Abstract
Background
The goal of this study was to define the role of T cell subsets in the pathogenesis of autoimmunity induced obliterative airway disease (OAD) by passive transfer of CD8+ or CD4+ T cells.
Methods
Antibodies (Abs) to MHC class I were administered intrabronchially into C57BL/6 animals. Lungs were analyzed by histopathology and immunohistochemistry. The CD8+ and CD4+ T cell subsets were purified from the lung infiltrating cells and intrabronchially transferred. Frequency of cells secreting IL-17, IFN-γ or IL-10 to self-antigens (self-Ags) was enumerated by ELISpot. Myeloperoxidase and Abs to self-Ags were determined by ELISA. Cytokine and growth factor expression was determined by qRT-PCR.
Results
Passive transfer of lung infiltrating CD8 T cells isolated following anti-MHC class I administration along with suboptimal dose induced significantly higher cellular infiltration (89.3±7.9% vs 62.8±10.1%, p <0.05) over CD4 transfer group. Further, Passive transfer of CD8 cells resulted in infiltration of neutrophils and macrophages suggesting early injury response. In contrast, passive transfer of CD4+ T cells induced significantly higher degree of luminal occlusion (29.3±5.6% vs 8.6±2.5%, p <0.05) and fibrosis (54.4±9.3% vs 10.2±2.4%, p <0.05) over CD8 group and B-cell infiltration leading to immune responses to lung associated self-Ags and fibrosis.
Conclusion
Ligation of MHC molecules by its specific Abs induced early injury with neutrophils, macrophages and CD8 T cells, which leads to exposure of cryptic self-Ags and their presentation by the infiltrating CD4+ T cells and B cells leading to the development of immune responses to self-Ags culminating to OAD.
Keywords: OAD, Alloimmunity, Autoimmunity, Treg, Th17
Introduction
In spite of advances in human lung transplantation techniques and post-operative management, chronic rejection characterized as bronchiolitis obliterans syndrome (BOS) remains the most important concern for the long term allograft function.1 There are several studies which demonstrated strong association between the development of antibodies (Abs) to mismatched donor HLA and development of BOS.2 This development of alloimmune responses precedes the development of BOS3, 4 and thus suggesting a potential pathogenic role for Abs to HLA.
To determine the mechanism by which Abs to donor MHC may lead to development of chronic rejection we developed a murine model of obliterative airway disease (OAD) of native lungs.5 In this model, intrabronchial administration of Abs to MHC class I to the native lungs of mice resulted in OAD including: cellular infiltration, luminal occlusion and fibrosis of the small airways, the central events seen during chronic human lung allograft rejection.5 Further depletion of T-regulatory cells (CD4+Foxp3+) accentuated the OAD while IL-17 neutralization abrogated development of OAD.6 We also reported that not only anti-MHC specific to Class I antigens but also to MHC class II can induce OAD.7 In this communication, we present evidence that Abs to MHC elicit early non-specific cellular immune responses characterized by infiltration of neutrophils and macrophages along with accumulation of CD8+ T cells. Passive transfer of CD8+ T cells along with suboptimal dose of MHC class I Abs induced primarily injury response while the CD4+ T cells orchestrated B cell infiltration, development of immune responses to self-antigens (Ags) leading to OAD.
Materials and Methods
Animals and adoptive transfer of T cell subsets
We utilized a murine model in which OAD, a correlate of BOS, was induced in the distal airways following intrabronchial administration of monoclonal Ab (mAb) specific to MHC class I Ags.5 All experiments were performed in compliance with the guidelines of the Institutional Laboratory Animal Care and Use Committee of Washington University School of Medicine. Murine mAb to H2Kb (IgG2a, endotoxin free, measured by LAL assay), was given at a dose of 200 μg/administration into wild-type C57BL/6 mice. Abs (200μg) was administered with a 20-gauge catheter into the lung on days 1, 2, 3, 6, and then weekly thereafter. For sub optimal dose, single dose (200μg) of Abs were given on day 1. To determine the role of CD8+ and CD4+ T cells in induction of autoimmunity following ligation of anti-MHC class I Abs, we administered varying concentration of (0.1, 1 and 10 million) CD8+ or CD4+ T cells positively selected from day 30 lungs of OAD animals and passively transferred intrabronchially into C57BL/6 mice on day 1 along with one dose of intrabronchial MHC class I Abs. The individual T cell subsets were positively selected by MACS beads (Miltenyi Biotec, NY) and the purity of cells selected was determined to be >90% by flow cytometry. The passively transferred animals were sacrificed on day 30 and analyzed as described below.
