Abstract
Primary sclerosing cholangitis (PSC) is a chronic immune-mediated disease of the liver of unclear etiology, characterized by chronic inflammation and fibrosis of bile ducts. It primarily affects middle aged men, and is associated with 4-fold increased mortality as compared to ageand gender-matched population. Progressive biliary and hepatic damage results in portal hypertension and hepatic failure in a significant majority of patients over a 10–15 year period from initial diagnosis. In addition, PSC confers a markedly increased risk of hepatobiliary cancer, including cholangiocarcinoma and gallbladder cancer as compared to the general population, and cancer is the leading cause of mortality in patients with PSC. It is associated with inflammatory bowel disease in 70% patients, and increases the risk of colorectal cancer almost 10-fold. Despite significant research efforts in this field, the pathogenic mechanisms of PSC are still incompletely understood, although growing evidence supports the role of genetic and immunologic factors. There are no proven medical therapies that alter the natural course of the disease. Thus, liver transplantation is the only available treatment for patients with advanced PSC, with excellent outcomes in this population.
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic cholestatic liver disease characterized by progressive inflammation and fibrosis of the intra-hepatic and extra-hepatic bile ducts, leading to cholestasis, progressive hepatic fibrosis and eventually to decompensated cirrhosis in a significant majority of patients over 10–15 years.1–7 In addition, since PSC confers a markedly increased risk of hepatobiliary and colorectal cancer (CRC) as compared to the general population, with cancer being the leading cause of mortality in patients with PSC, it should be considered a pre-malignant condition.8 Based on the uncertainty of etiopathogenesis, no effective medical therapy for halting the progression of PSC has been identified. Among eligible patients, liver transplantation (LT) is currently the only curative therapy for PSC. Despite advances in diagnostic modalities and endoscopic intervention, mortality rates from PSC have remained essentially unchanged over the past two decades.
EPIDEMIOLOGY AND RISK FACTORS
The incidence of PSC is estimated at 1 per 100,000 population, with evidence that the incidence is increasing over time based on a recent meta-analysis of population-based studies from Northern Europe, UK, USA and Canada.9 The highest annual incidence is reported in Norway (1.3 per 100,000), with a prevalence of 8.5 per 100,00010 and in this population PSC is the leading indication for LT.11 In the United States, the overall age- and sex-adjusted incidence of PSC is estimated at 0.9 per 100,000 population with a prevalence of 13.6 per 100,000.1 The median age at time of diagnosis ranges between 35–47 years, and 62–70% of patients are males.1, 3, 4, 7, 9, 10, 12 Approximately 58–77% patients have associated inflammatory bowel disease (IBD).
No clear environmental or clinical risk factors for the development of PSC have been identified. Some studies suggest that smoking and tonsillectomy may reduce the risk of PSC, independent of IBD.13–15 No significant relationship between previous appendectomy and risk of PSC is known.14, 15
PATHOGENESIS
The pathogenesis of PSC is continue to be elusive, and is reviewed in detail elsewhere.16 It is felt to be a complex immune-mediated disease, rather than a true autoimmune disease given the lack of classic features (male rather than female predisposition, lack of response to immunosuppressive medications and lack of highly-selective and pathogenic auto-antibodies).16 The most commonly accepted theory is that, in a genetically predisposed individual, an initial insult to cholangiocytes through environmental exposure to toxins or infections agents (such as bacterial translocation across a leaky gut) results in persistent immune-mediated damage with progressive destruction of bile ducts leading to chronic cholestasis and progressive fibrosis.
The genetic predisposition to PSC is supported by multiple studies which show almost a 100-fold increased risk of the disease in first-degree relatives of patients with PSC, as well as clustering within families.17 Genome-wide association studies in PSC have shown strong associations with HLA haplotypes, particularly HLA-DR3 (DRB1*0301) and HLA-B8 (B*0801).18 Polymorphisms of several non-HLA genes have also been shown to influence susceptibility to PSC such as those encoding intracellular adhesion molecule 1 (ICAM-1),19 tumor-necrosis factor,20 and matrix metalloproteinase 3 (MMP-3).21 However, most of these genetic associations are weak and have been difficult to reproduce. Given the strong association with IBD, the “leaky gut hypothesis” appears to be playing a fundamental role in describing the pathogenesis of PSC.22 It is hypothesized that bacterial products can translocate through an inflamed gut while gut-derived memory T-lymphocytes target the liver via aberrantly expressed adhesion molecules and contribute to cholangiocyte damage.23 This concept is also supported by the fact that pre-liver transplant colectomy may afford protection against recurrent PSC in the transplanted liver,24 as well as the presence of common peripheral anti-neutrophil cytoplasmic antibodies (pANCA) in both these disease.25 Nonetheless, this concept does not explain the pathogenesis of PSC in patients without IBD. Toxic, genetically- or chemically-modified bile-induced damage has also been proposed as a pathophysiologic mechanism in PSC primarily based on animal models, but there is inconclusive evidence to support this in humans.26
DIAGNOSIS
Clinical manifestations
The majority of patients (44–56%) are asymptomatic at time of diagnosis,1, 6 and the discovery of PSC is increasingly based on the investigation of abnormal serum liver tests and/or the incidental discovery of intra-hepatic bile duct dilatation on cross-sectional imaging. Notably, up to 17% of asymptomatic patients may have cirrhosis on liver biopsy at time of diagnosis.27 In asymptomatic patients, the estimated survival without decompensated cirrhosis at 7 years is 75% in comparison to 96% for age- and sex-matched healthy individuals.28
Among symptomatic patients, fatigue and pruritus are the initial presenting symptoms, and with progression, these patients tend to develop jaundice, abdominal pain and weight loss. Episodes of bacterial cholangitis are uncommon at presentation in the absence of dominant strictures or biliary manipulation,29 and usually present with fevers, chills, right upper quadrant pain and worsening liver biochemistries.
Biochemical and serological features
Elevated serum alkaline phosphatase is the hallmark of PSC, and found in 95% of patients. Serum alanine and aspartate aminotransferase levels are also usually elevated up to 2–3 times the upper limit of normal.30 Serum bilirubin level is typically normal at diagnosis in the majority of patients with high levels worrisome for advanced disease, superimposed choledocholithiasis, or malignancy.30, 31 A wide variety of non-organ specific auto-antibodies can be detected in the serum of patients with PSC, including anti-nuclear antibodies, anti-cardiolipin antibodies, thyroperoxidase, rheumatoid factor, and smooth muscle antibodies in keeping with underlying alterations in immune regulation.32 pANCA is detected approximately 30–80% of patients with PSC and like other auto-antibodies, lacks disease specificity.33, 34 None of these antibodies, however, are of clinicopathologic relevance nor do they reflect severity or prognosis of underlying disease. Hence, they are not routinely used in the diagnosis of PSC.
Radiographic features
Cholangiography is considered the ‘gold standard’ for diagnosis of PSC. The characteristic findings include short, multifocal, annular strictures alternating with normal or slightly dilated intervening segments, giving rise to the so-called ‘beads-on-a-string’ appearance.35 Long, confluent strictures may also be observed though these are more worrisome for development of superimposed cholangiocarcinoma (CCA). PSC involves both the intra- and extra-hepatic bile ducts in the majority of patients while an estimated 25% of patents only have isolated intra-hepatic involvement.29 Isolated involvement of the extra-hepatic bile ducts is very unusual (<5% patients), and should only be diagnosed if there is adequate visualization of intra-hepatic bile ducts. Gallbladder, cystic and pancreatic ducts may also be involved in patients with PSC. Magnetic resonance cholangiopancreatography (MRCP) has replaced endoscopic retrograde cholangiopancreatography (ERCP) as the initial diagnostic test of choice – it is non-invasive, lacks radiation exposure, has comparable sensitivity (80–90%) and specificity (>90%) to ERCP, and is more cost-effective.36–39. ERCP is associated with serious complications such as bacterial cholangitis and pancreatitis, and may be associated with post-procedural hospitalization in up to 10% of patients.40 Among symptomatic patients, ERCP is clearly required given the likelihood for biliary intervention. It is also useful in a subset of patients in whom MRCP provides sub-optimal visualization of intra-hepatic bile ducts.
