Abstract
Herpes simplex virus type 1 (HSV-1) glycoprotein gE functions as an immunoglobulin G (IgG) Fc receptor (FcγR) that promotes immune evasion. When an IgG antibody binds by the F(ab′)2 domain to an HSV antigen, the Fc domain of some of the same antibody molecules binds to the FcγR, which blocks Fc-mediated functions. gE is a type 1 membrane glycoprotein with a large ectodomain that is expressed on the virion envelope and infected-cell surface. Our goal was to determine if immunizing with gE protein fragments could produce antibodies that bind by the F(ab′)2 domain to gE and block the FcγR, as measured by competitively inhibiting nonimmune human IgG binding to the FcγR. Three gE peptides were constructed in baculovirus spanning almost the entire ectodomain and used to immunize mice and rabbits. Two fragments were highly effective at producing antibodies that bind by the F(ab′)2 domain and block the FcγR. The most potent of these two antibodies was far more effective at blocking the FcγR than antibodies that are only capable of binding by the Fc domains to the FcγR, including anti-gC, anti-gD, and nonimmune IgG. These results suggest that immunizing with gE fragments has potential for preventing immune evasion by blocking activities mediated by the HSV-1 FcγR.
Viruses have evolved diverse immune evasion strategies to survive in their natural hosts (43). HSV-1 encodes two glycoproteins, gC and gE, that target the humoral immune system (24). gC binds complement component C3b and blocks properdin (P) and C5 binding to C3b (12, 14). gE forms a noncovalent heterodimer complex with glycoprotein I (gI) that functions as an immunoglobulin G (IgG) Fc receptor (FcγR) (8, 11, 18, 19). Interactions between gE and gI increase Fc binding affinity because the gE/gI complex binds monomeric IgG, whereas gE alone binds IgG complexes but not monomers (7).
We previously showed that an IgG molecule targeted at a herpes simplex virus type 1 (HSV-1) membrane glycoprotein, such as gC or gD, binds by its F(ab′)2 domain to the antigen, while the Fc region of the same IgG molecule binds to the gE/gI complex to form an antibody bridge (11). Through antibody bridging, the FcγR inhibits IgG Fc-mediated activities, including C1q binding, antibody-dependent cellular cytotoxicity, and IgG binding to mammalian FcγR expressed on granulocytes (8, 45). Our studies to define the role of HSV-1 gE in immune evasion demonstrated that a gE mutant virus that does not bind IgG Fc is more susceptible to complement-enhanced antibody neutralization and antibody-dependent cellular cytotoxicity in vitro and is approximately 50-fold more susceptible to antibody and complement in vivo (8, 30).
Glycoproteins gC and gE inhibit different steps of the complement cascade; the former targets C3b, and the latter blocks C1q binding. Together, these two glycoproteins inhibit the complement cascade far more effectively than either alone, both in vitro and in vivo (25). We previously reported that blocking gC immune evasion domains reduces HSV-1 virulence (20). Recently, antibodies to pseudorabies virus were reported to block the pseudorabies virus FcγR (44). We now examined whether antibodies produced to gE can block IgG Fc binding to the HSV-1 FcγR. Three peptide fragments that span almost the entire HSV-1 gE ectodomain were expressed in baculovirus and used as immunogens. Antibodies produced to two gE fragments blocked nonimmune human IgG binding to the HSV-1 FcγR. The blocking activity was mediated most effectively by the anti-gE IgG F(ab′)2 domain; however, the Fc domain also contributed to blocking.
Other human pathogens encode FcγRs, including HSV-2, pseudorabies virus, varicella-zoster virus, cytomegalovirus, protozoa (schistosomes and trypanosomes), and bacteria (staphylococci and streptococci) (2-5, 9, 10, 21, 23, 26, 32, 33, 37, 39, 41, 46). Therefore, exploring means to block functions mediated by the HSV-1 FcγR may have broad implications for reducing virulence of many microbial pathogens.
MATERIALS AND METHODS
Cell cultures and virus strains.
COS-1 cells were grown at 37°C in 5% CO2 in an humidified incubator in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum, 20 μg of gentamicin per ml, and 20 mM HEPES (pH 7.3). Cells were infected with HSV-1 wild-type strain NS (13). Virus pools were prepared with African green monkey kidney (Vero) cells.
Construction of bac-gE24-224, bac-gE225-398, and bac-gE24-409 viruses.
Baculoviruses bac-gE24-224, bac-gE225-398, and bac-gE24-409 were constructed with ThermalAce DNA polymerase (Invitrogen Corp., Carlsbad, Calif.) PCR to amplify gE amino acids 24 to 224, 225 to 398, and 24 to 409 from pCMV3-gE (1). A six-histidine tag was incorporated into the 3′ primer in front of a stop codon. BamHI and PstI sequences were included on the 5′ and 3′ primers, respectively, and the BamHI-PstI fragment was cloned into pVT-Bac with a rapid DNA ligation kit (Roche Diagnostics, Indianapolis, Ind.) (42). This cloning strategy placed gE24-224, gE225-398, and gE24-409 sequences immediately 3′ of the honeybee mellitin signal sequence and under the control of the baculopolyhedrin promoter. Sf9 insect cells (Gibco-BRL, Grand Island, N.Y.) were cotransfected with pVT-Bac constructs and Baculogold DNA (PharMingen, San Diego, Calif.) to produce bac-gE24-224, bac-gE225-398, and bac-gE24-409 viruses.
Purification and identification of gE24-224, gE225-398, and gE24-409.
Baculoviruses were grown in Sf9 insect cells for the first three passages and in H5 insect cells (Gibco BRL) to obtain higher yields of protein expression at passage four. The supernatants from 150 ml of infected H5 cells were tested for protein expression by Western blot and used for protein purification. The supernatant fluids were passed through a nickel column (Qiagen Inc., Valencia, Calif.) and eluted with 50 to 250 mM imidazole. The eluted fragments were concentrated with an Ultrafree-15 centrifugal filter device (Millipore Corp., Bedford, Mass.), and identified by sodium dodecyl sulfate-4 to 15% polyacrylamide gel electrophoresis (SDS-PAGE) with GelCode Blue Stain Reagent (Pierce). Samples of the purified proteins were electrophoresed on SDS-4 to 15% PAGE, transferred to a nitrocellulose membrane, and probed with a mouse monoclonal antibody against the histidine hexamer tag, monoclonal antibody 1BA10, that recognizes gE sequences between amino acids 103 and 120, or rabbit polyclonal antibody R575 produced against gE amino acids 1 to 409 (1, 6, 30).