Histological Analyses
Lungs collected at days 30 were stained with H&E and Masson’s trichrome, and analyzed under Nikon ECLIPSE 55i (Melville, NY) microscope using NIS-Elements BR software (Melville, NY).6 Immunostaining for myeloperoxidase (MPO), CD11b, CD4, CD8, and CD19 infiltration was performed on frozen sections as described earlier.
Flow cytometry
Expression of specific cell surface markers was analyzed by flow cytometry. Lung infiltrating cells were collected by collagen digestion of lungs. The specific cell surface markers on the infiltrating cells were quantitated using fluorescent tagged Abs for CD11b (macrophage), CD19 (B cell), CD3 (T cell), CD8 (effector T cell) and CD4 (helper T cell) (Santa Cruz Biotech, CA). Cells were analyzed on LSR II flow cytometer (BD Biosciences, San Jose, CA) using FACSDiva software. All measurements were taken for 5,000 events that are a display of relative cell count.
Myeloperoxidase (MPO) Assay
Neutrophil activity was determined by MPO assay on sonicated lung extracts as described earlier.8
ELISpot assay
To enumerate the frequency of Ag specific cytokine secreting cells we performed ELISpot, as described previously.6, 9 The results were expressed as spots per million cells ± SEM.
ELISA
To analyze the serum concentration of Abs to Collagen V (ColV) and K-α1 Tubulin (Kα1T), ELISA plates (Nunc, Rochester, NY) were coated with ColV or Kα1T (1 μg/mL) in PBS over night at 4°C.5, 6 A sample was considered as positive if the values were over an average cutoff values of two SD above the mean obtained from normal sera (n=10). The data were represented as a mean ± SEM over a 5 different measurements.
Gene expression analysis
RT-PCR was performed to determine chemokines, and growth factors, based on mRNA transcription in the lungs harvested from day 30.5 The expression was analyzed by FAM-labeled mouse specific RT-PCR primers (Applied Biosystems, CA).
Statistical analysis
The statistical analyses were performed either using GraphPad5.0 (LaJolla, CA) or Origin 6.0 (Northampton, MA) and all the data is represented as a mean ± SEM over a 5 different measurements. Statistical significance at p-Value <0.05 was established by Student’s paired two-tailed t test.
Results
Kinetics of cellular infiltration following anti-MHC class I administration and development of OAD
We have previously demonstrated that intrabronchial administration of MHC class I (anti-H2Kb) Abs (on day 1, 2, 3, 6, and weekly) resulted in the development of OAD lesions in the lungs of C57BL/6 mice by day 30.5 We performed morphometric analyses to determine the cellular infiltration resulting in OAD. As shown in Table 1, temporal analyses demonstrated an increase in the frequency of neutrophils and macrophages by day 7; CD8+ T cells by day 15; and CD4+ T cells and B cells by day 30 (over the isotype Ab treated animals). These results suggest that ligation of MHC class I molecules with its specific Abs induces significant cellular infiltration into the lungs initially with innate and CD8+ T cells, followed by CD4+ T cells and B cells.
Table 1. Kinetic analyses of the cellular infiltration following MHC class I Abs administration in the alloimmune murine OAD model (n=5).