Histological features
Periductal fibrosis with inflammation, bile duct proliferation and ductopenia are the main histological findings in PSC. Notably, the characteristic ‘onion-skinning’ periductal fibrosis that is the hallmark for PSC is rarely observed.41 However, liver biopsy is not routinely necessary for confirming the diagnosis of PSC in patients with typical cholangiographic findings.42 It is useful for disease staging in PSC where inflammation and fibrosis involving portal (stage 1), periportal (stage 2), or septal (stage 3) areas can lead to cirrhosis, which represents stage 4.43 Non-invasive assessment of fibrosis with elastography-based imaging techniques has also been shown to be useful in patients with PSC.44 In addition, liver histology is critical in diagnosing patients with small-duct PSC and in patients where an overlap syndrome with autoimmune hepatitis (AIH) is suspected (see below). 42, 45
VARIANT PSC SYNDROMES
Small-duct PSC
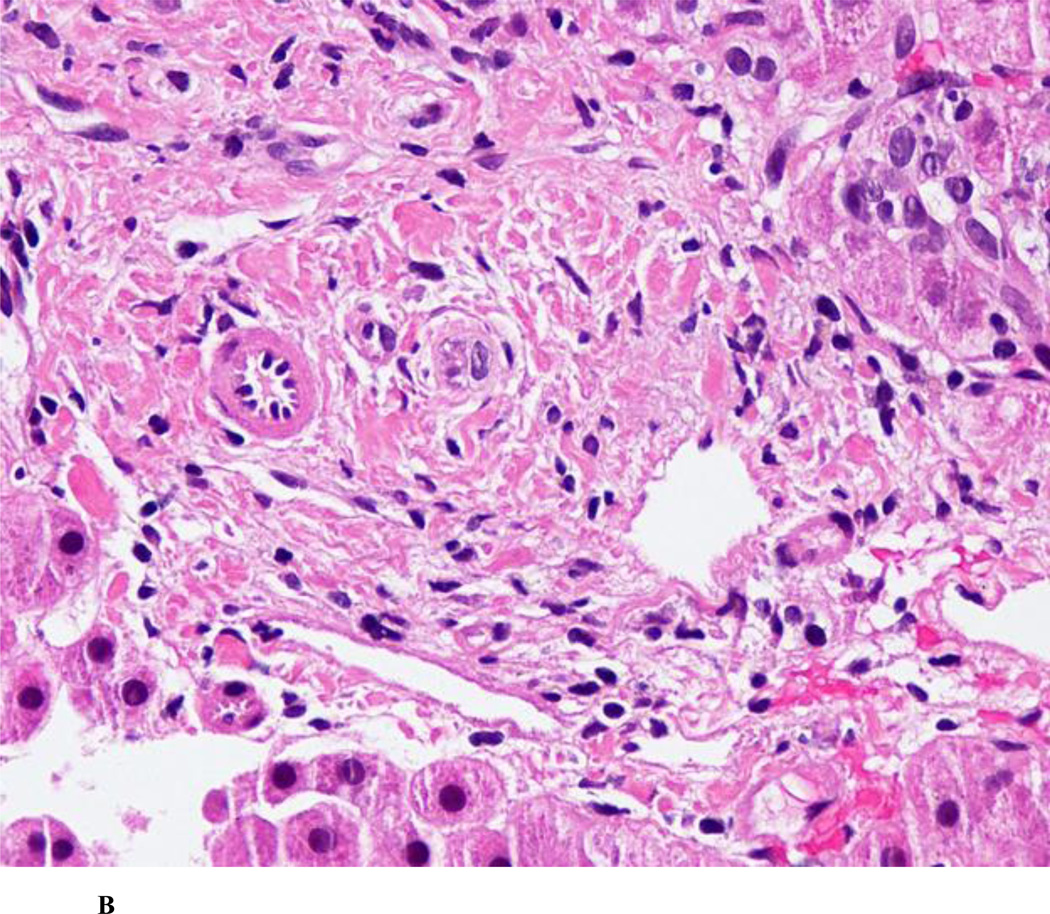
Small-duct PSC is diagnosed when patients have clinical, biochemical and histological features of PSC in the setting of a normal cholangiogram (Figure 2B). This represents ~11–17% patients with PSC based on population-based studies.1, 12 The diagnosis is difficult to confirm in the absence of IBD. Long-term follow-up studies have shown that approximately 25% of subjects progress to large-duct PSC over time. These patients have significantly longer transplantationfree survival rates as compared to patients with large-duct PSC. Small-duct PSC does not appear to predispose to the development of CCA in the absence of progression to large-duct PSC.46 Also, it appears that Crohn’s colitis may be associated with small-duct PSC than large-duct PSC.47 Children with ABCB4 deficiency (leading to a defect in the gene encoding the multidrug resistance protein 3) may be misdiagnosed as having small-duct PSC.48
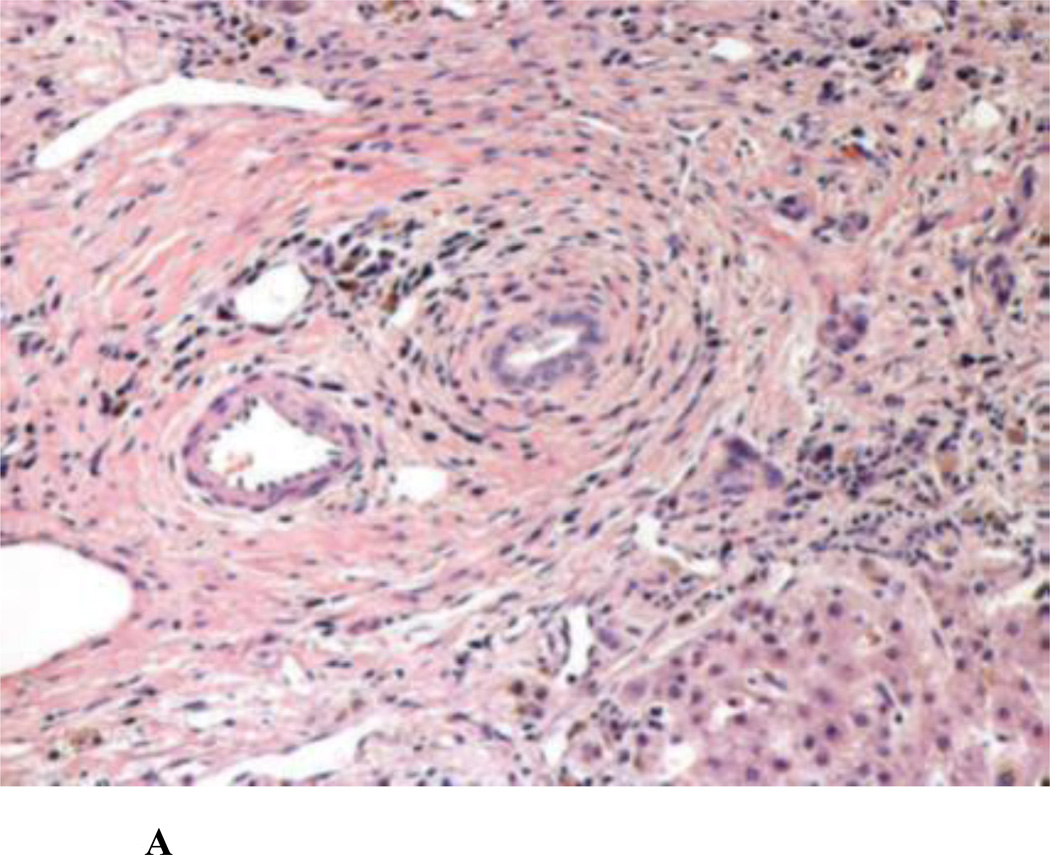
Figure 2.
