Abstract
We have investigated the expression of chromatin regulating genes in the prefrontal cortex and in the shell subdivision of the nucleus accumbens during protracted withdrawal in mice with increased ethanol drinking after chronic intermittent ethanol vapor exposure (CIE) and in mice with a history of non-dependent drinking. We observed that the methyl-CpG binding protein 2 (MeCP2) was one of the few chromatin-regulating genes to be differentially regulated by a history of dependence. As MeCP2 has the potential of acting as a broad gene regulator, we investigated sensitivity to ethanol and ethanol drinking in MeCP2308/Y mice, which harbor a truncated MeCP2 allele but have a milder phenotype than MeCP2 null mice. We observed that MeCP2308/Y mice were more sensitive to ethanol’s stimulatory and sedative effects than wild-type mice, drank less ethanol in a limited access 2 bottle choice (2BC) paradigm, and did not show increased drinking after induction of dependence with exposure to chronic intermittent ethanol vapors (CIE). Alcohol metabolism did not differ in MeCP2308/Y and wild-type (WT) mice. Additionally, MeCP2308/Y mice did not differ from WT mice in ethanol preference in a 24 hr paradigm nor in their intake of graded solutions of saccharin or quinine, suggesting that the MeCP2308/Y mutation did not alter taste function. Lastly, using the Gene Set Enrichment Analysis (GSEA) algorithm we found a significant overlap in the genes regulated by alcohol and by MeCP2. Together, these results suggest that MeCP2 contributes to the regulation of ethanol sensitivity and drinking.
Introduction
Methyl-CpG binding protein 2 (MeCP2) is a transcriptional regulator involved in chromatin remodeling and the modulation of RNA splicing that was originally identified through its affinity for methylated cytosines (Lewis et al., 1992). Mutations of MeCP2 are responsible for Rett syndrome (RTT), a phenotypically variable spectrum disorder characterized by cognitive impairment, motor disabilities, autistic features, seizures, and anxiety (Chahrour and Zoghbi, 2007). MeCP2 aberrations have been implicated in a constellation of neuropsychiatric abnormalities, and, interestingly, both loss of function and gain in MeCP2 dosage can be associated with similar neurological phenotypes (Chahrour and Zoghbi, 2007).
MeCP2 is capable of interacting with multiple proteins involved, chromatin, DNA, and RNA regulation (Guy et al., 2011). In particular, MeCP2 has been shown to be able to both activate and repress transcription of specific target genes and to modulate splicing (Guy et al., 2011). Interestingly, MeCP2 deficiency-induced functional abnormalities are largely reversible, as shown by mice in which MeCP2 expression is rescued in adulthood, resulting in reverting their neurological phenotype (Guy et al., 2007). The consequences of MeCP2 loss of function in the brain are complex and involve both changes in excitatory and inhibitory neurotransmission with contrasting effects in different brain regions and neuronal systems (Shepherd and Katz, 2011).
Here we investigated the expression of a battery of genes involved in chromatin regulation in mice with a history of ethanol dependence and observed that MeCP2 expression was significantly increased both in the medial prefrontal cortex (PFC) and in the shell subdivision of the nucleus accumbens (NAc), two brain regions relevant to ethanol reinforcement. Thus, for an initial investigation of the potential role of MeCP2 in the effects of ethanol we used a mouse model generated by the group of Dr. Zoghbi with a truncation of MeCP2 at amino acid 308 resulting in the loss of the C-terminal region of the protein (Shahbazian et al., 2002). These mice, MeCP2308/Y or MeCP2tm1Hz present with mild tremor and stereotypic forelimb motions reminiscent of RETT (Shahbazian et al., 2002) but a majority of MeCP2308/Y male mice survives to adulthood and have a milder phenotype than mice lacking MeCP2 completely (Chahrour and Zoghbi, 2007). We observed that MeCP2308/Y mice were more sensitive than their wild-type (WT) counterparts both to the stimulatory effect of a moderate dose of ethanol as well as to the intoxicating effects of a higher dose of ethanol. Additionally, while MeCP2308/Y mice did not differ from WT mice in ethanol preference in a 24 hr 2 bottle choice (2BC) test, they drank significantly less in a 2 hr limited access 2BC paradigm and did not increase their ethanol intake after intermittent exposure to ethanol vapors as did WT mice. Lastly, using the Gene Set Enrichment Analysis (GSEA) algorithm we found a significant overlap in the genes regulated by alcohol and by MeCP2. These results suggest that Mecp2-regulated genes modulate ethanol sensitivity and intake.
Materials and Methods
Mice
Male wild-type C57BL/6J obtained from Jackson Laboratories were used for chronic intermittent ethanol exposure (CIE). Following CIE, mice were dissected with the help of a brain mold at 10 days after withdrawal to investigate the expression of chromatin-regulating genes. To probe the effect of MeCP2 on ethanol sensitivity and intake, we used hemizygous MeCP2308/Y male mice originally generated by the Zoghbi group in 129S7/SvEvBrd-Hprtb-m32-derived AB2.2 embryonic stem (ES) cells and then backcrossed to the C57BL/6J over 12 generations. MeCP2308/Y male mice and matched WT mice were obtained from Jackson Laboratory (129S7/SvEvBrdxC57BL/6J). Mice were aged 14–16 weeks of age at the beginning of the studies.