Immunizations with gE fragments.
Five 8- to 9-week-old female BALB/c mice (Charles River Laboratories, Wilmington, Mass.) were immunized intraperitoneally with 10 μg of purified gE24-224, gE225-398, or gE24-409, and three mice were injected with phosphate-buffered saline as controls. Complete Freund's adjuvant was used for primary immunizations and incomplete Freund's adjuvant for booster injections at intervals of 10 to 14 days. Serum was collected 2 weeks after the third to fifth immunization. Two rabbits each were immunized with purified gE24-224, gE225-398, and gE24-409 (Cocalico Biologicals, Inc., Reamstown, Pa.), and serum was collected after the fourth to sixth immunization.
Preparation of rabbit IgG and F(ab′)2 fragments.
Rabbit preimmune and immune IgGs were purified through HiTrap protein G HP columns (Amersham Biosciences AB, Uppsala, Sweden). IgG was purified from R118 anti-gC and R122 anti-gD rabbit sera. These antibodies were produced by immunizing rabbits with baculovirus proteins comprising the ectodomains of HSV-1 gC (gC467t) and gD (gD306t) (38, 40). IgG F(ab′)2 fragments were generated from IgG with ImmunoPure F(ab′)2 preparation kits (Pierce, Rockford, Ill.) according to the manufacturer's instructions.
Antibody detection by ELISA.
Purified gE24-224, gE225-398, and gE24-409 fragments were diluted to 2 μg/ml in Dulbecco's phosphate-buffered saline (DuPBS, pH 7.1), and 200 ng was used to coat Covalink NH 96 well-microtiter plates (Nalge Nunc International, Naperville, Ill.). The coated plates were incubated overnight at 4°C, and blocked for 2 h at 37°C with 5% (wt/vol) nonfat milk in DuPBS. IgG samples were diluted to 2 μg/100 μl in DuPBS and 0.1% bovine serum albumin. A 1:1,000 dilution of horseradish peroxidase-conjugated, affinity-purified donkey anti-rabbit IgG (H+L) or sheep anti-mouse IgG (H+L) (Amersham Life Science, Piscataway, N.J.) was prepared in DuPBS and 0.01% bovine serum albumin. The reaction was developed by adding 1 mg of ABTS per ml in buffer solution (Roche, Mannheim, Germany) for 10 min at room temperature, and the optical density (OD) was measured at 405 nm with an enzyme-linked immunosorbent assay (ELISA) plate reader (Dynatech, Chantilly, Va.).
Flow cytometry assays to determine whether antibodies block IgG Fc binding to HSV-1-infected cells.
The assay included two components: first measuring whether antibodies bind to HSV-1-infected cells, and then determining if the bound antibodies block biotin-labeled nonimmune human IgG binding to the HSV-1 FcγR. For antibody binding, COS-1 cells were infected with wild-type virus at a multiplicity of infection of 2 for 16 h, dispersed with cell dissociation buffer (Life Technologies Inc., Rockville, Md.), and treated with 0.05U neuraminidase (Sigma Chemical Co., St. Louis, Mo.), which enhances IgG Fc binding (29). Binding of IgG antibodies to HSV-1-infected cell surfaces was measured with fluorescein isothiocyanate-conjugated F(ab′)2 fragments against mouse or rabbit IgG or against rabbit IgG F(ab′)2 fragments. Cells were fixed with 1% paraformaldehyde and analyzed by FACScan flow cytometry (Becton-Dickinson, San Jose, Calif.). Antibody binding was reported as mean fluorescence intensity (MFI). For assays to measure blocking of IgG binding, neuraminidase-treated HSV-1-infected cells were incubated with IgG or F(ab′)2 fragments and then 10 μg of biotin-labeled nonimmune human IgG was added, which was detected with streptavidin-R-phycoerythrin (Sigma) (36). The percent blocking was calculated as [(MFI without blocking antibody − MFI with blocking antibody)/MFI without blocking antibody] × 100.
RESULTS
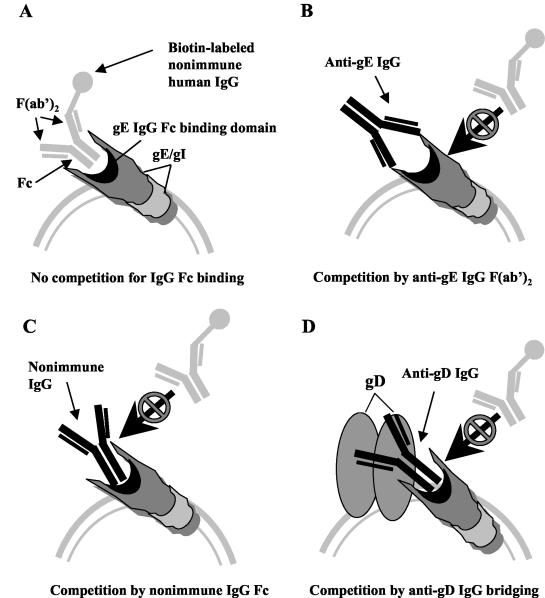
IgG molecules potentially can bind to the HSV-1 FcγR by either the Fc domains or the F(ab′)2 domains, the latter if the antibodies are directed against gE or gI. Binding by the Fc domain occurs when nonimmune IgG binds to the HSV-1 FcγR or when antibody bridging develops, which refers to the F(ab′)2 domain binding to its target, such as gC or gD, and the Fc domain of the same antibody molecule binding to the HSV-1 FcγR. The goal of our study was to determine whether gE immunization can induce antibodies that bind to gE by the F(ab′)2 domains and effectively compete with nonimmune human IgG for binding to the HSV-1 FcγR (Fig. 1, model B).
FIG. 1.
Potential mechanisms for blocking biotin-labeled nonimmune human IgG binding to the HSV-1 FcγR (gE/gI). (A) In the absence of competition, biotin-labeled nonimmune human IgG binds with its Fc domain to the HSV-1 FcγR. (B) The anti-gE IgG F(ab′)2 domain binds to gE and competes with biotin-labeled nonimmune human IgG for binding to the HSV-1 FcγR. (C) Unlabeled nonimmune IgG competes with biotin-labeled nonimmune human IgG for binding to the HSV-1 FcγR. (D) Antibody bridging by IgG directed against another glycoprotein (shown as gD) competes with biotin-labeled nonimmune IgG. The IgG F(ab′)2 domain binds to gD and the IgG Fc domain binds to the HSV-1 FcγR.