A. Classic ‘onion skin’ fibrosis, causing obliteration of the bile ducts in primary sclerosing cholangitis [Photograph courtesy: Thomas Smyrk, M.D., Department of Pathology, Mayo Clinic, Rochester]
B. Ductopenia, without onion-skinning fibrosis in small-duct primary sclerosing cholangitis [Photograph courtesy: Thomas Smyrk, M.D., Department of Pathology, Mayo Clinic, Rochester]
Overlap syndrome with autoimmune hepatitis
Overlap syndrome with AIH and PSC should be suspected if a patient with typical cholangiographic findings is noted to have a 5–10 fold elevation in serum aminotransferases, increased level of serum auto-antibodies and/or hypergammaglobulinemia and histological evidence of moderate to severe interface hepatitis.29 This variant of PSC is diagnosed in 1.4–17% patients with PSC, and most commonly in children and young adults.49–51 In this subset of patients, medical therapy with prednisone and/or azathioprine is recommended. Data on the clinical presentation, treatment response, and long-term clinical outcomes in adults with overlap syndrome is lacking. Likewise, overlap syndrome should also be suspected in a patient with AIH who is noted to have concomitant IBD and/or an incomplete response to medical therapy.29
IgG4-related sclerosing cholangitis
IgG4-related sclerosing cholangitis is a recently recognized entity thought to represent the biliary manifestation of a multi-organ, steroid-responsive disorder characterized by increased IgG4 production.52 This is the most common cause of biliary strictures in patients with autoimmune pancreatitis, but it may occur in the absence of characteristic pancreatic involvement. In a subset of patients with IgG4-related sclerosing cholangitis identified from a cohort of patients with autoimmune pancreatitis, the disease primarily affected males in the 7th decade of life. Patients frequently present with painless jaundice (77%) and are less likely to have associated IBD.53 IgG4-related sclerosing cholangitis is usually responsive to treatment with corticosteroids, though relapse is frequent after steroid withdrawal especially for patients with proximal strictures. Diagnostic criteria have been proposed for IgG4-related sclerosing cholangitis based on characteristic histological and cholangiographic findings, elevations in serum IgG4, systemic organ involvement and response to corticosteroids.53
In contrast, there is a subset of patients with classic PSC who have elevated serum IgG4 levels and no other features of IgG4-related disease. In a retrospective analysis of 127 patients with PSC, an estimated 9% were noted to have elevated IgG4 levels although biopsies demonstrating infiltration of IgG4-positive plasma cells in bile ducts were not routinely available.54 These patients were usually older than patients with PSC and normal IgG4 levels, less likely to have associated IBD and a shorter time to LT. Hence, it is recommended that serum IgG4 levels be tested in all patients with suspected PSC, and if elevated, to consider and evaluation for IgG4-related disease where a trial of steroid therapy may be considered.29 It should be noted that increased serum IgG4 levels have also been more commonly observed in patients with typical PSC who have or ultimately develop CCA.52
COMPLICATIONS AND ASSOCIATED CONDITIONS
Cholangiocarcinoma
The lifetime prevalence of CCA is 5–10% in patients with PSC, a risk that is 160-fold higher than that seen in the general population.55–57 Several risk factors for CCA in PSC have been recognized including older age at PSC diagnosis, smoking, alcohol use, elevated bilirubin, a longer duration of associated IBD, presence of colorectal neoplasia in patients with IBD, proctocolectomy, variceal bleeding, and polymorphism of the NKG2D gene.57 Duration of PSC does not appear to be a risk for development of CCA as 30–50% patients with CCA are diagnosed within 1 year of diagnosis of PSC.57, 58
The diagnosis of CCA is very difficult to confirm. It should be suspected in patients with an acute decline in clinical status including unexplained weight loss and/or worsening of serum liver biochemistries. In these patients, imaging with MRI/MRCP as well as an assessment of serum CA 19-9 level is warranted. The demonstration of an intra-hepatic mass lesion with characteristic imaging features (i.e., malignant appearing mass with delayed venous phase enhancement) has virtually a 100% sensitivity and specificity for the diagnosis of CCA.59 However, most early stage CCAs are ductal in location and current non-invasive imaging modalities have poor positive predictive values in identifying these lesions. Likewise, serum CA 19-9 has a limited diagnostic utility as it can be elevated in patients with bacterial cholangitis or significant intra-hepatic cholestasis. Furthermore, it is virtually undetectable in 7% of the normal population who are negative for the Lewis antigen.57 A diagnostic cut-off level of 130 U/ml has a sensitivity and specificity of 79% and 98% for diagnosing CCA although CA 19-9 has never been shown to increase the detection of localized or early-stage CCA. Patients with a dominant stricture should undergo ERCP with attempts at obtaining biopsies and brush cytology. Unfortunately, the sensitivity of brush cytology is low at 18–40%.29 Recent studies have demonstrated that fluorescence in situ hybridization (FISH) may increase the yield of conventional cytology. The demonstration of polysomy by FISH analysis of cytologic specimens has demonstrated a sensitivity of 41% and a specificity of 98% for the diagnosis of CCA in PSC patients.60
Surveillance for CCA is recommended in patients with PSC. However, an evidence-based approach based on prospective data collection has not been identified. Most experts recommend annual imaging (using MRI/MRCP or ultrasound) along with serum CA 19-9 level measurement. In patients noted to have abnormalities in either one of these, invasive testing with ERCP using conventional brush cytology and FISH is recommended.57 Table 1 shows cancer surveillance recommendations for patients with PSC.
Table 1.
Cancer surveillance in Primary Sclerosing Cholangitis. Due to the markedly increased risk of cancer in patients with PSC, it should be considered a premalignant condition.
Cholangiocarcinoma
|
Gallbladder Cancer
|
Colorectal Cancer
|
Hepatocellular Cancer
|
Pancreatic Cancer
|
[Abbreviations: CCA – Cholangiocarcinoma; CRC – Colorectal cancer; ERCP – Endoscopic retrograde cholangiopancreatography; FISH – Fluorescence in situ hybridization; IBD – Inflammatory bowel disease; IPAA – Ileal pouch anal anastomosis; MRI – Magnetic resonance imaging, MRCP – Magnetic resonance cholangiopancreatography]
Colorectal Neoplasia
PSC is associated with IBD in about 60–80% patients based on numerous observational studies and a meta-analysis of population-based studies.61 Ulcerative colitis (UC) is the most common type of IBD identified in 48–86% patients; Crohn’s disease can be seen in 13–25% patients. Conversely, PSC is the most common hepatobiliary complication seen in patients with IBD, and is seen in about 5% patients.29 IBD often precedes PSC or is diagnosed concomitantly; in a subset of patients IBD may develop after diagnosis of PSC, even after LT. Hence, it is recommended that all patients with PSC undergo colonoscopy with random biopsies at diagnosis to detect IBD. If the initial colonoscopy with biopsies is negative for IBD, then a repeat colonoscopy should be repeated every 5 years.