Twenty four hours 2 bottle choice (2BC) ethanol preference
MeCP2 and WT mice were single housed and presented with two 50 ml conical tubes both filled with water and allowed to habituate for one week. After the habituation period one of the two bottles was replaced with an ethanol solution in escalating concentrations (3%, 6%, 9%,12%,15%,20% v/v) starting on Monday of each week. Both bottles were weighed daily Monday through Friday and mice weighed each Wednesday. Bottle positions were switched daily to avoid side preferences. Both 50 ml bottles were replaced with a single 500 ml water bottle over the weekends. Ethanol preference was calculated as the ratio of ethanol intake over total fluid intake.
Chronic intermittent ethanol exposure (CIE)
The present paradigm was based on previous studies by the group of Howard Becker that showed increased ethanol drinking effect after repeated bouts of vapor exposure (Becker and Lopez, 2004; Lopez and Becker, 2005; Griffin et al., 2009) with some modifications (Finn et al., 2007). Briefly, C57BL/6J mutant and WT mice had access to two bottles, one containing water and the other containing 20% (v/v) ethanol, for 2 hours starting 3 hours into the dark phase for 15 days. Each bout of ethanol vapor exposure consisted of 16 hours per day for 4 days. Before exposure to ethanol vapor, mice were injected intraperitoneally (IP) with a solution of ethanol (1.5 g/kg, 15% w/v in saline) containing 68.1mg/kg pyrazole and immediately placed into ethanol vapor chambers (La Jolla Ethanol Research, CA). Tail blood sampling for blood ethanol level (BAL) determination was carried out daily. Target BAL were 150–200 mg%. Seventy-two hours following removal from the chambers, mice received access to water vs. 20% (v/v) ethanol for 2 hours, and again over the next 4 days. The following week, mice were re-exposed to the ethanol vapor/control conditions and again tested for two-bottle choice drinking for 5 days. Three vapor bouts followed by two-bottle choice were carried out. Mice were weighed every 4–6 days throughout the 2BC sessions and daily during the vapor exposure bouts. Food and water were available ad libitum and mice were group housed except during the ethanol drinking sessions.
Ethanol sensitivity
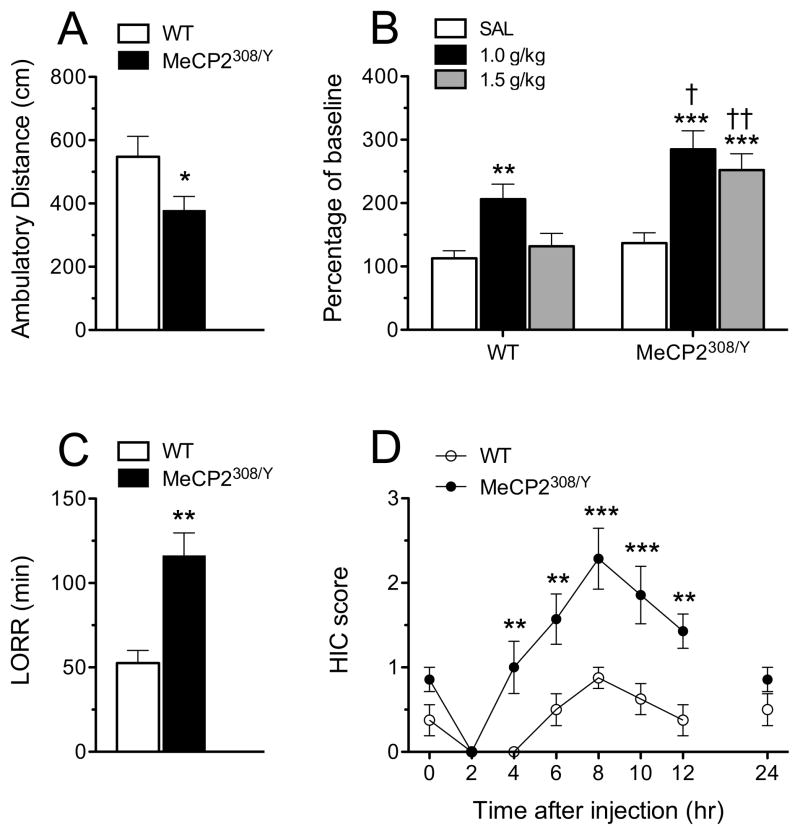
An ethanol-naive cohort of MeCP2308/Y and WT mice was tested for sensitivity to a high dose of ethanol by injecting them with 3.5 g/kg ethanol (15% w/v) IP and assessing loss of righting reflex (sedation), body temperature reductions, and acute physical withdrawal signs. Mice (n = 7 MeCP2308/Y, n = 8 WT) were first tested for the presence of baseline handling-induced convulsions (HIC) using a scale adapted by Crabbe and colleagues (Terdal and Crabbe, 1994). Briefly, this procedure involves lifting the mouse by the tail and observing it for possible convulsions. If none occur, the mouse is gently spun 180 degrees by rubbing the tail between the thumb and forefinger. Convulsions are scored on a 6-point scale ranging from facial grimace to severe tonic-clonic convulsions. Baseline scores are typically very low and C57BL/6J mice show very mild, but significant increases between 6 and 12 hr following a high dose of ethanol (Roberts et al., 1992). HIC were scored at 2, 4, 6, 8, 10, 12 and 24 hr post ethanol. Baseline body temperatures were taken using a rectal probe coated in lubricant and inserted 2 cm. Temperature measurements were repeated 60 and 120 min following the ethanol injection. Immediately following the ethanol injection, the mice were tested every 10 sec for loss of righting reflex (LORR), or the inability of the mouse to turn over on to its feet and stomach after being placed on its back. The mice were then tested for regain of righting reflex (RORR) every 5 minutes. At RORR, tail blood was collected for blood ethanol level determination and the time of LORR was calculated. One week later all mice were once again injected with 3.5 g/kg ethanol and tail blood was sampled for blood ethanol determination at 30, 60, 120, 180, and 300 min post injection. A second