Production of gE fragments in baculovirus.
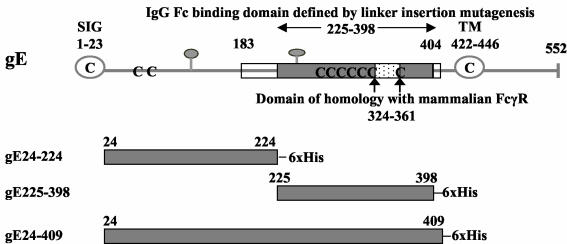
Three gE fragments were prepared that span almost the entire gE ectodomain, gE24-224, gE225-398, and gE24-409 (Fig. 2). Our prior studies used two approaches to define the gE IgG Fc binding domain. One method involved constructing gE-1/gD-1 fusion proteins, which localized the IgG Fc binding domain between gE amino acids 183 and 404, while the other approach used linker insertion mutagenesis and identified amino acids 225 to 398 as required for IgG Fc binding (6). The amino acid boundaries of the gE fragments shown in Fig. 2 were selected based on the linker insertion mutagenesis studies; however, we were uncertain which fragment was likely to be the best immunogen.
FIG. 2.
Features of HSV-1 gE. Stick diagram of HSV-1 strain NS gE (top) showing the signal peptide (SIG, open circle), transmembrane domain (TM, open circle), potential N-linked glycosylation sites (gray balloons), and cysteine residues (C). The gray shaded box indicates the margins of the IgG Fc binding domain as defined by linker insertion mutagenesis (amino acids 225 to 398), the white boxes show the amino and carboxyl termini outer margins of the IgG Fc binding domain based on studies with gE/gD fusion proteins (amino acids 183 to 404), and the white speckled box marks the domain of homology with mammalian FcγRs (6). The gray shaded boxes (bottom three figures) represent the regions of HSV-1 gE expressed in baculovirus, which include gE protein coding sequences from amino acids 24 to 224 (gE24-224), 225 to 398 (gE225-398), and 24 to 409 (gE24-409). 6× His, six histidine residues added at the carboxyl terminus. The amino acid sequence of the ectodomain of NS gE differs from the published HSV-1 strain 17 gE sequence in that NS gE contains an insertion of two amino acids (Gly and Glu) at positions 186 and 187 (H. M. Friedman, unpublished observation) (27). The numbering system used in the current paper differs from that in our previous publications based on these two added amino acids (1, 6, 30).
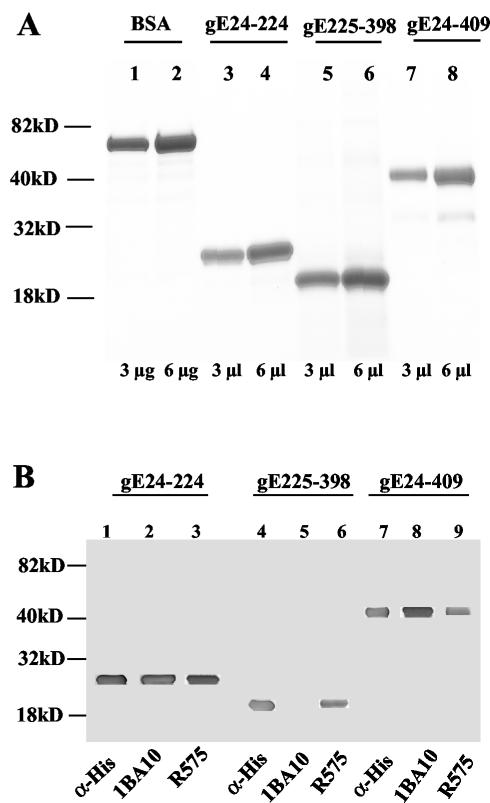
Supernatant fluids of baculovirus-infected cells yielded 6 to 8 mg of fragments gE24-224, gE225-398, and gE24-409 per liter, which were purified on a nickel column and concentrated to 1 mg/ml. GelCode Blue staining on SDS-PAGE estimated purity at ≥95% (Fig. 3A). The gE24-224 and gE24-409 fragments reacted with anti-His monoclonal antibody, anti-gE monoclonal antibody 1BA10 and rabbit polyclonal antibody R575. Fragment gE225-398 reacted with anti-His monoclonal antibody, rabbit polyclonal antibody R575, and as expected, it did not bind monoclonal antibody 1BA10, which recognizes amino acid sequences between 103 and 120 that were not included in this fragment (Fig. 3B) (1, 6).
FIG. 3.
SDS-PAGE and Western blot of bac-gE fragments. (A) Purified fragments secreted from H5 cells infected with bac-gE24-224, bac-gE225-398, and bac-gE24-409 viruses were identified with GelCode Blue Stain Reagent by SDS-4 to 15% PAGE. Molecular mass markers are shown on the left. (A) Lanes 1 and 2, bovine serum albumin (BSA) at 3 μg and 6 μg, respectively; lanes 3 and 4, 5 and 6, and 7 and 8, purified gE24-224, gE225-398, and gE24-409 preparations at volumes of 3 μl and 6 μl, respectively. (B) Western blot of bac-gE fragments. Lanes 1 to 3, 4 to 6, and 7 to 9, purified gE24-224, gE225-398, and gE24-409, respectively, probed with anti-His monoclonal antibody (lanes 1, 4, and 7), anti-gE monoclonal antibody 1BA10 that detects gE sequences between amino acids 103 and 120 (lanes 2, 5, and 8), and rabbit anti-gE polyclonal antibody R575 (lanes 3, 6, and 9).
Baculovirus gE immunization of mice and rabbits.