| Cellular Infiltration % | Day 7 | Day 15 | Day 30 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Class I | Isotype | Class I | Isotype | Class I | Isotype | |
|
| ||||||
| Neutrophils | 33.1±5.6% | 2.7±0.8% | 29.6±5.1% | 1.9±0.5% | 23.4±6.3% | 4.1±1.3% |
| Macrophages | 12.3±1.9% | 1.8±0.6% | 11.7±2.3% | 2.1±0.5% | 8.9±1.7% | 2.3±1.2% |
| CD8 T cells | 2.2±0.7% | 2.1±0.6% | 10.3±1.9% | 3.7±1.2% | 12.3±3.1% | 4.5±2.3% |
| CD4 T cells | 3.1±0.9% | 2.2±0.4% | 7.2±2.3% | 6.5±1.7% | 32.2±7.1% | 8.2±3.1% |
| B-cells | 1.2±0.5% | 0.9±0.5% | 1.6±0.7% | 0.9±0.3% | 14.3±3.2% | 2.1±0.9% |
|
Epi/Endothelial
cells |
48.1±9.1% | 90.3±5.1% | 39.6±12.5% | 84.9±6.7% | 10.9±8.9% | 78.8±7.9% |
|
| ||||||
| Total | 100 % | 100% | 100% | 100% | 100% | 100% |
Adoptive transfer of CD8 T cells along with suboptimal dose of MHC class I antibodies causes increased cellular infiltration and CD4 T cells resulted in enhanced luminal occlusion and fibrosis
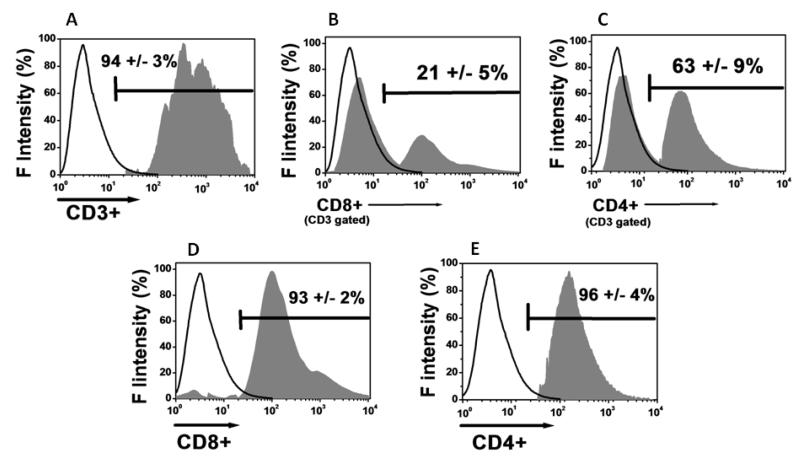
To determine the role of T cell subsets in the development of OAD following intrabronchial administration of MHC class I Abs (anti-H2Kb), we performed adoptive transfer of CD8+ and CD4+ T cell subsets isolated from the lung infiltrating lymphocytes. As shown in figure 1, purified CD3+ T cells demonstrated a 21 ± 5% of CD8+ T cells (figure 1B) and 63 ± 9% of CD4+ T cells (figure 1C). This suggests that, both CD8+ and CD4+ T cell phenotypes play a role in the pathogenesis of OAD. To determine the contribution of the individual T cell subsets in inducing OAD, we passive transferred these purified CD8 (figure 1D) and CD4 (figure 1E) T cells from the total T cell population (CD3+) by MACS beads purification procedure (purity>90%).
Figure 1.
Isolation of lung infiltrating lymphocytes (LILs) on day 30 from our murine alloimmune model (n=5) following MHC class I Abs administration on day 1, 2, 3, 6 and weekly. (A) MACS beads purified CD3+ T cells with a purity of 94±3%. The CD8+ T cells in the CD3+ fraction amounted for 21±5% (B), and CD4+ T cell fraction amounted for 63±9% (C). The CD3+ T cells were further positively selected for CD8+ (D) and CD4+ (E) T cells with >90% purity. The data were obtained by pooling of LILs from 5 different animals which developed OAD and represented as mean ± SEM.