ethanol-naive cohort of mice (n = 8 MeCP2308/Y, n = 8 WT) was tested for sensitivity to stimulatory effects of low to moderate ethanol doses. In particular, mice were placed in the center of the open field chambers (41.5 cm × 21.5 cm) that automatically recorded activity via photo beam breaks (Med Associates, VT). Ambulatory distance data (cm) was collected in 10 min intervals. Thirty min later, mice were injected saline or ethanol (1.0 g/kg or 1.5 g/kg, i.p., 15% w/v in saline) and ambulatory distance data was collected in 10 min intervals for 30 min. Ambulatory distance in the 10 min intervals prior to and following injection of saline or ethanol were used for comparison. As MeCP2308/Y mice showed a small but significantly lower baseline locomotor activity compared to wild-type mice (Fig. 2A), alcohol induced locomotor activity changes were expressed as percentage of baseline ambulatory distance (Fig. 2B). Analysis of data was performed using two-tailed t-test for baseline (10 min prior injection) and two-way (genotype X dose) ANOVA with repeated measures followed by a Bonferroni post hoc test for ethanol stimulated locomotor activity (10 min following injection). Following this procedure this second cohort of mice was used to replicate the LORR data obtaining the same significant difference between MeCP2308/Y and WT (p<0.01, t = 4.271, n= 8 for each group).
Figure 2.
Ethanol sensitivity of MeCP2308/Y mice. A, MeCP2308/Y mice showed significantly lower locomotor activity compared to WT mice (* p<0.05, t = 2.169, n= 8 for each group) in the baseline measurement (10 min prior to injection). B, Acute ethanol (1.0 or 1.5 g/kg, i.p. in saline) induced a significantly greater increase in locomotor activity in MeCP2308/Y than in wild-type mice (10 min after injection). ** p<0.01, *** p<0.001, compared to SAL; † p<0.05, †† p<0.01, compared to WT (n=8, respectively, 2-way ANOVA with Bonferroni post hoc test). C, Duration of the loss of righting reflex induced by 3.5 g/kg ethanol. MeCP2308/Y mice required significantly longer time to recover their righting responses (** p<0.01, t = 4.087, n = 7 for each group). D, Handling-induced convulsions following 3.5g/kg ethanol. MeCP2308/Y mice showed greater severity of ethanol-induced withdrawal symptoms. RM two-way ANOVA revealed significant main effects of time (F7,91=16.27, p<0.0001) and genotype (F1,91=21.54, p<0.001), and a significant interaction of time and genotype (F7,91=4.127, p<0.0001). N = 8 for the WT group and n = 7 for the MeCP2308/Y group. ** p<0.01 and *** p<0.001 versus WT mice at the same point (Bonferroni post hoc test).
Intake of non-alcohol tastants
Separate groups of naive MeCP2308/Y and WT mice were used. Intake of saccharin (0.033 and 0.066%) and quinine hemisulfate (0.015, 0.06 mM) was tested in 2BC with one tube containing water and the other the tastant solution. Each concentration was offered for 4 days, with bottle position alternated daily. Preference was measured as g of tastant solution drank/total fluid intake over 24 h.
RT-PCR
RT-PCR was carried out as previously described (Ahmed et al., 2005; Repunte-Canonigo et al., 2007) from brain regions of individual mice (n=5–8) using an iQ5 Real-Time PCR Detection System (BioRad, Hercules, CA). The relative amounts of target mRNA were determined by theΔ Ct method using β-actin for normalization (Livak and Schmittgen, 2001).
Gene Set Enrichment Analysis (GSEA)
GSEA (Subramanian et al., 2005) was used to compare the genes regulated by alcohol and MeCP2, as previously described (Repunte-Canonigo et al., 2010). Specifically, for GSEA, we used as the gene set a list of genes differentially expressed in the anterior cingulated cortex by repeated administration of an intoxicating dose of alcohol in wild type mice (Repunte-Canonigo et al., 2010), as defined by t-test (p<0.05). This gene set was used to interrogate a reference set obtained from the comparison of MeCP2 null vs. wild-type mice (Urdinguio et al., 2008) ranked based on their t-statistics. Significance testing was performed by shuffling the gene-labels 1,000 times.