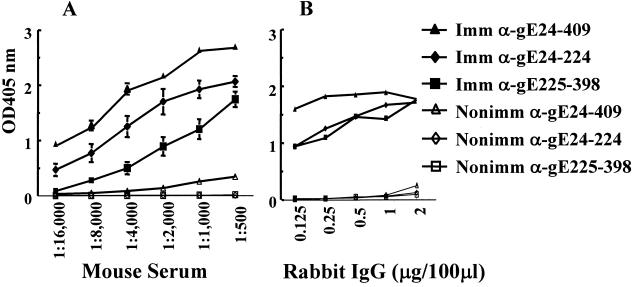
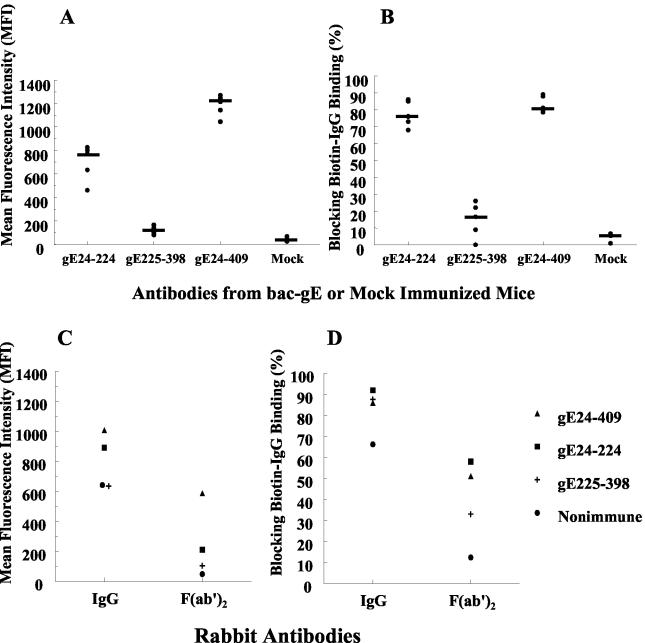
Studies were performed to determine if antibodies produced to the various gE baculovirus fragments bind to gE and block IgG Fc binding to the HSV-1 FcγR. Mice were immunized with gE24-224, gE225-398, or gE24-409 or mock-immunized as controls. Mouse serum was collected after the fifth immunizations with gE24-224 and gE225-398 or third immunizations with gE24-409 and tested for antibody titers by ELISA against the corresponding immunogens. Mice produced antibodies that reacted with the immunizing antigen, while mock-immunized control mice failed to produce antibodies against the three gE fragments (Fig. 4A). Rabbits were immunized six times with gE24-224 or gE225-398 and four times with gE24-409. IgG was purified and tested by ELISA against the corresponding inducing immunogen. Antibodies reacted with the immunogen for each of the gE fragments, while rabbit nonimmune IgG failed to react with any of the gE fragments (Fig. 4B). Generally, antibody levels were highest against the gE24-409 fragment.
FIG. 4.
Studies with murine and rabbit antibodies. (A) Antibodies in mouse serum measured against the inducing immunogen. Data are plotted as means ± standard error of the mean of five mice immunized with bac-gE fragments or three mock-immunized mice. (B) Rabbit IgG measured against the inducing immunogen (n = 1, 2, and 2 for gE24-224, gE225-398, and gE24-409 IgG, respectively). Imm, immune; Nonimm, nonimmune.
Blocking the HSV-1 FcγR with mouse antibodies.
Flow cytometry assays were performed 16 h postinfection to evaluate whether antibodies bind to gE expressed on HSV-1-infected cells. Antibodies were used at a 1:10 dilution of serum, which is a concentration 50-fold higher than that used in Fig. 4A. Even at this high concentration, antibodies from gE225-398-immunized mice showed low levels of binding, which were only slightly greater than serum from mock-immunized mice. In contrast, antibodies from gE24-224- and gE24-409-immunized mice bound to gE (Fig. 5A). Unlike human or rabbit IgG, murine IgG Fc does not bind to the HSV-1 FcγR; therefore, binding by mouse antibodies can only be mediated by the IgG F(ab′)2 domain (17). Therefore, these results indicate that the gE24-224 and gE24-409 F(ab′)2 antibody domains bind to gE and that gE225-398 produces antibodies that barely react with gE expressed on infected cells.
FIG. 5.
Murine and rabbit antibody binding to gE and blocking biotin-labeled nonimmune human IgG binding to the FcγR. (A) Infected cells were incubated with serum from immunized mice (n = 5 per group) or mock-immunized controls (n = 3). Serum (1:10 dilution) was evaluated for binding to gE on infected cells by flow cytometry. (B) Serum (undiluted) was added to infected cells to block binding of 10 μg of biotin-labeled nonimmune human IgG. Bars indicate median values for each group. (C) Rabbit anti-gE IgG (2 μg) and F(ab′)2 (1.4 μg) fragment binding to gE was measured by flow cytometry. Results are the average of four to seven determinations for IgG and two determinations for F(ab′)2 assays. (D) Rabbit anti-gE IgG (20 μg) or F(ab′)2 fragments (14 μg) were incubated with infected cells prior to adding 10 μg of biotin-labeled nonimmune human IgG. Results are the mean of three to five determinations for IgG and one determination for F(ab′)2 fragments.
Experiments were performed to determine if antibodies produced after mouse immunizations block binding of biotin-labeled nonimmune human IgG to the HSV-1 FcγR. Mouse serum was used undiluted in these blocking assays. If antibodies in mouse serum bind to gE and block the FcγR, reduced levels of biotin-IgG binding should be detected (see Fig. 1B) compared with controls (see Fig. 1A). Antibodies produced to gE24-224 and gE24-409 were effective at blocking the HSV-1 FcγR (median blocking of 76% and 80%, respectively), while antibodies to gE225-398 showed little blocking (median blocking of 17%), despite being used as undiluted serum (Fig. 5B). Therefore, antibodies to gE24-224 and gE24-409 bind to gE by their F(ab′)2 domains and block IgG Fc binding to the HSV-1 FcγR.
Blocking the HSV-1 FcγR with rabbit antibodies.
Studies were performed with IgG purified from immunized rabbits to further examine the mechanisms by which antibodies competitively inhibit binding of biotin-labeled nonimmune human IgG to the HSV-1 FcγR. Since rabbit anti-gE IgG can bind to the HSV-1 FcγR by either the F(ab′)2 or Fc domain, each of the mechanisms for blocking the HSV-1 FcγR shown in Fig. 1B to 1D is potentially possible, including antibody bridging where the IgG F(ab′)2 domain binds to one gE molecule and the IgG Fc domain binds to the HSV-1 FcγR on another gE molecule (11). Rabbit IgG was added at a concentration of 2 μg per 106 infected cells. At this IgG concentration, the three anti-gE antibodies showed comparable binding by ELISA (Fig. 4B). IgG produced to gE24-409, gE24-224, and gE225-398 and nonimmune IgG each bound to HSV-1-infected cells, as detected by flow cytometry, although gE24-409 and gE24-224 IgG showed greater binding than the other two IgG molecules (Fig. 5C).