Multiple studies suggest that IBD in PSC may represent a unique phenotype characterized by a high prevalence of pancolitis, mild histological inflammation, and usually a mild or quiescent clinical course.61–66 Some studies suggest that patients with PSC-IBD may have higher incidence of backwash ileitis and rectal sparing,62 though this has not been universally observed.61, 63, 65 Even in patients with Crohn’s disease associated with PSC, colonic involvement is more likely (>90%) and ileal involvement is uncommon.61, 64, 67 Patients with PSC-IBD who undergo ileal pouch anal anastomosis (IPAA) have a higher incidence of acute and chronic pouchitis,62, 68, 69 as compared to subjects with IBD alone. In addition, patients with PSC-IBD who undergoing Brooke ileostomy creation have a significantly higher rate of developing difficult-to-treat peristomal varices due to portal hypertension from progressive PSC.62, 70 Hence, it is preferable the patients with PSC with associated UC undergo IPAA if surgical management is required, or, in patients with endoscopic and histologic rectal sparing, ileorectal anastomosis may be considered.
Patients with PSC-IBD have a significantly higher risk of colorectal dysplasia and cancer (CRC) than patients with IBD alone. A meta-analysis of 11 studies reported a pooled odds ratio of 4.26 (95% CI, 2.80–6.48), for developing CRC.71 The cumulative incidence of development of CRC or dysplasia in PSC/UC patients versus UC alone is 9% and 2% after 10 years and 20%–31% and 5% after 20 years of disease duration, respectively. Hence, in patients with PSC-IBD, colonoscopic surveillance is recommended every 1–2 years from time of PSC diagnosis.29, 72 Colonic neoplasia tends to occur proximally in 2/3rd of patients.73 This risk of colonic neoplasia continues to be high even after LT, with an almost 10-fold higher risk than patients undergoing LT for other indications. Thus, annual surveillance colonoscopy with a minimum of 32 biopsies is required to exclude colorectal neoplasia (Table 1).
Gallbladder Neoplasia
Gallbladder abnormalities such as gallstones (25%) and PSC involving the gallbladder or cystic duct (15%), are seen in up to 41% patients with PSC.45 Patients also have an increased frequency of gallbladder mass lesions, with an estimated prevalence of 3–14% versus 0.35% in the general population.74 In a study of 286 patients with PSC, 10/18 mass lesions represented gallbladder adenocarcinoma while an additional 9 patients were noted to have gallbladder epithelial dysplasia without a mass lesion.75 It appears that gallbladder adenocarcinoma appears to follow an adenoma-carcinoma sequence secondary to PSC-induced chronic inflammation.76 Based on these observations, it is recommended that patients with PSC undergo annual surveillance for gallbladder mass lesions. While early studies suggested that any patient with PSC and gallbladder polyps should consider cholecystectomy, the procedure is associated with significant liver-related morbidity in up to 40% patients, especially in those with cirrhosis. More recent data suggests that polyp size ≥0.8cm has 100% sensitivity and 70% specificity in predicting the presence of gallbladder neoplasia.77 Hence, we recommend close surveillance with imaging annually in patients with PSC and small gallbladder polyps and to consider prophylactic cholecystectomy for lesions > 8 mm in the setting of intact hepatic synthetic function (Table 1).29
Due to the markedly increased risk of cancer in patients with PSC, it should be considered a pre-malignant condition warrant close surveillance.
Pruritus
Pruritus develops in 40–60% patients with PSC and is associated with significant impairment of health-related quality of life.30 The severity is independent of histological stage and cholangiographic extent of disease, though extra-hepatic biliary structuring can influence intensity of symptoms. It is mediated by bile salt retention and endogenous opioid ligand accumulation.78 Medical management strategies are similar to those employed for patients with primary biliary cirrhosis.78 The management of pruritus is outlined in Table 2.40, 79
Table 2.
Management of extra-hepatic, non-neoplastic complications of primary sclerosing cholangitis
Dominant Stricture
|
Pruritus
|
Metabolic Bone Disease
|
Malabsorption
|
Metabolic bone disease and nutritional deficiencies
Osteoporosis (defined as t-score ≤-2.5 on bone mineral densitometry) is seen in 10–15% of patients with PSC while osteopenia (t-score between −1.0–2.5) is seen in 30% patients.80 The risk of metabolic bone disease is higher with advanced age, low body mass index and long duration of associated IBD. In patients with PSC being evaluated for LT, osteoporosis develops in 30–50% patients.81 Screening and management recommendations are outlined in Table 2.29
Fat-soluble vitamin deficiency occurs in 50–85% of patients with advanced PSC. Decreased secretion of conjugated bile acids in the small intestine can contribute to steatorrhea, or it may develop secondary to co-existing conditions such as celiac disease and chronic pancreatitis. Oral supplementation of vitamin A, D, and E is recommended in patients with PSC when present.29, 82
Complications of portal hypertension
Cirrhotic-stage PSC patients develop portal hypertension and associated complications such as ascites, hepatic encephalopathy and variceal bleeding. In addition, a small subset of patients with PSC may develop pre-cirrhotic, pre-sinusoidal portal hypertension secondary to nodular regenerative hyperplasia and/or obliterative portal venopathy.83 The management of complications from portal hypertension in PSC similar to other underlying chronic liver disease etiologies.29
TREATMENT OF PRIMARY SCLEROSING CHOLANGITIS
Medical Management
To date, there are no medical therapies that have been proven to alter the natural course of PSC. The most comprehensively studied agent is UDCA, 7-β-epimer of chenodeoxycholic acid that improves hepatobiliary secretion, is hepato-protective, has immunomodulatory properties, and is very effective in the management of primary biliary cirrhosis.84 An initial placebo-controlled randomized controlled trial demonstrated that standard dose UDCA (13–15mg/kg/day) was no superior to placebo at a median follow-up 2.2 years with regards to important clinical end points although serum liver biochemistries were substantially improved.85 Subsequent trials with higher weight-based doses of UDCA have shown no clinical benefit over placebo treated groups as well. Furthermore, a multicenter trial of UDCA at 28–30mg/kg/day was associated with a two-fold increase in disease progression as compared to placebo treated subjects.86 A meta-analysis of 8 RCTs comprising 567 patients (5 studies with standard dose UDCA, 3 studies with high-dose UDCA) showed UDCA was comparable to placebo with no differences in mortality, need for LT, histologic progression, or development of CCA.87 Therefore, current available evidence does not support the use of UDCA for the treatment of PSC. A novel bile acid, 24-norursodeoxycholic acid has shown promise as an anti-fibrotic, anti-inflammatory and anti-proliferative agent in a mouse model of cholangitis and biliary fibrosis where the reversal of sclerosing cholangitis within 4 weeks of treatment has been observed.88 Human studies are currently in progress.
There is some evidence that UDCA may modify the risk of colorectal neoplasia, with standard dose UDCA decreasing the risk of colorectal neoplasia89, 90 while high-dose UDCA potentially increases the risk.91 Based on a meta-analysis of 6 studies reporting on 41 cases of advanced colorectal neoplasia (CRC and/or high-grade dysplasia) in 546 patients with PSC-IBD, we recently showed that standard-dose UDCA was associated with a 63% risk reduction.92 Based on these results, standard dose UDCA has been recommended as a chemopreventive agent for reduction in CRC risk in patients with PSC and associated IBD by the American Gastroenterological Association.72
Several other medical therapies such as immunosuppressive agents (prednisolone, budesonide, azathioprine, methotrexate, tacrolimus, cyclosporine, mycophenolate mofetil), anti-tumor necrosis factor antagonists (infliximab, etanercept), anti-fibrotic agents (silymarin, pirfenidone, pentoxifylline, colchicine, penicillamine) and systemic antibiotics (vancomycin, minocycline, metronidazole) have been tried in the management of PSC, but none of them has demonstrated clinical benefits to date.29, 84
Management of Biliary Strictures
A dominant stricture is defined by a stenosis diameter ≤1.5mm in the common bile duct and ≤1mm in the common hepatic duct. It develops in 45–58% patients with PSC.29 In a patient with stable PSC, the presence of clinical deterioration with worsening pruritus, jaundice or bacterial cholangitis warrants evaluation by ERCP to exclude a dominant stricture. Furthermore, progressive biliary dilation on imaging, rising biochemical indices and/or constitutional symptoms such as weight loss also requires evaluation by ERCP to exclude CCA masquerading as a dominant stricture.