Results
Alcohol dependence and withdrawal differentially regulate MeCP2 expression in the brain
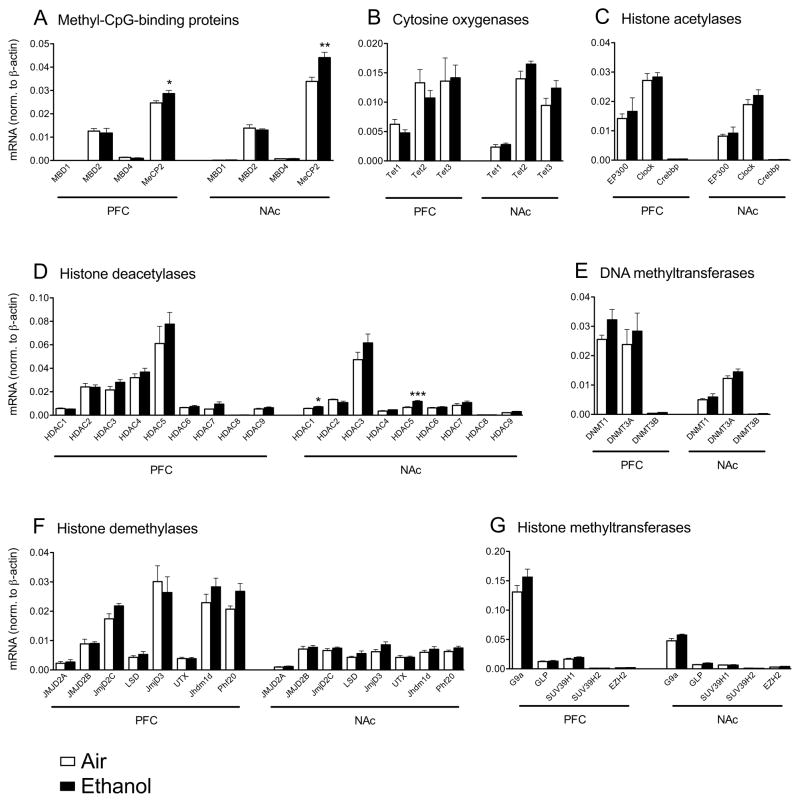
We investigated the effect of a history of dependence in a limited access paradigm on the expression of a panel of genes whose products contribute to the regulation of chromatin and gene expression in ethanol-drinking mice. Specifically, C57BL/6J mice were trained to drink ethanol in a 2 hr 2 bottle choice (2BC) limited access paradigm. Following the establishment of a stable drinking baseline, mice were either exposed to 3 repeated bouts of ethanol vapor to induce increased drinking or exposed to air in parallel for control. After the third bout of ethanol vapor exposure, the vapor-exposed mice consumed on average 4.85 (± 0.13) g/kg, while mice exposed to air in the same apparatus for control drank 2.19 (± 0.19) g/kg. The expression of genes involved in chromatin regulation was determined by quantitative RT-PCR in the PFC and NAc shell ten days after the end of the last bout of alcohol vapor. We observed that only a few of the genes under investigation were significantly different between vapor exposed mice that showed increased drinking after repeated intermittent vapor exposure and the air-exposed controls (Fig. 1). Interestingly, MeCP2 was significantly increased both in the medial prefrontal cortex (PFC) and shell regions of the NAc shell by history of ethanol dependence (Fig. 1A). Other chromatin-related genes that showed significant changes included HDAC1 and HDAC5 that were increased in the NAc shell of dependent mice (Fig. 1D).
Figure 1. Differential expression of chromatin regulating genes during ethanol withdrawal.
Few genes were affected by CIE in PFC and NAc shell. A, Among Methyl-CpG-binding proteins, MeCP2 mRNA was increased in both PFC and NAc shell. B, There were no differences among cytosine oxygenases and C, histone acetylases. D, Among histone deacetylases, HDAC1 and 5 were increased in NAc shell. E, DNA methyltransferases, F, Histone demethylases, and G, Histone methyltransferases were not affected by CIE (n = 5–8, * p< 0.05; ** p< 0.01; *** p< 0.001 versus air for each gene, t-test).
MeCP2308/Y mice are more sensitive to the stimulatory and sedative effects of ethanol and have greater withdrawal severity
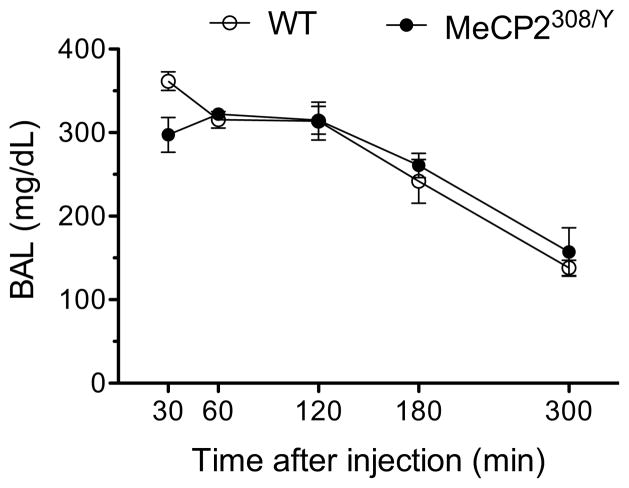
The above results showing that a history of dependence affects the regulation of MeCP2 in the brain provided the rationale to examine the effect of MeCP2 in ethanol drinking and sensitivity. To this end we tested hemizygous MeCP2308/Y mice that are characterized by the expression of a truncated form of MeCP2 and show a mild symptomology that allows most mice to reach adulthood. Consistent with previous observations (Shahbazian et al., 2002), MeCP2308/Y mice traveled a shorter distance (Fig. 2A). In addition, we observed that MeCP2308/Y mice were more sensitive than matched WT mice both to the stimulatory and sedative effects of alcohol. In particular, MeCP2308/Y were significantly more sensitive to the stimulatory effect of ethanol at doses of both 1.0 g/kg and 1.5 g/kg (Fig. 2B). Additionally, MeCP2308/Y mice had a dramatically longer duration of loss of righting reflex (LORR) times than WT mice when treated with an intoxicating dose of ethanol of 3.5 g/kg (Fig. 2C: t = 4.2, p < 0.01), suggesting considerably greater sedative effects of alcohol in these mutant mice. MeCP2308/Y mice also had a more severe acute withdrawal response than WT mice (Fig. 2D) reflected in significantly increased handling-induced convulsions (HIC) scores 4–12 hr post ethanol (F(1,13) = 21.5, p < 0.001). Blood ethanol levels obtained in a separate test one week later from the same cohort of mice showed no difference between the MeCP2308/Y and WT groups of mice, indicating that the greater ethanol sensitivity of MeCP2308/Y was not due to differences in ethanol metabolism (Fig. 3). Lastly, while there were no significant differences in body temperatures before or following ethanol administration (3.5 g/kg), there was a trend toward lower body temperatures in MeCP2308/Y mice at the 120 min time point (t = −1.8, p = 0.09). Together, these data suggest that MeCP2 mice are more sensitive both to the stimulatory and sedative effects of ethanol as well as to the subsequent excitatory rebound (withdrawal) effects.