IgG F(ab′)2 fragments were added to infected cells (1.4 μg per 106 infected cells) to examine whether binding was mediated at least partially by the F(ab′)2 domain. Binding was greatest with F(ab′)2 fragments from rabbits immunized with gE24-409, while gE24-224 showed less binding, and gE225-398 and nonimmune F(ab′)2 fragments showed little or no binding (Fig. 5C). These results are consistent with those shown in Fig. 5A in that binding was greatest for the gE24-409 F(ab′)2 domain, while the gE225-398 F(ab′)2 domain showed little binding. These results also indicate that rabbit IgG binds by the Fc domain, since nonimmune IgG binds while F(ab′)2 fragments fail to bind.
Rabbit IgG and F(ab′)2 fragments were then evaluated for their ability to block IgG Fc binding to the HSV-1 FcγR. IgG (20 μg per 106 infected cells) produced to all three gE fragments competed for binding of 10 μg of biotin-labeled nonimmune human IgG (blocking by 86% to 92%), as did 20 μg of nonimmune rabbit IgG (blocking by 67%) (Fig. 5D). IgG F(ab′)2 fragments (14 μg per 106 infected cells) to gE24-224 and gE24-409 were also effective at blocking IgG Fc binding to the FcγR (blocking by 59% and 51%, respectively), while gE225-398 F(ab′)2 fragments had low levels of blocking activity (33%), which is consistent with low binding noted in Fig. 5C. Nonimmune F(ab′)2 fragments showed little blocking (14%); however, the fact that any blocking occurred suggests that some intact IgG remained in the F(ab′)2 preparation (Fig. 5D). These results indicate that rabbit IgG can compete with nonimmune human IgG for binding to the HSV-1 FcγR by the Fc domain (results with nonimmune IgG) and by the F(ab′)2 domain (results with gE24-224 and gE24-409 F[ab′]2 fragments). Therefore, the mechanisms shown in Fig. 1B and 1C are clearly operative. Whether antibody bridging between two gE molecules also contributes to blocking biotin-labeled IgG binding cannot be determined from these experiments.
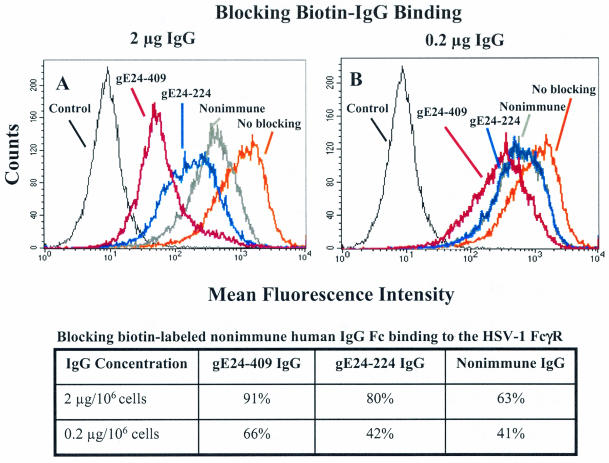
Additional studies were performed to compare the blocking activity of rabbit gE24-224, gE24-409, and nonimmune IgG with IgG concentrations of 2 μg and 0.2 μg per 106 cells, which are 10-fold and 100-fold lower, respectively, than used in Fig. 5D. These IgG concentrations were used to block binding of 10 μg of biotin-labeled nonimmune human IgG. At 2 μg, gE24-409 IgG was more effective than gE24-224 IgG, and both were more active than nonimmune IgG (Fig. 6A). The blocking activities of anti-gE IgG were less at 0.2 μg; however, differences were still detected comparing gE24-409 and gE24-224 IgG (Fig. 6B). At this lower IgG concentration, no differences were noted between gE24-224 IgG and nonimmune IgG (Fig. 6B). Results are shown in tabular form below the flow cytometry figures to facilitate comparison with results shown in Fig. 5D that used higher concentrations of rabbit IgG (20 μg). The results at the two lower IgG concentrations suggest that gE24-409 IgG is more effective at blocking IgG Fc binding to the HSV-1 FcγR than gE24-224 IgG. Blocking by gE24-409 IgG at 0.2 μg (66%) was comparable to preimmune IgG at 100-fold higher concentrations (67%, Fig. 5D), which indicates that large differences exist comparing blocking activity of rabbit anti-gE and nonimmune IgG.
FIG. 6.
Flow cytometry assays to measure blocking of biotin-labeled nonimmune human IgG binding to the HSV-1 FcγR by rabbit gE24-224, gE24-409, or nonimmune IgG. Infected cells were incubated with 2 μg of IgG per 106 cells (A) or 0.2 μg of IgG per 106 cells (B). The no-blocking curve refers to binding of biotin-labeled nonimmune human IgG in the absence of blocking antibody. Control, unstained cells. The table displays results as percent blocking.
Comparisons between rabbit anti-gE, anti-gC, and anti-gD IgG.
We compared the ability of IgG antibody produced to the ectodomains of gC, gD, and gE to block biotin-labeled nonimmune human IgG binding to the HSV-1 FcγR. As discussed above, anti-gE IgG blocks the FcγR by both the F(ab′)2 and Fc domains. Anti-gC or anti-gD IgG can only block by the Fc domain; however, these IgG molecules are capable of antibody bridging, since their F(ab′)2 domains are targeted against other glycoproteins (see Fig. 1D). This series of experiments examined whether antibodies that can bind to gE by their F(ab′)2 domains are more effective at blocking biotin-labeled nonimmune human IgG binding than antibodies directed at other glycoproteins, such as gC or gD, that can only bind by their Fc domains.