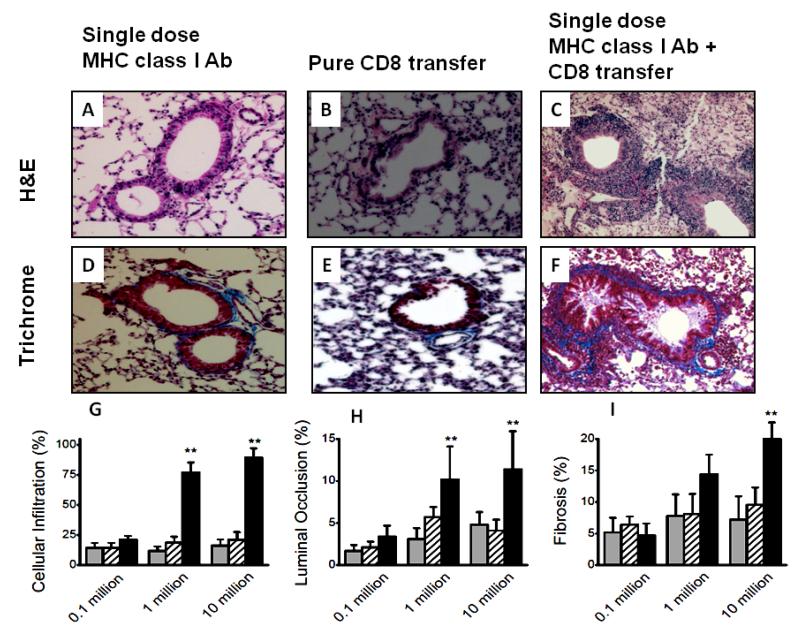
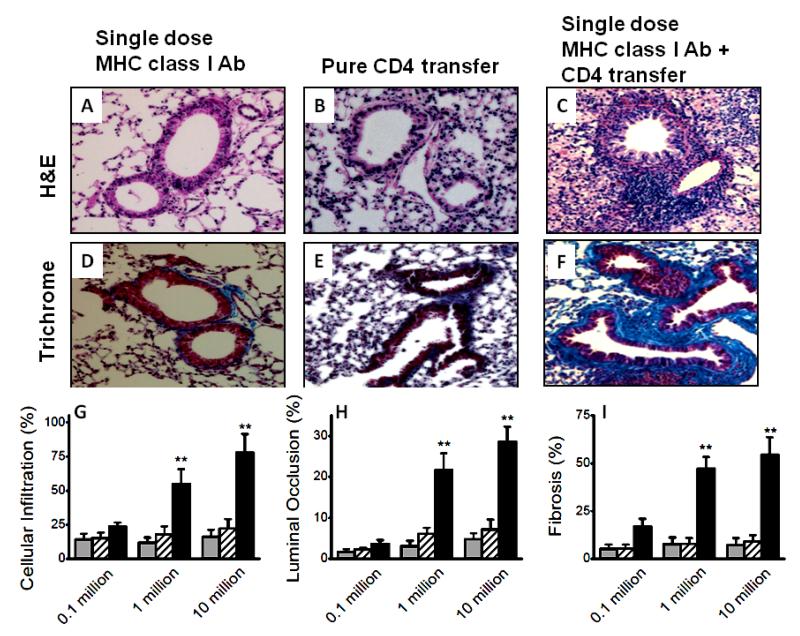
To determine the role of individual T cell subset in inducing OAD, we analyzed five cohorts: Control group, single administration of MHC class I Ab on day 1 along with equivalent number of splenocytes transferred from naïve animals (control); purified CD8+ T cell transfer groups, CD8 T cell with and without single administration of MHC class I Abs (suboptimal dose of MHC class I Abs); purified CD4+ T cell transfer groups, CD4 T cell along with and without suboptimal MHC class I Abs. Intrabronchial administration of either suboptimal MHC class I Abs (figure 2A and D) or adoptive transfer of CD8+ T cell subset alone (figure 2B and E) did not result in OAD. Following adoptive transfer of CD8+ T cell subsets along with suboptimal MHC class I Abs, the animals developed OAD including cellular infiltration, luminal occlusion and fibrosis (figure 2C and F). Further, morphometric analysis (figure 2G-I) demonstrated that adoptive transfer of CD8+ T cells along with suboptimal MHC class I Abs induced OAD in a dose dependent manner. In a similar set of experiments, passive transfer of lung infiltrating CD4+ T cells from OAD animals to wild-type animals along with suboptimal MHC class I Abs induced OAD (Figure 3C and F). As with CD8 passive transfer, CD4 transfer alone in the absence of suboptimal MHC class I Abs did not induce OAD lesions (Figure 3B and D) indicating an important role for anti-MHC in priming the airway epithelium.
Figure 2.