The ideal endoscopic technique has not been established, but some non-randomized studies suggest that balloon dilatation alone and dilatation with stent placement are equally efficacious, but the latter is associated with a significantly higher risk of complications including bacterial cholangitis, as compared to balloon dilation alone.93 Hence, stenting is usually reserved for strictures that are refractory to dilatation. In a subset of patients with failed endoscopic cannulation, sphincterotomy alone may be helpful if the sphincter of Oddi is affected by PSC, but is usually reserved to facilitate dilatation, stent placement or stone extraction.94
In patients with proximal dominant strictures or complex biliary anatomy, and in patients with failed attempts at endoscopic intervention, percutaneous trans-hepatic cholangiographic approach is required. Non-transplant surgical procedures with resection of the extra-hepatic biliary stricture and Roux Y hepaticojejunostomy, with or without operative stent insertion, may be considered for selected non-cirrhotic patients with PSC who have failed endoscopic and/or radiologic intervention.95 Biliary bypass procedure with cholangioenterostomy is infrequently performed as most dominant strictures are hilar.96
Complications from endoscopic therapy have been reported in 7–20% of PSC patients, including pancreatitis, cholangitis, biliary tract perforation and hemorrhage.40, 97 Hence, prophylactic antimicrobial therapy is recommended for all patients with PSC undergoing endoscopic or percutaneous manipulation of the biliary tree.29
Liver Transplantation
LT is currently the only available therapeutic modality for patients with advanced stage PSC, and fortunately excellent long-term patient and graft outcomes have been observed for both deceased donor and living donor LT.45, 98 In addition to standard indications for LT (progressive liver failure, complications of portal hypertension, hepatocellular carcinoma), there are a number of specific indications for PSC such as intractable pruritus, recurrent bacterial cholangitis and perihilar CCA. Of note, a recent multicenter analysis of LT for CCA demonstrated 5-year recurrence-free survival rates of 65% in carefully selected patients.99 Patients with PSC who undergo LT are more prone to acute and chronic cellular rejection although it does not impact graft survival. Recurrent PSC is reported in 30%–50% patients within 10 years after transplantation.24, 100 The diagnosis is based on combination of radiological, histological and biochemical findings after excluding other causes of post-transplant biliary strictures such as hepatic artery thrombosis, ductopenic rejection and ABO incompatibility.101 The long-term natural history of recurrent PSC is poorly understood as 30% of patients may develop progressive disease over a 10-year follow-up period leading to re-transplantation or death.102
The management of PSC patients following LT is similar to strategies used for other liver transplant recipients. However, two important issues should be kept in mind. About 30% of patients with associated IBD may develop worsening of disease activity after LT. Active IBD at the time of LT, discontinuation of 5-aminosalicylate at the time of LT, use of tacrolimus-based immunosuppression and infrequent use of azathioprine may be associated with an unfavorable course of IBD after LT.103, 104 Approximately ~14–30% patients with PSC may develop de novo IBD up to 10 years after transplantation. Finally, the risk of CRC is 10-fold higher in patients with PSC and IBD undergoing transplantation, as compared to other indications, and may be higher than pre-transplant risk. Hence, annual colonoscopic surveillance for CRC should be continued after LT in patients with PSC-IBD.29
PROGNOSIS
PSC is an insidious and slowly progressive disease without effective medical therapy. While the median time from diagnosis to death or LT is 12 years,105 there is significant inter-patient variability in prognosis depending on the stage of disease at diagnosis as well as inherent differences in the phenotype of PSC (small duct v. large duct). Several prognostic models for PSC have been described to determine risk factors for disease progression and the best time for LT.2, 3, 7, 45, 106, 107 The most recent modification of the Mayo PSC risk score based on age, bilirubin, serum AST, albumin and history of variceal bleeding as prognostic parameters is helpful to predict prognosis in early PSC.107 Recent studies have shown that cholangiographic findings, particularly the presence of a dominant stricture, may modify the prognosis of PSC and should be included in prognostic models.5, 108 In advanced PSC, the Model for End Stage Liver Disease (MELD) score is more predictive of mortality than any of the PSC-specific prognostic models. However, there is no consensus regarding the optimal model and hence, the use of prognostic models for predicting clinical outcomes in an individual patient is not recommended by the AASLD.29 Additionally, none of the prognostic models is able to predict the development of CCA in patients with PSC.
CONCLUSIONS
PSC is a chronic cholestatic liver disease which impacts survival and quality of life of affected patients. Complications related to chronic cholestasis and portal hypertension from progressive disease as well as hepatobiliary and colorectal cancer, occur in a significant number of patients. To date, no medical therapies have been shown to modify the natural course of PSC except for LT.
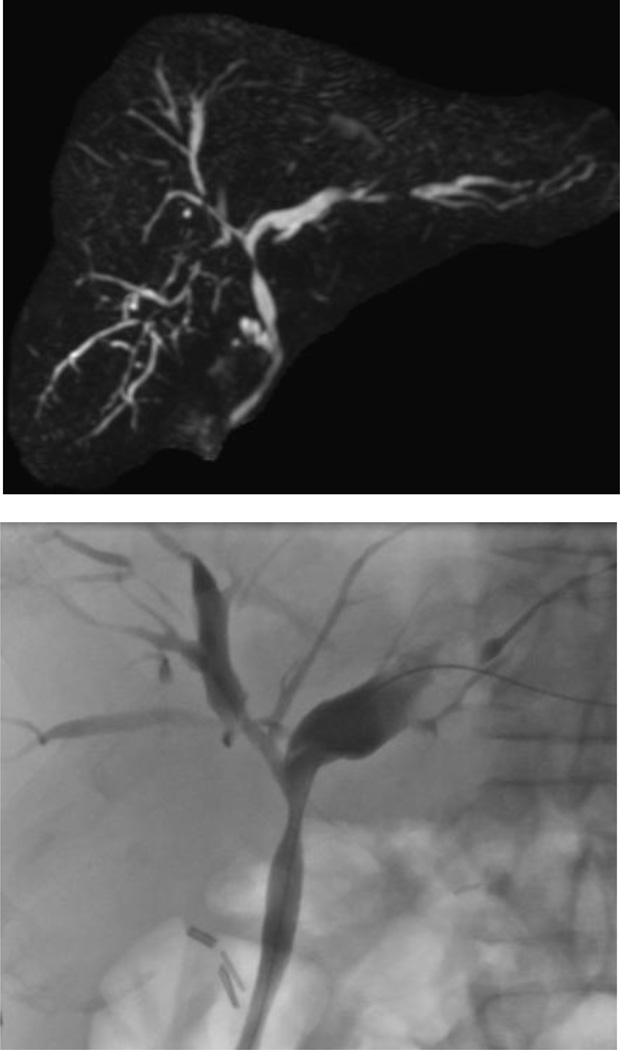
Figure 1.
Typical cholangiographic findings of primary sclerosing cholangitis. (a) MRCP demonstrating multiple strictures and dilatations of the biliary tree, affecting the intrahepatic and extrahepatic biliary tree; (b) ERCP with typical findings of pruning and beaded appearance of the biliary tree.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No relevant disclosures.