Figure 3.
Blood alcohol levels following 3.5 g/kg ethanol. MeCP2308/Y mice showed similar blood alcohol concentrations and rate of decay after injection of ethanol compared with WT controls. There was a significant main effect of time (F4,40=45.22, p<0.0001), but no significant main effect of genotype (F1,40=0.052, NS; RM two-way ANOVA; N = 6 for each group).
MeCP2308/Y mice consume less alcohol in a limited access paradigm and do not show dependence-induced increased drinking
We first trained hemizygous MeCP2308/Y and WT mice to consume ethanol in a 24 h 2BC ethanol preference test at increasing concentrations of ethanol (Fig. 4A). Ethanol intake of MeCP2308/Y and WT mice increased similarly with increasing ethanol concentrations and did not differ between the two genotypes although a minor trend toward lower ethanol consumption was seen in the MeCP2308/Y mice at the higher ethanol concentrations tested (12–20% v/v). MeCP2308/Y mice also drank equal amounts of graded solutions of saccharin or quinine as WT mice, supporting that taste function was not affected by this MeCP2 mutation (Supplemental Fig. 1), as also indicated by equivalent alcohol intake in the 24 h paradigm.
Figure 4.
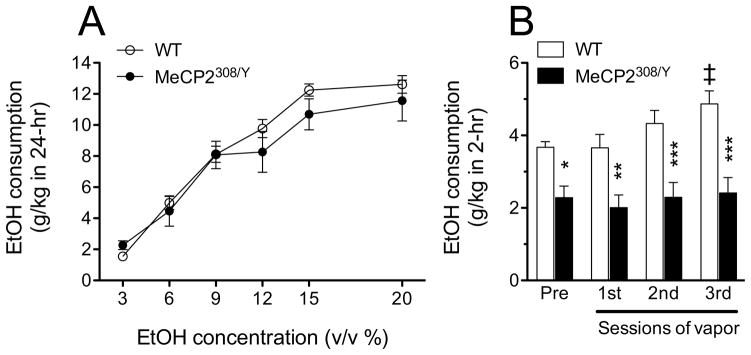
Ethanol intake of wild-type (WT) and MeCP2308/Y mice in 24 hr (A) and 2 hr (B) 2BC drinking test. A) MeCP2308/Y and WT mice consumed ethanol to a similar degree in a dose-related manner. There was a significant main effect of ethanol dose (F5,85=84.65, p<0.0001), but no significant main effect of genotype (F1,85=0.39, NS) and interaction of genotype and ethanol dose (F5,85=1.083, NS; RM two-way ANOVA; N = 9–10). B) MeCP2308/Y mice consumed significantly less ethanol in limited access 2 hr drinking sessions before and after vapor treatments, compared with WT mice. The vapor treatments induced a significant increased ethanol intake in WT mice, but not in the MeCP2308/Y mice. Significant main effects of genotype (F1,48=23.68, p<0.001) and session (F3,48=3.94, p=0.014) were revealed by RM two-way ANOVA. * p< 0.05; ** p< 0.01; *** p< 0.001 vs. WT mice at the same point, ‡ p<0.01 vs. before vapor (Bonferroni post hoc test).
We then tested MeCP2308/Y and WT mice in a limited access 2BC paradigm. Ethanol consumption in this paradigm was significantly lower in MeCP2308/Y than in WT mice (Fig. 4B). Mice were then subjected to repeated bouts of ethanol vapors to induce dependence as outlined in the Materials and Methods above. In WT mice ethanol intake progressively increased and, after 3 bouts of ethanol vapors, WT mice showed significantly increased ethanol drinking over their baseline levels (Fig. 4B). Conversely, the alcohol intake of MeCP2308/Y mice did not change after vapor exposure and remained significantly lower than that of WT mice throughout the study (Fig. 4B). The lack of increased ethanol drinking after the CIE procedure in MeCP2308/Y mice cannot be explained by differences in BALs as average results from the final round of vapor were 195.21 (± 16.43) for WT and 192.71 (± 13.12) mg/100 ml from MeCP2308/Y mice. Also supporting the specificity of the lower ethanol consumption in MeCP2308/Y mice, water intake was higher in these mice compared to WT mice (significant main effect of genotype: F(1,48) = 6.465, p=0.0217) and was reflected in a lower alcohol preference (significant main effect of genotype: F1,48= 7.437, p=0.0149) in MeCP2308/Y as compared to WT mice (Supplemental Fig. 2A–C). Therefore, decreased alcohol consumption by MeCP2308/Y in this limited access paradigm was selective and did not reflect an effect on overall consummatory behavior. Thus, MeCP2308/Y mice consume less alcohol than WT mice under limited access conditions and do not show increased drinking after vapor exposure in the CIE procedure.