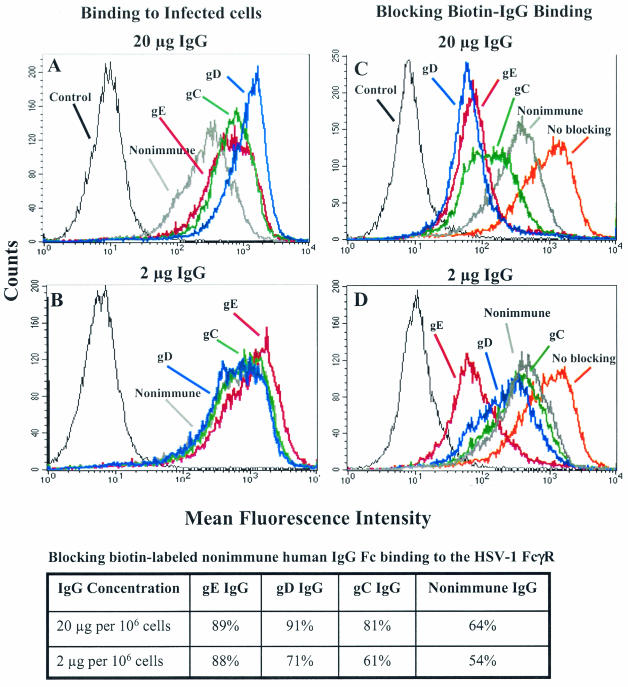
At 20 μg per 106 cells, gE IgG and gC IgG had comparable levels of binding, while gD IgG showed greater binding to HSV-1-infected cells (Fig. 7A). At 2 μg all IgGs bound to infected cells at similar levels (Fig. 7B). At 20 μg, gE and gD IgGs were approximately equally effective at blocking 10 μg of biotin-labeled nonimmune human IgG binding to the HSV-1 FcγR, and more active than gC or nonimmune IgG (Fig. 7C). At 2 μg, gE IgG showed greater blocking activity than gD, gC, or nonimmune IgG (Fig. 7D). Results of blocking experiments are shown in tabular form below the figures. At 2 μg, gE IgG blocked biotin-labeled nonimmune human IgG binding comparable to gD or gC IgG used at 10-fold higher concentrations. These results indicate that F(ab′)2 binding to gE is more effective at blocking the HSV-1 FcγR than nonimmune IgG Fc binding or Fc binding by antibody bridging.
FIG. 7.
Flow cytometry assays to measure rabbit antibody binding to HSV-1-infected cells and blocking of biotin-labeled nonimmune human IgG binding to the FcγR. (A and B) Binding of rabbit anti-gC, anti-gD, anti-gE, or nonimmune IgG at 20 μg and 2 μg. (C and D) Blocking of biotin-labeled nonimmune human IgG binding to the HSV-1 FcγR by anti-gC, anti-gD, anti-gE, or nonimmune IgG at 20 μg and 2 μg. Antibody to gE24-409 was used as the anti-gE IgG. The no-blocking curve refers to binding of biotin-labeled nonimmune human IgG in the absence of blocking antibody. Control, unstained cells. The table displays the results shown in panel C and D as percent blocking.
DISCUSSION
This report presents evidence that immunization with baculovirus gE fragments can produce antibodies that block nonimmune human IgG binding to the HSV-1 FcγR. Immunogens gE24-224 and gE24-409 were more effective than gE225-398, which was chosen because of the high concentration of FcγR sequences within this fragment. Hopp-Woods antigenicity analysis suggests that the most immunogenic gE ectodomain is located between amino acids 160 and 197 (included in gE24-224 and gE24-409), while the region from amino acids 225 to 398 has low predicted immunogenicity (16). The gE 225-to-398 domain includes seven of nine cysteine residues in the ectodomain; therefore, this fragment may have assumed an abnormal conformation because of faulty disulfide bonding. However, the fragment was secreted into the supernatant fluids of baculovirus-infected cells, suggesting that conformation was sufficiently maintained for the protein to traverse the secretory pathway. An alternative explanation for low immunogenicity comes from studies of envelope glycoproteins of human and simian immunodeficiency viruses, where N-linked carbohydrates or conformational changes in the protein mask critical epitopes (22, 34). Despite the blunted responses to gE225-398, results with the other two gE peptides support the hypothesis that gE immune evasion domains can be blocked by antibody F(ab′)2 fragments.
Studies with mouse and rabbit antibodies defined several mechanisms by which gE IgG binds to the HSV-1 FcγR and competes for binding of biotin-labeled nonimmune human IgG. These mechanisms include gE IgG F(ab′)2 binding to domains involved in FcγR activity, nonimmune IgG Fc binding, and antibody bridging from HSV-1 membrane glycoproteins, such as gC and gD, to gE/gI. It is possible that antibody bridging also occurs from one gE/gI complex to another; however, no conclusions can be reached based on the current study. The results shown in Fig. 7C support our previous findings that antibody bridging blocks the FcγR more effectively than nonimmune IgG, since both gC and gD are more active than nonimmune IgG (11).
The current findings indicate that gE IgG is effective at blocking the FcγR at approximately 10-fold lower IgG concentrations than gC or gD IgG, and approximately 100-fold lower concentrations than nonimmune IgG. These results suggest that targeting gE domains by immunization is an effective approach to produce FcγR-blocking antibodies. gE-mediated immune evasion contributes significantly to HSV-1 pathogenesis (25, 30). The Fc portion of IgG plays an important role in antibody-mediated host defense against HSV-1 infection, since mice passively immunized with rabbit or human HSV-1 IgG survive lethal HSV-1 challenge, while those given (Fab′)2 portions are not protected (15, 28, 31). Targeting gE domains to prevent antibody bridging of HSV IgG may enable the IgG Fc portions to function in antibody-dependent cellular cytotoxicity and complement activation, which would improve host defense against the virus.
Our immunization studies in mice and rabbits indicate that the gE24-224 fragment induces antibodies that block nonimmune human IgG binding to the HSV-1 FcγR. These findings suggest that the region of HSV-1 gE required for FcγR activity may extend further towards the N terminus than originally defined with linker insertion mutagenesis (6). Results from the current study support our findings with gE/gD fusion peptides that mapped the FcγR domain on gE as located between residues 183 and 404 (6). Rizvi et al. used limited proteolytic analysis to study the interaction between gE and gI (35). They found that the N-terminal domain of HSV-1 gE forms a stable complex with gI, and that this gE/gI complex binds the Fc domain of IgG. Only the first 188 amino acids of gE were required for this activity. In our nomenclature, we include the signal peptide in the numbering; therefore, residue 188 of Rizvi et al. is equivalent to amino acid 210 in our reports. The Rizvi et al. findings are consistent with our current results and suggest that the gE FcγR domain includes amino acids upstream of gE225-398. Resolving the crystal structure of IgG Fc binding to the gE/gI complex will be required to definitively establish the FcγR binding sites.