REFERENCES
- 1.Bambha K, Kim WR, Talwalkar J, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364–1369. doi: 10.1016/j.gastro.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Broome U, Olsson R, Loof L, et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610–615. doi: 10.1136/gut.38.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrant JM, Hayllar KM, Wilkinson ML, et al. Natural history and prognostic variables in primary sclerosing cholangitis. Gastroenterology. 1991;100:1710–1717. doi: 10.1016/0016-5085(91)90673-9. [DOI] [PubMed] [Google Scholar]
- 4.Kingham JG, Kochar N, Gravenor MB. Incidence, clinical patterns, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology. 2004;126:1929–1930. doi: 10.1053/j.gastro.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 5.Ponsioen CY, Vrouenraets SM, Prawirodirdjo W, et al. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut. 2002;51:562–566. doi: 10.1136/gut.51.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tischendorf JJ, Hecker H, Kruger M, et al. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: A single center study. Am J Gastroenterol. 2007;102:107–114. doi: 10.1111/j.1572-0241.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- 7.Wiesner RH, Grambsch PM, Dickson ER, et al. Primary sclerosing cholangitis: natural history, prognostic factors and survival analysis. Hepatology. 1989;10:430–436. doi: 10.1002/hep.1840100406. [DOI] [PubMed] [Google Scholar]
- 8.Bergquist A, Ekbom A, Olsson R, et al. Hepatic and extrahepatic malignancies in primary sclerosing cholangitis. J Hepatol. 2002;36:321–327. doi: 10.1016/s0168-8278(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 9.Molodecky NA, Kareemi H, Parab R, et al. Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 2011;53:1590–1599. doi: 10.1002/hep.24247. [DOI] [PubMed] [Google Scholar]
- 10.Boberg KM, Aadland E, Jahnsen J, et al. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99–103. doi: 10.1080/00365529850166284. [DOI] [PubMed] [Google Scholar]
- 11.Karlsen TH. The Nordic Liver Transplant Registry. Annual Report 2009. 2009;Volume 2012 [Google Scholar]
- 12.Kaplan GG, Laupland KB, Butzner D, et al. The burden of large and small duct primary sclerosing cholangitis in adults and children: a population-based analysis. Am J Gastroenterol. 2007;102:1042–1049. doi: 10.1111/j.1572-0241.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- 13.Loftus EV, Jr, Sandborn WJ, Tremaine WJ, et al. Primary sclerosing cholangitis is associated with nonsmoking: a case-control study. Gastroenterology. 1996;110:1496–1502. doi: 10.1053/gast.1996.v110.pm8613055. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell SA, Thyssen M, Orchard TR, et al. Cigarette smoking, appendectomy, and tonsillectomy as risk factors for the development of primary sclerosing cholangitis: a case control study. Gut. 2002;51:567–573. doi: 10.1136/gut.51.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Erpecum KJ, Smits SJ, van de Meeberg PC, et al. Risk of primary sclerosing cholangitis is associated with nonsmoking behavior. Gastroenterology. 1996;110:1503–1506. doi: 10.1053/gast.1996.v110.pm8613056. [DOI] [PubMed] [Google Scholar]
- 16.Pollheimer MJ, Halilbasic E, Fickert P, et al. Pathogenesis of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2011;25:727–739. doi: 10.1016/j.bpg.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergquist A, Lindberg G, Saarinen S, et al. Increased prevalence of primary sclerosing cholangitis among first-degree relatives. J Hepatol. 2005;42:252–256. doi: 10.1016/j.jhep.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Karlsen TH, Franke A, Melum E, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138:1102–1111. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, Cullen SN, Li JH, et al. Susceptibility to primary sclerosing cholangitis is associated with polymorphisms of intercellular adhesion molecule-1. J Hepatol. 2004;40:375–379. doi: 10.1016/j.jhep.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell SA, Grove J, Spurkland A, et al. Association of the tumour necrosis factor alpha-308 but not the interleukin 10 -627 promoter polymorphism with genetic susceptibility to primary sclerosing cholangitis. Gut. 2001;49:288–294. doi: 10.1136/gut.49.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satsangi J, Chapman RW, Haldar N, et al. A functional polymorphism of the stromelysin gene (MMP-3) influences susceptibility to primary sclerosing cholangitis. Gastroenterology. 2001;121:124–130. doi: 10.1053/gast.2001.25527. [DOI] [PubMed] [Google Scholar]
- 22.O'Mahony CA, Vierling JM. Etiopathogenesis of primary sclerosing cholangitis. Semin Liver Dis. 2006;26:3–21. doi: 10.1055/s-2006-933559. [DOI] [PubMed] [Google Scholar]
- 23.Grant AJ, Lalor PF, Salmi M, et al. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet. 2002;359:150–157. doi: 10.1016/S0140-6736(02)07374-9. [DOI] [PubMed] [Google Scholar]
- 24.Fosby B, Karlsen TH, Melum E. Recurrence and rejection in liver transplantation for primary sclerosing cholangitis. World J Gastroenterol. 2012;18:1–15. doi: 10.3748/wjg.v18.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terjung B, Sohne J, Lechtenberg B, et al. p-ANCAs in autoimmune liver disorders recognise human beta-tubulin isotype 5 and cross-react with microbial protein FtsZ. Gut. 2010;59:808–816. doi: 10.1136/gut.2008.157818. [DOI] [PubMed] [Google Scholar]
- 26.Fickert P, Fuchsbichler A, Wagner M, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Okolicsanyi L, Fabris L, Viaggi S, et al. Primary sclerosing cholangitis: clinical presentation, natural history and prognostic variables: an Italian multicentre study. The Italian PSC Study Group. Eur J Gastroenterol Hepatol. 1996;8:685–691. [PubMed] [Google Scholar]
- 28.Porayko MK, Wiesner RH, LaRusso NF, et al. Patients with asymptomatic primary sclerosing cholangitis frequently have progressive disease. Gastroenterology. 1990;98:1594–1602. doi: 10.1016/0016-5085(90)91096-o. [DOI] [PubMed] [Google Scholar]
- 29.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 30.Talwalkar JA, Lindor KD. Primary sclerosing cholangitis. Inflamm Bowel Dis. 2005;11:62–72. doi: 10.1097/00054725-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200–206. [PubMed] [Google Scholar]
- 32.Angulo P, Peter JB, Gershwin ME, et al. Serum autoantibodies in patients with primary sclerosing cholangitis. J Hepatol. 2000;32:182–187. doi: 10.1016/s0168-8278(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 33.Mulder AH, Horst G, Haagsma EB, et al. Prevalence and characterization of neutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 1993;17:411–417. [PubMed] [Google Scholar]
- 34.Bansi DS, Fleming KA, Chapman RW. Importance of antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis and ulcerative colitis: prevalence, titre, and IgG subclass. Gut. 1996;38:384–389. doi: 10.1136/gut.38.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacCarty RL, LaRusso NF, Wiesner RH, et al. Primary sclerosing cholangitis: findings on cholangiography and pancreatography. Radiology. 1983;149:39–44. doi: 10.1148/radiology.149.1.6412283. [DOI] [PubMed] [Google Scholar]
- 36.Textor HJ, Flacke S, Pauleit D, et al. Three-dimensional magnetic resonance cholangiopancreatography with respiratory triggering in the diagnosis of primary sclerosing cholangitis: comparison with endoscopic retrograde cholangiography. Endoscopy. 2002;34:984–990. doi: 10.1055/s-2002-35830. [DOI] [PubMed] [Google Scholar]