MeCP2 and alcohol regulate overlapping gene expression programs
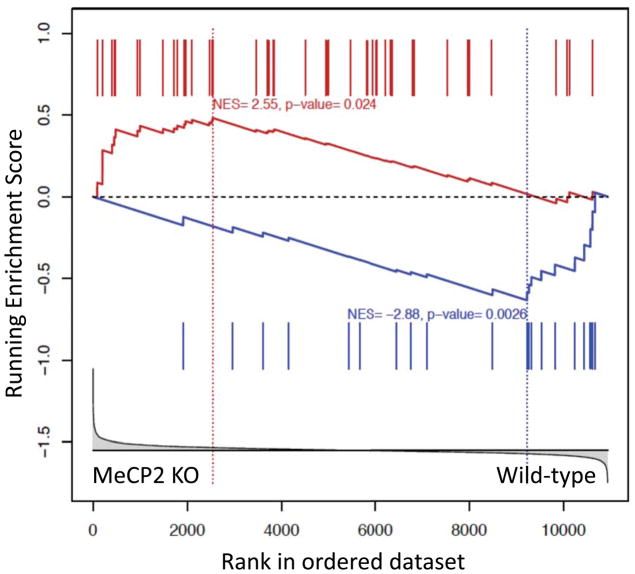
We then used the GSEA algorithm (Subramanian et al., 2005) to compare the gene expression programs regulated by alcohol and MeCP2 in the cortex. Since MeCP2 effects on gene expression are known to be small (Tudor et al., 2002), we compared two reasonably strong perturbations. In particular, we determined the degree of enrichment of the set of genes differentially expressed by alcohol in the cortex of wild-type mice (Repunte-Canonigo et al., 2010) in a dataset of genes differentially regulated in the cortex of MeCP2 null mice vs. wild-type littermates (Urdinguio et al., 2008). GSEA revealed a highly statistically significant concordance between the genes differentially expressed in the two datasets (Fig. 5) (Fig. 5 and Supplemental Table 1). Thus, MeCP2 and alcohol regulate overlapping gene expression programs in the cortex.
Figure 5.
MeCP2 and alcohol regulate overlapping gene expression programs. GSEA was used to compare the alcohol-induced gene expression changes in the cortex of wild-type mice (Repunte-Canonigo et al., 2010) to gene expression differences in the cortex of MeCP2 null vs. wild-type mice (Urdinguio et al., 2008). GSEA indicates that genes differentially expressed by alcohol in the cortex are enriched in differentially expressed genes in the cortex of MeCP2 null vs. wild-type mice (NES=2.55, p=0.024 for genes with increased expression by alcohol and NES=−2.88, p=0.0026 for genes with decreased expression). In particular, the expression data set from the comparison of MeCP2 null vs. wild-type mice was ranked based on the t-statistics (X-axis, bottom panel) and interrogated with 2 gene sets consisting of the differentially expressed genes showing increased expression in response to alcohol administration (red bars show their location in the ranked gene set of MeCP2 regulated genes) or decreased expression in response to alcohol administration (blue bars). The plots in each of the panels represent the enrichment score (ES, Y-axis) of genes with increased (red plot) or decreased (blue plot) expression in response to alcohol administration in the list of genes ranked by their differential expression in the cortex of MeCP2 null and wild-type mice. The asymmetrical distributions observed for ES (red higher toward the left-hand side and blue lower toward the right-hand side) indicates their enrichment in the dataset of MeCP2 regulated genes. The vertical dotted lines, also called leading edges, indicate the points at which the running ES reach their maximum scores.
Discussion
Here we showed that MeCP2 was one of the few differentially regulated genes in the PFC and NAc shell of mice with a history of ethanol dependence. Two other chromatin regulatory genes showed differential regulation, HDAC1, 5 in the NAc shell, which may contribute to the gene expression changes in this region.
MeCP2308/Y mice, which harbor a truncated MeCP2 allele, were more sensitive than WT to the stimulatory effect of moderate doses of ethanol and to the intoxicating effects of higher doses of ethanol. Greater ethanol sensitivity has been usually associated with reduced voluntary intake both in animal models and humans (Harris et al., 1995; Crabbe et al., 1999; Hodge et al., 1999; Schuckit, 2000; Crabbe, 2001; Le et al., 2001; Wallace et al., 2007). Indeed, MeCP2308/Y drank significantly less in a 2 hr limited access 2 bottle choice (2BC) paradigm, albeit they did not differ in their ethanol intake in a 24 hr 2BC test, possibly reflecting that, depending on the drinking bout sizes, even an overall intake of 12 g/kg may in the 24 hr 2BC may never have exceeded metabolic capacity and led to elevations in BAL. In fact, in this paradigm, drinking is episodic, often occurs over the 24 hr period, and it is not clear whether mice drink to pharmacologically significant levels (Rhodes et al., 2005). However, equivalent ethanol consumption by MeCP2308/Y and WT mice in the 24 hr 2BC paradigm suggests that taste and other consummatory functions are not impacted by the MeCP2308/Y mutation. This is also supported by equal intake of graded solutions of saccharin or quinine by MeCP2308/Y and WT mice (Supplemental Fig. 1).