Induction of OAD by adoptive transfer of CD8+ T cell subsets from LILs of C57BL/6 animals with OAD (following administration of 200 μg of Abs to MHC class I intrabronchially to C57Bl/6 mice on days 1, 2, 3, and 6, and weekly thereafter) into naïve animals with single dose of Abs to MHC class I. Representative H&E stain, (A-C); trichrome stain, (D-F); of the sections taken from day 30, following suboptimal single dose 200 μg MHC class I Abs administration (A and D), pure 10 million CD8+ T cell transfer (B and E) and CD8+ T cell transfer along with suboptimal single dose 200 μg MHC class I Abs administration (C and F). Morphometric analyses on the sections to measure cellular infiltration (G), luminal occlusion (H), and fibrosis (I) from various groups. A dose dependent increase in lesions upon adoptive transfer of 0.1, 1 and 10 million CD8 T cell transfer; suboptimal single dose 200 μg MHC class I Ab administration (grey), pure CD8 T cell transfer (cross-line) and CD8 T cell transfer along with suboptimal single dose 200 μg MHC class I Abs administration (black). Representative of 5 different sections and presented as mean ± SEM; the significance (p-value <0.05) was determined by student t-test; (*) p-value <0.05, statistically significant different between the comparison groups; (#) p-value >0.5, statistically insignificant difference between the comparison groups.
Figure 3.
Induction of OAD by adoptive transfer of CD4+ T cell subsets from LILs of C57BL/6 animals with OAD (following administration of 200 μg of Abs to MHC class I intrabronchially to C57Bl/6 mice on days 1, 2, 3, and 6, and weekly thereafter) into naïve animals with single dose of Abs to MHC class I. Representative H&E stain, (A-C); trichrome stain, (D-F); of the sections taken from day 30, following suboptimal single dose 200 μg MHC class I Abs administration (A and D), pure 10 million CD4+ T cell transfer (B and E) and CD4+ T cell transfer along with suboptimal single dose 200 μg MHC class I Abs administration (C and F). Morphometric analyses on the sections to measure cellular infiltration (G), luminal occlusion (H), and fibrosis (I) from various groups. A dose dependent increase in lesions upon adoptive transfer of 0.1, 1 and 10 million CD4 T cell transfer; suboptimal single dose 200 μg MHC class I Abs administration (grey), pure CD4 T cell transfer (cross-line) and CD4 T cell transfer along with suboptimal single dose 200 μg MHC class I Abs administration (black). Representative of 5 different sections and presented as mean ± SEM. The significance (p-value <0.05) was determined by student t-test; (*) p-value <0.05, statistically significant different between the comparison groups; (#) p-value >0.5, statistically insignificant difference between the comparison groups.
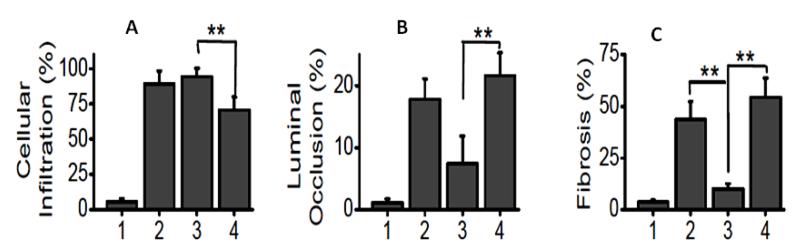
The CD8+ T cell transfer group induced significantly higher cellular infiltration (89.3±7.9% vs 62.8±10.1%, p <0.05) over CD4 transfer group (figure 4A). In contrast, CD4 transfer group induced significantly higher degree of luminal occlusion (29.3±5.6% vs 8.6±2.5%, p <0.05) and fibrosis (54.4±9.3% vs 10.2±2.4%, p <0.05) over CD8 group (Figure 4B and C). These results demonstrate that CD8+ T cells induced initial epithelial damage by innate cells, while, CD4+ T cells induced fibrosis and luminal occlusion.
Figure 4.
Comparision of cellular infiltration (A), luminal occlusion (B) and fibrosis (C) among various groups: (1) suboptimal single dose MHC class I Abs administration group; (2) native alloimmune OAD group; (3) CD8 transfer along with suboptimal single dose MHC class I administration group; and (4) CD4 transfer along with suboptimal single dose MHC class I Abs administration group. Representative of 5 different sections and presented as mean ± SEM; the significance (p-value <0.05) was determined by student t-test; (*) p-value <0.05, statistically significant different between the comparison groups; (#) p-value >0.5, statistically insignificant difference between the comparison groups.