An unexpected result from our studies was the finding that the gE24-224 fragment is a potential candidate immunogen for future vaccine studies. This baculovirus fragment was initially selected to serve as a “negative control” for the gE225-398 fragment, which we expected to be the preferred immunogen. Although the gE24-224 fragment was less effective at blocking the FcγR than gE24-409, a possible advantage of the gE24-224 immunogen is the exclusion of the domain of homology with mammalian FcγRs (amino acids 324 to 361). Therefore, this fragment does not carry the risk of causing autoimmunity via cross-reacting antibodies. Additional studies are required to evaluate whether gE24-409 or gE225-398 actually induce cross-reacting antibodies.
Future studies can evaluate the role of gE immunization in preventing gE-mediated immune evasion in animal models. If successful, pilot studies may be warranted in primates. Our future goal is to block both gC and gE immune evasion domains by immunization. We propose adding gE and gC to other HSV-1 immunogens, such as gD, with the goal of inducing potent T-cell and B-cell immunity and blocking the ability of virus to evade antibody and complement responses. Such vaccines provide a novel approach for prevention or treatment of HSV-1 infections in humans.
Acknowledgments
This work was supported by Public Health Service grants AI 33063 from NIAID, HL 28220 from NHLBI, and DE 14152 from NIDCR. Xiaoqing Lin was a trainee on NIH grant T32 AR 07490 (to N. A. Kefalides).
We thank Zhimei Wang and Ming Jiang for technical assistance and Roselyn Eisenberg and Gary Cohen for critical assessment of the manuscript and for providing rabbit antibodies R118 and R122 to gC and gD, respectively.
REFERENCES
- 1.Basu, S., G. Dubin, M. Basu, V. Nguyen, and H. M. Friedman. 1995. Characterization of regions of herpes simplex virus type 1 glycoprotein E involved in binding the Fc domain of monomeric IgG and in forming a complex with glycoprotein I. J. Immunol. 154:260-267. [PubMed] [Google Scholar]
- 2.Bjorck, L., and G. Kronvall. 1984. Purification and some properties of streptococcal protein G, a novel IgG-binding reagent. J. Immunol. 133:969-974. [PubMed] [Google Scholar]
- 3.Christensen, P., B. G. Johansson, and G. Kronvall. 1976. Interaction of streptococci with the Fc fragment of IgG. Acta Pathol. Microbiol. Scand. Sect. C Immunol. 84:73-76. [DOI] [PubMed] [Google Scholar]
- 4.Deisenhofer, J., T. A. Jones, R. Huber, J. Sjodahl, and J. Sjoquist. 1978. Crystallization, crystal structure analysis and atomic model of the complex formed by a human Fc fragment and fragment B of protein A from Staphylococcus aureus. Hoppe-Seylers Zeit. Physiol. Chem. 359:975-985. [DOI] [PubMed] [Google Scholar]
- 5.De Miranda-Santos, I. K., and A. Compos-Neto. 1981. Receptor for immunoglobulin Fc on pathogenic but not on nonpathogenic protozoa of the Trypanosomatidae. J. Exp. Med. 154:1732-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubin, G., S. Basu, D. L. Mallory, M. Basu, R. Tal-Singer, and H. M. Friedman. 1994. Characterization of domains of herpes simplex virus type 1 glycoprotein E involved in Fc binding activity for immunoglobulin G aggregates. J. Virol. 68:2478-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubin, G., I. Frank, and H. M. Friedman. 1990. Herpes simplex virus type 1 encodes two Fc receptors which have different binding characteristics for monomeric immunoglobulin G (IgG) and IgG complexes. J. Virol. 64:2725-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubin, G., E. Socolof, I. Frank, and H. M. Friedman. 1991. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 65:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favoreel, H. W., H. J. Nauwynck, P. Van Oostveldt, T. C. Mettenleiter, and M. B. Pensaert. 1997. Antibody-induced and cytoskeleton-mediated redistribution and shedding of viral glycoproteins, expressed on pseudorabies virus-infected cells. J. Virol. 71:8254-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsgren, A., and J. Sjoquist. 1966. “Protein A” from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J. Immunol. 97:822-827. [PubMed] [Google Scholar]
- 11.Frank, I., and H. M. Friedman. 1989. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J. Virol. 63:4479-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman, H. M., G. H. Cohen, R. J. Eisenberg, C. A. Seidel, and D. B. Cines. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309:633-635. [DOI] [PubMed] [Google Scholar]
- 13.Friedman, H. M., E. J. Macarak, R. R. MacGregor, J. Wolfe, and N. A. Kefalides. 1981. Virus infection of endothelial cells. J. Infect. Dis. 143:266-273. [DOI] [PubMed] [Google Scholar]
- 14.Fries, L. F., H. M. Friedman, G. H. Cohen, R. J. Eisenberg, C. H. Hammer, and M. M. Frank. 1986. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J. Immunol. 137:1636-1641. [PubMed] [Google Scholar]
- 15.Hayashida, I., S. Nagafuchi, Y. Hayashi, Y. Kino, R. Mori, H. Oda, N. Ohtomo, and A. Tashiro. 1982. Mechanism of antibody-mediated protection against herpes simplex virus infection in athymic nude mice: requirement of Fc portion of antibody. Microbiol. Immunol. 26:497-509. [DOI] [PubMed] [Google Scholar]
- 16.Hopp, T. P., and K. R. Woods. 1981. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. USA 78:3824-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson, P. J., E. B. Myhre, and J. Blomberg. 1985. Specificity of Fc receptors induced by herpes simplex virus type 1: comparison of immunoglobulin G from different animal species. J. Virol. 56:489-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, D. C., and V. Feenstra. 1987. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J. Virol. 61:2208-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, D. C., M. C. Frame, M. W. Ligas, A. M. Cross, and N. D. Stow. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 62:1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Judson, K. A., J. M. Lubinski, M. Jiang, Y. Chang, R. J. Eisenberg, G. H. Cohen, and H. M. Friedman. 2003. Blocking immune evasion as a novel approach for prevention and treatment of herpes simplex virus infection. J. Virol. 77:12639-12645. [DOI] [PMC free article] [PubMed]