In the 2 hr 2BC limited access paradigm, MeCP2308/Y mice drank less alcohol than WT both at baseline and after each bout of alcohol vapor exposure (Fig. 4B). MeCP2308/Y mice also drank more water than WT mice and their alcohol preference was significantly lower than that of WT mice (Supplemental Fig. 2A,C). MeCP2308/Y mice alcohol intake, water intake, total fluid intake and alcohol preference did not change through the study (Fig. 4B, Supplemental Fig. 2). The greater water intake and lower alcohol preference of MeCP2308/Y mice suggests that their decreased alcohol consumption is not caused by non-specific effects such as hypolocomotor activity on consummatory behavior but is due to the pharmacological effect of alcohol. The difference in ethanol sensitivity and drinking between MeCP2308/Y and WT mice in the limited access 2BC paradigm test could not be due to differences in ethanol metabolism. In fact, alcohol metabolism in the two genotypes did not differ (Fig. 3) and BALs measured after the final round of CIE were not different between MeCP2308/Y and WT mice. Altogether, these results appear consistent with lower alcohol consumption in MeCP2308/Y mice being due to their greater sensitivity of the pharmacological effects of alcohol (Fig. 2) and suggests that MeCP2308/Y mice are either avoiding ethanol’s pharmacological effects or are requiring less ethanol to achieve the same pharmacological effects as WT mice.
The lack of increased drinking in MeCP2308/Y after 3 bouts of intermittent exposure to ethanol vapors, which was sufficient to induce increased drinking in the WT mice, together with the long-lasting dysregulation of MeCP2 measured in C57BL/6J mice showing increased drinking in the CIE paradigm, and the overlap of alcohol- and MeCP2-regulated genes suggest that MeCP2-regulated genes are required for the transition to dependent drinking.
To compare gene expression programs regulated by alcohol and MeCP2 we used GSEA that allows the comparison of global gene expression perturbations induced by alcohol and by MeCP2 deficiency (Fig. 5, Supplemental Table 1). The GSEA results showed a highly statistically significant overlap between the genes affected by MeCP2 deficiency and alcohol administration. Gene expression changes associated with manipulations of MeCP2 are typically of low magnitude, which made the breadth of its gene expression targets elusive in early studies (Tudor et al., 2002). Similarly, scant gene expression changes are typically seen during protracted withdrawal from alcohol vapor, e.g. (Repunte-Canonigo et al., 2007). Thus, in order to maximize the power of the GSEA global approach, we compared a dataset of gene expression differences in the cortex of MeCP2 null mice vs. wild-type control mice (Urdinguio et al., 2008) with genes differentially expressed in the mouse cortex after repeated administration of an intoxicating dose of alcohol (Repunte-Canonigo et al., 2010). A highly significant overlap was observed, which indicates that MeCP2 and alcohol regulate overlapping gene expression programs in the cortex. It is worth noting that this significant overlap was seen between the genes differentially expressed by the two perturbations, MeCP2 deficiency and alcohol, despite the differences in the two experimental designs, microarray platform, and dissections (Urdinguio et al., 2008; Repunte-Canonigo et al., 2010).
Consistent with a role in long-term gene expression regulation, MeCP2 was also implicated in neuroadaptive changes induced by stimulants like methamphetamine and cocaine (Deng et al., 2010; Feng and Nestler, 2010; Im et al., 2010; Sadri-Vakili et al., 2010), although probably not in the incubation of heroin craving (Theberge et al., 2012). It is therefore possible that MeCP2 may be implicated in the regulation of specific sets of genes in a drug-specific manner. Electrophysiological analyses of MeCP2 mutant mice showed that MeCP2 regulates multiple cell functions and loss of MeCP2 results in complex changes that involve changes in both excitatory and inhibitory postsynaptic currents, and intrinsic excitability (Shepherd and Katz, 2011). Additionally, mounting evidence also points to a role of glia in the effects of MeCP2 deficiency (Lioy et al., 2011). However, the neurologic phenotype of MeCP2 mutant mice is not believed to be neurodegenerative in nature as it can be largely reversed by post-developmental re-expression of MeCP2 (Guy et al., 2007). Therefore future studies will be needed to investigate the consequence of anatomically localized loss or gain of function of MeCP2 on ethanol’s effects and gene expression regulation.
In conclusion, we observed that MeCP2 was one of the few chromatin-regulating genes to be differentially regulated in brain regions relevant to alcohol reinforcement in mice during protracted withdrawal in the CIE paradigm of dependence-associated increased drinking. MeCP2308/Y mice were more sensitive to ethanol’s stimulatory and sedative effects than WT mice, drank less ethanol in a limited access 2BC paradigm, and did not show increased drinking after induction of dependence with exposure to ethanol vapors with CIE. Lastly, a significant overlap in the genes regulated by alcohol and by MeCP2 was identified using the GSEA algorithm. These results suggest that MeCP2-regulated genes contribute to ethanol sensitivity and drinking and are critical in the transition to dependence.