- 37.Angulo P, Pearce DH, Johnson CD, et al. Magnetic resonance cholangiography in patients with biliary disease: its role in primary sclerosing cholangitis. J Hepatol. 2000;33:520–527. doi: 10.1034/j.1600-0641.2000.033004520.x. [DOI] [PubMed] [Google Scholar]
- 38.Berstad AE, Aabakken L, Smith HJ, et al. Diagnostic accuracy of magnetic resonance and endoscopic retrograde cholangiography in primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2006;4:514–520. doi: 10.1016/j.cgh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Talwalkar JA, Angulo P, Johnson CD, et al. Cost-minimization analysis of MRC versus ERCP for the diagnosis of primary sclerosing cholangitis. Hepatology. 2004;40:39–45. doi: 10.1002/hep.20287. [DOI] [PubMed] [Google Scholar]
- 40.Bangarulingam SY, Gossard AA, Petersen BT, et al. Complications of endoscopic retrograde cholangiopancreatography in primary sclerosing cholangitis. Am J Gastroenterol. 2009;104:855–860. doi: 10.1038/ajg.2008.161. [DOI] [PubMed] [Google Scholar]
- 41.Ludwig J. Surgical pathology of the syndrome of primary sclerosing cholangitis. Am J Surg Pathol. 1989;13 Suppl 1:43–49. [PubMed] [Google Scholar]
- 42.Burak KW, Angulo P, Lindor KD. Is there a role for liver biopsy in primary sclerosing cholangitis? Am J Gastroenterol. 2003;98:1155–1158. doi: 10.1111/j.1572-0241.2003.07401.x. [DOI] [PubMed] [Google Scholar]
- 43.Ludwig J, Barham SS, LaRusso NF, et al. Morphologic features of chronic hepatitis associated with primary sclerosing cholangitis and chronic ulcerative colitis. Hepatology. 1981;1:632–640. doi: 10.1002/hep.1840010612. [DOI] [PubMed] [Google Scholar]
- 44.Corpechot C, El Naggar A, Poujol-Robert A, et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118–1124. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- 45.Mendes F, Lindor KD. Primary sclerosing cholangitis: overview and update. Nat Rev Gastroenterol Hepatol. 2010;7:611–619. doi: 10.1038/nrgastro.2010.155. [DOI] [PubMed] [Google Scholar]
- 46.Bjornsson E, Olsson R, Bergquist A, et al. The natural history of small-duct primary sclerosing cholangitis. Gastroenterology. 2008;134:975–980. doi: 10.1053/j.gastro.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 47.Bjornsson E. Small-duct primary sclerosing cholangitis. Curr Gastroenterol Rep. 2009;11:37–41. doi: 10.1007/s11894-009-0006-6. [DOI] [PubMed] [Google Scholar]
- 48.Davit-Spraul A, Gonzales E, Baussan C, et al. The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects. Semin Liver Dis. 2010;30:134–146. doi: 10.1055/s-0030-1253223. [DOI] [PubMed] [Google Scholar]
- 49.Kaya M, Angulo P, Lindor KD. Overlap of autoimmune hepatitis and primary sclerosing cholangitis: an evaluation of a modified scoring system. J Hepatol. 2000;33:537–542. doi: 10.1034/j.1600-0641.2000.033004537.x. [DOI] [PubMed] [Google Scholar]
- 50.Floreani A, Rizzotto ER, Ferrara F, et al. Clinical course and outcome of autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. Am J Gastroenterol. 2005;100:1516–1522. doi: 10.1111/j.1572-0241.2005.41841.x. [DOI] [PubMed] [Google Scholar]
- 51.Beuers U, Rust C. Overlap syndromes. Semin Liver Dis. 2005;25:311–320. doi: 10.1055/s-2005-916322. [DOI] [PubMed] [Google Scholar]
- 52.Bjornsson E, Chari ST, Smyrk TC, et al. Immunoglobulin G4 associated cholangitis: description of an emerging clinical entity based on review of the literature. Hepatology. 2007;45:1547–1554. doi: 10.1002/hep.21685. [DOI] [PubMed] [Google Scholar]
- 53.Ghazale A, Chari ST, Zhang L, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706–715. doi: 10.1053/j.gastro.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Mendes FD, Jorgensen R, Keach J, et al. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101:2070–2075. doi: 10.1111/j.1572-0241.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 55.Burak K, Angulo P, Pasha TM, et al. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523–526. doi: 10.1111/j.1572-0241.2004.04067.x. [DOI] [PubMed] [Google Scholar]
- 56.Kornfeld D, Ekbom A, Ihre T. Survival and risk of cholangiocarcinoma in patients with primary sclerosing cholangitis. A population-based study. Scand J Gastroenterol. 1997;32:1042–1045. doi: 10.3109/00365529709011222. [DOI] [PubMed] [Google Scholar]
- 57.Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology. 2011;54:1842–1852. doi: 10.1002/hep.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Claessen MM, Vleggaar FP, Tytgat KM, et al. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50:158–164. doi: 10.1016/j.jhep.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 59.Charatcharoenwitthaya P, Enders FB, Halling KC, et al. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–1117. doi: 10.1002/hep.22441. [DOI] [PubMed] [Google Scholar]
- 60.Moreno Luna LE, Kipp B, Halling KC, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–1072. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56:1181–1188. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 62.Loftus EV, Jr, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joo M, Abreu-e-Lima P, Farraye F, et al. Pathologic features of ulcerative colitis in patients with primary sclerosing cholangitis: a case-control study. Am J Surg Pathol. 2009;33:854–862. doi: 10.1097/PAS.0b013e318196d018. [DOI] [PubMed] [Google Scholar]
- 64.O'Toole A, Alakkari A, Keegan D, et al. Primary sclerosing cholangitis and disease distribution in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10:439–441. doi: 10.1016/j.cgh.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 65.Sokol H, Cosnes J, Chazouilleres O, et al. Disease activity and cancer risk in inflammatory bowel disease associated with primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3497–3503. doi: 10.3748/wjg.14.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hagness M, Foss A, Line PD, et al. Liver Transplantation for Non-Resectable Liver Metastases from Colorectal Cancer. Liver Transplantation. 2012;18:S140–S140. [Google Scholar]
- 67.Halliday JS, Djordjevic J, Lust M, et al. A unique clinical phenotype of primary sclerosing cholangitis associated with Crohn's disease. J Crohns Colitis. 2012;6:174–181. doi: 10.1016/j.crohns.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 68.Fleshner PR, Vasiliauskas EA, Kam LY, et al. High level perinuclear antineutrophil cytoplasmic antibody (pANCA) in ulcerative colitis patients before colectomy predicts the development of chronic pouchitis after ileal pouch-anal anastomosis. Gut. 2001;49:671–677. doi: 10.1136/gut.49.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lepisto A, Karkkainen P, Jarvinen HJ. Prevalence of primary sclerosing cholangitis in ulcerative colitis patients undergoing proctocolectomy and ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2008;14:775–779. doi: 10.1002/ibd.20384. [DOI] [PubMed] [Google Scholar]
- 70.Wiesner RH, LaRusso NF, Dozois RR, et al. Peristomal varices after proctocolectomy in patients with primary sclerosing cholangitis. Gastroenterology. 1986;90:316–322. doi: 10.1016/0016-5085(86)90926-1. [DOI] [PubMed] [Google Scholar]
- 71.Soetikno RM, Lin OS, Heidenreich PA, et al. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48–54. doi: 10.1067/mge.2002.125367. [DOI] [PubMed] [Google Scholar]
- 72.Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–745. doi: 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 73.Thackeray EW, Charatcharoenwitthaya P, Elfaki D, et al. Colon neoplasms develop early in the course of inflammatory bowel disease and primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2011;9:52–56. doi: 10.1016/j.cgh.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 74.Brandt DJ, MacCarty RL, Charboneau JW, et al. Gallbladder disease in patients with primary sclerosing cholangitis. AJR Am J Roentgenol. 1988;150:571–574. doi: 10.2214/ajr.150.3.571. [DOI] [PubMed] [Google Scholar]