- 21.Keller, R., R. Peitchel, J. N. Goldman, and M. Goldman. 1976. An IgG-Fc receptor induced in cytomegalovirus-infected human fibroblasts. J. Immunol. 116:772-777. [PubMed] [Google Scholar]
- 22.Kwong, P. D., M. L. Doyle, D. J. Casper, C. Cicala, S. A. Leavitt, S. Majeed, T. D. Steenbeke, M. Venturi, I. Chaiken, M. Fung, H. Katinger, P. W. Parren, J. Robinson, D. Van Ryk, L. Wang, D. R. Burton, E. Freire, R. Wyatt, J. Sodroski, W. A. Hendrickson, and J. Arthos. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678-682. [DOI] [PubMed] [Google Scholar]
- 23.Litwin, V., W. Jackson, and C. Grose. 1992. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. J. Virol. 66:3643-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lubinski, J., T. Nagashunmugam, and H. M. Friedman. 1998. Viral interference with antibody and complement. Semin. Cell Dev. Biol. 9:329-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubinski, J. M., M. Jiang, L. Hook, Y. Chang, C. Sarver, D. Mastellos, J. D. Lambris, G. H. Cohen, R. J. Eisenberg, and H. M. Friedman. 2002. Herpes simplex virus type 1 evades the effects of antibody and complement in vivo. J. Virol. 76:9232-9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacCormac, L. P., and J. E. Grundy. 1996. Hum. cytomegalovirus induces an Fc gamma receptor (Fc gammaR) in endothelial cells and fibroblasts that is distinct from the human cellular Fc gammaRs. J. Infect. Dis. 174:1151-1161. [DOI] [PubMed] [Google Scholar]
- 27.McGeoch, D. J., A. Dolan, S. Donald, and F. J. Rixon. 1985. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J. Mol. Biol. 181:1-13. [DOI] [PubMed] [Google Scholar]
- 28.McKendall, R. R. 1985. IgG-mediated viral clearance in experimental infection with herpes simplex virus type 1: role for neutralization and Fc-dependent functions but not C′ cytolysis and C5 chemotaxis. J. Infect. Dis. 151:464-470. [DOI] [PubMed] [Google Scholar]
- 29.McTaggart, S. P., W. H. Burns, D. O. White, and D. C. Jackson. 1978. Fc receptors induced by herpes simplex virus. I. Biologic and biochemical properties. J. Immunol. 121:726-730. [PubMed] [Google Scholar]
- 30.Nagashunmugam, T., J. Lubinski, L. Wang, L. T. Goldstein, B. S. Weeks, P. Sundaresan, E. H. Kang, G. Dubin, and H. M. Friedman. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 72:5351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oakes, J. E., and R. N. Lausch. 1981. Role of Fc fragments in antibody-mediated recovery from ocular and subcutaneous herpes simplex virus infections. Infect. Immun. 33:109-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Para, M. F., L. Goldstein, and P. G. Spear. 1982. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. J. Virol. 41:137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahman, A. A., M. Teschner, K. K. Sethi, and H. Brandis. 1976. Appearance of IgG (Fc) receptor(s) on cultured human fibroblasts infected with human cytomegalovirus. J. Immunol. 117:253-258. [PubMed] [Google Scholar]
- 34.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 35.Rizvi, S. M., and M. Raghavan. 2001. An N-terminal domain of herpes simplex virus type Ig E is capable of forming stable complexes with gI. J. Virol. 75:11897-11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saldanha, C. E., J. Lubinski, C. Martin, T. Nagashunmugam, L. Wang, H. van Der Keyl, R. Tal-Singer, and H. M. Friedman. 2000. Herpes simplex virus type 1 glycoprotein E domains involved in virus spread and disease. J. Virol. 74:6712-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schalen, C., L. Truedsson, K. K. Christensen, and P. Christensen. 1985. Blocking of antibody complement-dependent effector functions by streptococcal IgG Fc-receptor and staphylococcal protein A. Acta Pathol. Microbiol. Immunol. Scand. Sect. B Microbiol. 93:395-400. [DOI] [PubMed] [Google Scholar]
- 38.Sisk, W. P., J. D. Bradley, R. J. Leipold, A. M. Stoltzfus, M. Ponce de Leon, M. Hilf, C. Peng, G. H. Cohen, and R. J. Eisenberg. 1994. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 68:766-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sjodahl, J. 1977. Repetitive sequences in protein A from Staphylococcus aureus. Arrangement of five regions within the protein, four being highly homologous and Fc-binding. Eur. J. Biochem. 73:343-351. [DOI] [PubMed] [Google Scholar]
- 40.Tal-Singer, R., C. Peng, M. Ponce De Leon, W. R. Abrams, B. W. Banfield, F. Tufaro, G. H. Cohen, and R. J. Eisenberg. 1995. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J. Virol. 69:4471-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarleton, R. L., and W. M. Kemp. 1981. Demonstration of IgG-Fc and C3 receptors on adult Schistosoma mansoni. J. Immunol. 126:379-384. [PubMed] [Google Scholar]
- 42.Tessier, D. C., D. Y. Thomas, H. E. Khouri, F. Laliberte, and T. Vernet. 1991. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene 98:177-183. [DOI] [PubMed] [Google Scholar]
- 43.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 44.Van de Walle, G. R., H. W. Favoreel, H. J. Nauwynck, and M. B. Pensaert. 2003. Antibody-induced internalization of viral glycoproteins and gE-gI Fc receptor activity protect pseudorabies virus-infected monocytes from efficient complement-mediated lysis. J. Gen. Virol. 84:939-947. [DOI] [PubMed] [Google Scholar]
- 45.Van Vliet, K. E., L. A. De Graaf-Miltenburg, J. Verhoef, and J. A. Van Strijp. 1992. Direct evidence for antibody bipolar bridging on herpes simplex virus-infected cells. Immunology 77:109-115. [PMC free article] [PubMed] [Google Scholar]
- 46.Westmoreland, D., S. St Jeor, and F. Rapp. 1976. The development by cytomegalovirus-infected cells of binding affinity for normal human immunoglobulin. J. Immunol. 116:1566-1570. [PubMed] [Google Scholar]