Supplementary Material
Acknowledgments
Research was supported by National Institutes of Health grants AA017371, AA020960, AA013191, and AA013523.
Literature cited
- Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M, Koob GF, Repunte-Canonigo V, Sanna PP. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Use of genetic analyses to refine phenotypes related to alcohol tolerance and dependence. Alcohol Clin Exp Res. 2001;25:288–292. [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Buck KJ, Cunningham CL, Belknap JK. Identifying genes for alcohol and drug sensitivity: recent progress and future directions. Trends Neurosci. 1999;22:173–179. doi: 10.1016/s0166-2236(99)01393-4. 174_00001393. [DOI] [PubMed] [Google Scholar]
- Deng JV, Rodriguiz RM, Hutchinson AN, Kim IH, Wetsel WC, West AE. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat Neurosci. 2010;13:1128–1136. doi: 10.1038/nn.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Nestler EJ. MeCP2 and drug addiction. Nat Neurosci. 2010;13:1039–1041. doi: 10.1038/nn0910-1039. [DOI] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12–41) Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Cheval H, Selfridge J, Bird A. The role of MeCP2 in the brain. Annu Rev Cell Dev Biol. 2011;27:631–652. doi: 10.1146/annurev-cellbio-092910-154121. [DOI] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, McQuilkin SJ, Paylor R, Abeliovich A, Tonegawa S, Wehner JM. Mutant mice lacking the gamma isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of gamma-aminobutyrate type A receptors [see comments] Proc Natl Acad Sci U S A. 1995;92:3658–3662. doi: 10.1073/pnas.92.9.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. java/Propub/neuro/nn1199_1997.fulltext java/Propub/neuro/nn1199_1997.abstract. [DOI] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Kiianmaa K, Cunningham CL, Engel JA, Ericson M, Soderpalm B, Koob GF, Roberts AJ, Weiss F, Hyytia P, Janhunen S, Mikkola J, Backstrom P, Ponomarev I, Crabbe JC. Neurobiological processes in alcohol addiction. Alcohol Clin Exp Res. 2001;25:144S–151S. doi: 10.1111/j.1530-0277.2001.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Lewis JD, Meehan RR, Henzel WJ, Maurer-Fogy I, Jeppesen P, Klein F, Bird A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992;69:905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- Lim JP, Zou ME, Janak PH, Messing RO. Responses to ethanol in C57BL/6 versus C57BL/6 x 129 hybrid mice. Brain and behavior. 2012;2:22–31. doi: 10.1002/brb3.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, Mandel G. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Repunte-Canonigo V, Lutjens R, van der Stap LD, Sanna PP. Increased expression of protein kinase A inhibitor alpha (PKI-alpha) and decreased PKA-regulated genes in chronic intermittent alcohol exposure. Brain Res. 2007;1138:48–56. doi: 10.1016/j.brainres.2006.09.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte-Canonigo V, van der Stap LD, Chen J, Sabino V, Wagner U, Zorrilla EP, Schumann G, Roberts AJ, Sanna PP. Genome-wide gene expression analysis identifies K-ras as a regulator of alcohol intake. Brain Res. 2010;1339:1–10. doi: 10.1016/j.brainres.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Crabbe JC, Keith LD. Genetic differences in hypothalamic-pituitary-adrenal axis responsiveness to acute ethanol and acute ethanol withdrawal. Brain Res. 1992;579:296–302. doi: 10.1016/0006-8993(92)90064-g. [DOI] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, Pierce RC, Cha JH. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Genetics of the risk for alcoholism. Am J Addict. 2000;9:103–112. doi: 10.1080/10550490050173172. [DOI] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Katz DM. Synaptic microcircuit dysfunction in genetic models of neurodevelopmental disorders: focus on Mecp2 and Met. Curr Opin Neurobiol. 2011;21:827–833. doi: 10.1016/j.conb.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terdal ES, Crabbe JC. Indexing withdrawal in mice: matching genotypes for exposure in studies using ethanol vapor inhalation. Alcohol Clin Exp Res. 1994;18:542–547. doi: 10.1111/j.1530-0277.1994.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Theberge FR, Pickens CL, Goldart E, Fanous S, Hope BT, Liu QR, Shaham Y. Association of time-dependent changes in mu opioid receptor mRNA, but not BDNF, TrkB, or MeCP2 mRNA and protein expression in the rat nucleus accumbens with incubation of heroin craving. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2784-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor M, Akbarian S, Chen RZ, Jaenisch R. Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc Natl Acad Sci U S A. 2002;99:15536–15541. doi: 10.1073/pnas.242566899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdinguio RG, Lopez-Serra L, Lopez-Nieva P, Alaminos M, Diaz-Uriarte R, Fernandez AF, Esteller M. Mecp2-null mice provide new neuronal targets for Rett syndrome. PloS one. 2008;3:e3669. doi: 10.1371/journal.pone.0003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MJ, Newton PM, Oyasu M, McMahon T, Chou WH, Connolly J, Messing RO. Acute functional tolerance to ethanol mediated by protein kinase Cepsilon. Neuropsychopharmacology. 2007;32:127–136. doi: 10.1038/sj.npp.1301059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.