- 75.Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48:598–605. doi: 10.1016/j.jhep.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 76.Lewis JT, Talwalkar JA, Rosen CB, et al. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasiadysplasia- carcinoma sequence. Am J Surg Pathol. 2007;31:907–913. doi: 10.1097/01.pas.0000213435.99492.8a. [DOI] [PubMed] [Google Scholar]
- 77.Eaton JE, Thackeray EW, Lindor KD. Likelihood of malignancy in gallbladder polyps and outcomes following cholecystectomy in primary sclerosing cholangitis. Am J Gastroenterol. 2012;107:431–439. doi: 10.1038/ajg.2011.361. [DOI] [PubMed] [Google Scholar]
- 78.Bunchorntavakul C, Reddy KR. Pruritus in chronic cholestatic liver disease. Clin Liver Dis. 2012;16:331–346. doi: 10.1016/j.cld.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 79.EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 80.Angulo P, Grandison GA, Fong DG, et al. Bone disease in patients with primary sclerosing cholangitis. Gastroenterology. 2011;140:180–188. doi: 10.1053/j.gastro.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guichelaar MM, Kendall R, Malinchoc M, et al. Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transpl. 2006;12:1390–1402. doi: 10.1002/lt.20874. [DOI] [PubMed] [Google Scholar]
- 82.Jorgensen RA, Lindor KD, Sartin JS, et al. Serum lipid and fat-soluble vitamin levels in primary sclerosing cholangitis. J Clin Gastroenterol. 1995;20:215–219. doi: 10.1097/00004836-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 83.Abraham SC, Kamath PS, Eghtesad B, et al. Liver transplantation in precirrhotic biliary tract disease: Portal hypertension is frequently associated with nodular regenerative hyperplasia and obliterative portal venopathy. Am J Surg Pathol. 2006;30:1454–1461. doi: 10.1097/01.pas.0000213286.65907.ea. [DOI] [PubMed] [Google Scholar]
- 84.Culver EL, Chapman RW. Systematic review: management options for primary sclerosing cholangitis and its variant forms - IgG4-associated cholangitis and overlap with autoimmune hepatitis. Aliment Pharmacol Ther. 2011;33:1273–1291. doi: 10.1111/j.1365-2036.2011.04658.x. [DOI] [PubMed] [Google Scholar]
- 85.Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. N Engl J Med. 1997;336:691–695. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 86.Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Triantos CK, Koukias NM, Nikolopoulou VN, et al. Meta-analysis: ursodeoxycholic acid for primary sclerosing cholangitis. Aliment Pharmacol Ther. 2011;34:901–910. doi: 10.1111/j.1365-2036.2011.04822.x. [DOI] [PubMed] [Google Scholar]
- 88.Halilbasic E, Fiorotto R, Fickert P, et al. Side chain structure determines unique physiologic and therapeutic properties of norursodeoxycholic acid in Mdr2−/− mice. Hepatology. 2009;49:1972–1881. doi: 10.1002/hep.22891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tung BY, Emond MJ, Haggitt RC, et al. Ursodiol use is associated with lower prevalence of colonic neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Ann Intern Med. 2001;134:89–95. doi: 10.7326/0003-4819-134-2-200101160-00008. [DOI] [PubMed] [Google Scholar]
- 90.Pardi DS, Loftus EV, Jr, Kremers WK, et al. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–893. doi: 10.1053/gast.2003.50156. [DOI] [PubMed] [Google Scholar]
- 91.Eaton JE, Silveira MG, Pardi DS, et al. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011;106:1638–1645. doi: 10.1038/ajg.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh S, Pardi DS, Loftus EV, et al. Ursodeoxycholic Acid and Risk of Colorectal Neoplasia in Patients with Primary Sclerosing Cholangitis: A Meta-analyis. Inflamm Bowel Dis. 2012;18:S29. doi: 10.1097/MIB.0b013e318286fa61. [DOI] [PubMed] [Google Scholar]
- 93.Kaya M, Petersen BT, Angulo P, et al. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. Am J Gastroenterol. 2001;96:1059–1066. doi: 10.1111/j.1572-0241.2001.03690.x. [DOI] [PubMed] [Google Scholar]
- 94.Johnson GK, Geenen JE, Venu RP, et al. Endoscopic treatment of biliary tract strictures in sclerosing cholangitis: a larger series and recommendations for treatment. Gastrointest Endosc. 1991;37:38–43. doi: 10.1016/s0016-5107(91)70618-4. [DOI] [PubMed] [Google Scholar]
- 95.Cameron JL, Pitt HA, Zinner MJ, et al. Resection of hepatic duct bifurcation and transhepatic stenting for sclerosing cholangitis. Ann Surg. 1988;207:614–622. doi: 10.1097/00000658-198805000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Myburgh JA. Surgical biliary drainage in primary sclerosing cholangitis. The role of the Hepp-Couinaud approach. Arch Surg. 1994;129:1057–1062. doi: 10.1001/archsurg.1994.01420340071012. [DOI] [PubMed] [Google Scholar]
- 97.Baluyut AR, Sherman S, Lehman GA, et al. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. Gastrointest Endosc. 2001;53:308–312. doi: 10.1016/s0016-5107(01)70403-8. [DOI] [PubMed] [Google Scholar]
- 98.Graziadei IW, Wiesner RH, Marotta PJ, et al. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology. 1999;30:1121–1127. doi: 10.1002/hep.510300501. [DOI] [PubMed] [Google Scholar]
- 99.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98. e3. doi: 10.1053/j.gastro.2012.04.008. quiz e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Egawa H, Taira K, Teramukai S, et al. Risk factors for recurrence of primary sclerosing cholangitis after living donor liver transplantation: a single center experience. Dig Dis Sci. 2009;54:1347–1354. doi: 10.1007/s10620-009-0773-9. [DOI] [PubMed] [Google Scholar]
- 101.Graziadei IW, Wiesner RH, Batts KP, et al. Recurrence of primary sclerosing cholangitis following liver transplantation. Hepatology. 1999;29:1050–1056. doi: 10.1002/hep.510290427. [DOI] [PubMed] [Google Scholar]
- 102.Alabraba E, Nightingale P, Gunson B, et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15:330–340. doi: 10.1002/lt.21679. [DOI] [PubMed] [Google Scholar]
- 103.Haagsma EB, Van Den Berg AP, Kleibeuker JH, et al. Inflammatory bowel disease after liver transplantation: the effect of different immunosuppressive regimens. Aliment Pharmacol Ther. 2003;18:33–44. doi: 10.1046/j.1365-2036.2003.01613.x. [DOI] [PubMed] [Google Scholar]
- 104.Verdonk RC, Dijkstra G, Haagsma EB, et al. Inflammatory bowel disease after liver transplantation: risk factors for recurrence and de novo disease. Am J Transplant. 2006;6:1422–1429. doi: 10.1111/j.1600-6143.2006.01333.x. [DOI] [PubMed] [Google Scholar]
- 105.LaRusso NF, Shneider BL, Black D, et al. Primary sclerosing cholangitis: summary of a workshop. Hepatology. 2006;44:746–764. doi: 10.1002/hep.21337. [DOI] [PubMed] [Google Scholar]
- 106.Dickson ER, Murtaugh PA, Wiesner RH, et al. Primary sclerosing cholangitis: refinement and validation of survival models. Gastroenterology. 1992;103:1893–1901. doi: 10.1016/0016-5085(92)91449-e. [DOI] [PubMed] [Google Scholar]
- 107.Kim WR, Therneau TM, Wiesner RH, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc. 2000;75:688–694. doi: 10.4065/75.7.688. [DOI] [PubMed] [Google Scholar]
- 108.Rudolph G, Gotthardt D, Kloters-Plachky P, et al. Influence of dominant bile duct stenoses and biliary infections on outcome in primary sclerosing cholangitis. J Hepatol. 2009;51:149–155. doi: 10.1016/j.jhep.2009.01.023. [DOI] [PubMed] [Google Scholar]