from the criterial recollection task. Journal of Memory and Language. 2008;58:1095–1111. [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995 Jul 14;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FI. The effects of age on the neural correlates of episodic encoding. Cerebral Cortex. 1999;9:805–814. doi: 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: Aging, attention, and control. Psychology and Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Wood CC. Letter: The epsilon-adjustment procedure for repeated-measures analyses of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr On the neural generators of the P300 component of the event-related potential. Psychophysiology. 1993;30:90–97. doi: 10.1111/j.1469-8986.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr . Event-related potential insights into the neurobiology of memory systems. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Vol. 10. Amsterdam, Netherlands: Elsevier; 1995. pp. 135–163. [Google Scholar]
- Köhler S, Paus T, Buckner RL, Milner B. Effects of left inferior prefrontal stimulation on episodic memory formation: A two-stage fMRI-rTMS study. Journal of Cognitive Neuroscience. 2004;16:178–188. doi: 10.1162/089892904322984490. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Madden DJ. Attention. In: Craik FIM, Salthouse TA, editors. The handbook of cognition and aging. Vol. 3. New York: Psychology Press; 2008. pp. 189–249. [Google Scholar]
- Langenecker SA, Nielson KA. Frontal recruitment during response inhibition in older adults replicated with fMRI. NeuroImage. 2003;20:1384–1392. doi: 10.1016/S1053-8119(03)00372-0. [DOI] [PubMed] [Google Scholar]
- Luo L, Hendriks T, Craik FI. Age differences in recollection: Three patterns of enhanced encoding. Psychology and Aging. 2007;22:269–280. doi: 10.1037/0882-7974.22.2.269. [DOI] [PubMed] [Google Scholar]
- Machizawa MG, Kalla R, Walsh V, Otten LJ. The time course of ventrolateral prefrontal cortex involvement in memory formation. Journal of Neurophysiology. 2010;103:1569–1579. doi: 10.1152/jn.90937.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RE, Rugg MD. Age effects on brain activity associated with episodic memory retrieval: An electrophysiological study. Brain. 1998;121:861–873. doi: 10.1093/brain/121.5.861. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Stern Y, Rosen J, Leventhal J. Depression, intellectual impairment, and Parkinson disease. Neurology. 1981;31:645–650. doi: 10.1212/wnl.31.6.645. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: An ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Mecklinger A. Interfacing mind and brain: A neurocognitive model of recognition memory. Psychophysiology. 2000;37:565–582. [PubMed] [Google Scholar]
- Morcom AM, Rugg MD. Effects of age on retrieval cue processing as revealed by ERPs. Neuropsychologia. 2004;42:1525–1542. doi: 10.1016/j.neuropsychologia.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Nessler D, Friedman D, Johnson R, Jr, Bersick M. Does repetition engender the same retrieval processes in young and older adults? NeuroReport. 2007;18:1837–1840. doi: 10.1097/WNR.0b013e3282f16d9f. [DOI] [PubMed] [Google Scholar]
- Nessler D, Johnson R, Bersick M, Friedman D. On why the elderly have normal semantic retrieval but deficient episodic encoding: A study of left inferior frontal ERP activity. NeuroImage. 2006;30:299–312. doi: 10.1016/j.neuroimage.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Nessler D, Johnson R, Jr, Bersick M, Friedman D. Age-related ERP differences at retrieval persist despite age-invariant performance and left-frontal negativity during encoding. Neuroscience Letters. 2008;432:151–156. doi: 10.1016/j.neulet.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langenecker SA, Garavan H. Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychology and Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Transforming experience into memory: Observations of mind and brain. Trends in Cognitive Sciences. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Smith AD, Lautenschlager G, Earles JL, Frieske D, Zwahr M, Gaines CL. Mediators of long-term memory performance across the life span. Psychology and Aging. 1996;11:621–637. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Prince SE, Tsukiura T, Cabeza R. Distinguishing the neural correlates of episodic memory encoding and semantic memory retrieval. Psychological Science. 2007;18:144–151. doi: 10.1111/j.1467-9280.2007.01864.x. [DOI] [PubMed] [Google Scholar]
- Raposo A, Han S, Dobbins IG. Ventrolateral prefrontal cortex and self-initiated semantic elaboration during memory retrieval. Neuropsychologia. 2009;47:2261–2271. doi: 10.1016/j.neuropsychologia.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: Reorganizing discoveries about the aging mind. Current Opinion in Neurobiology. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Rossi S, Innocenti I, Polizzotto NR, Feurra M, De Capua A, Ulivelli M, Cappa SF. Temporal dynamics of memory trace formation in the human prefrontal cortex. Cerebral Cortex. 2011;21:368–373. doi: 10.1093/cercor/bhq103. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Sharbrough F, Chatrian GE, Lesser RP, Luders H, Nuwer M, Picton TW. Guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1990;8:200–202. [PubMed] [Google Scholar]
- Smith EE, Geva A, Jonides J, Miller A, Reuter-Lorenz P, Koeppe RA. The neural basis of task-switching in working memory: Effects of performance and aging. Proceedings of the National Academy of Sciences, USA. 2001;98:2095–2100. doi: 10.1073/pnas.98.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Levine B. Ageing and autobiographical memory for emotional and neutral events. Memory. 2007;15:129–144. doi: 10.1080/09658210601119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proceedings of the National Academy of Sciences, USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott CT, Friedman D, Ritter W, Fabiani M, Snodgrass JG. Episodic priming and memory for temporal source: Event-related potentials reveal age-related differences in prefrontal functioning. Psychology and Aging. 1999;14:390–413. doi: 10.1037//0882-7974.14.3.390. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. Vol. 2. New York: Oxford University Press; 1983. [Google Scholar]
- Vilberg KL, Moosavi RF, Rugg MD. The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Research. 2006;1122:161–170. doi: 10.1016/j.brainres.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegesin DJ, Friedman D, Varughese N, Stern Y. Age-related changes in source memory retrieval: An ERP replication and extension. Cognitive Brain Research. 2002;13:323–338. doi: 10.1016/s0926-6410(01)00126-4. [DOI] [PubMed] [Google Scholar]
- Wilding EL. In what way does the parietal ERP old/new effect index recollection? International Journal of Psychophysiology. 2000;35:81–87. doi: 10.1016/s0167-8760(99)00095-1. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Sen NM, Chong H, Riis JL, McGinnis SM, Holcomb PJ, Daffner KR. ERP correlates of item recognition memory: Effects of age and performance. Brain Research. 2009;1250:218–231. doi: 10.1016/j.brainres.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]