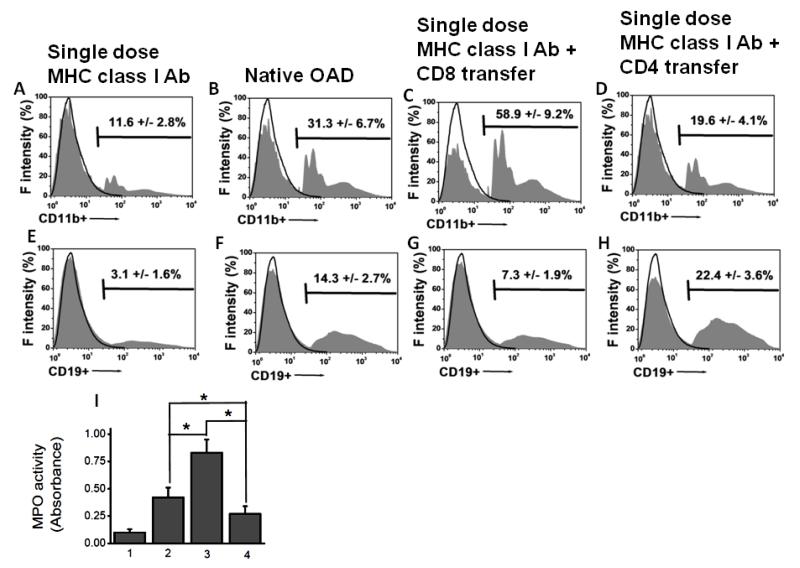
Adoptive transfer of CD8+ T cells along with suboptimal MHC class I Abs resulted in increased neutrophil and macrophage infiltration, whereas CD4+ T cells resulted in increased B cell infiltration
To determine the differential role of T cell subpopulations in inducing preferential cellular infiltration, phenotype analyses on the infiltrating cell following passive transfer of CD8+ or CD4+T cells was performed. On day 30, lung infiltrating cells were isolated using collagenase digestion of the lungs following which flow cytometric analysis of lung infiltrating cells was performed. As shown in figure 5A-D, CD8+ T cell transfer along with suboptimal MHC class I Abs induced increased infiltration of CD11b+ cells in comparison to the CD4+ T cell transfer groups. Further CD8 transfer also induced increased infiltration of MPO positive neutrophils (figure 5I) over CD4 groups. There was also significant (p<0.05) increase in macrophage and neutrophil infiltration in native alloimmune OAD animals over CD4 transfer group. In contrast, CD4+ T cell transfer group induced higher CD19+ B cells infiltration over CD8+ T cell transfer and (figure 5E-H). These results demonstrate that CD8+ T cells promote infiltration of innate immune cells (neutrophils, monocytes and macrophages) likely due to the initial lung injury response, while CD4+ T cells are involved in late injury response including recruitment and activation of B cells which are involved in the production of Abs to lung associated self-Ags. Further, the regulatory T cell (Treg) frequency in various cohorts (figure not shown) were as follows: isotype treated day 30 (14±3% of CD4+T cells), MHC antibody treated day 30 (1.8±0.5% of CD4+T cells), CD8 transfer with suboptimal MHC antibody at day 30 (1.1±0.7% of CD4+T cells), CD4 transfer with suboptimal MHC antibody at day 30 (0.7±0.4% of CD4+T cells). Therefore, these results demonstrate that the differential role of OAD lesions upon CD8 vs CD4+T cell passive transfer is not due to Treg.
Figure 5.
Analyses of the frequency of the phenotype of the infiltrating cells on day 30 following adoptive transfer of T cell subsets from OAD (following administration of 200 μg of Abs to MHC class I intrabronchially to C57Bl/6 mice on days 1, 2, 3, and 6, and weekly thereafter) into naïve animals with single dose of Abs to MHC class I. (A-D) Analyses of CD11b+ infiltrating cells, representing monocyte/macrophage infiltration. The frequency of CD11b+ infiltrating cell phenotype among various groups include: sub optimal single dose MHC class I Abs group = 11.6±2.8% (A); native OAD group= 31.3±6.7% (B); CD8 transfer along with single dose MHC class I Abs administration group = 58.9±9.2% (C); and CD4 transfer along with single dose MHC class I Abs administration group = 19.6±4.1% (D). There was a statistically significant difference between the native alloimmune OAD and CD8 (p-value <0.05); CD8 and CD4 (p-value <0.01), and the native alloimmune OAD and CD8 (p-value <0.05) groups. (E-H). Analyses of CD19+ infiltrating cells, representing B-cell infiltration. The frequency of CD19+ infiltrating cell phenotype among various groups include: suboptimal MHC class I Ab group = 3.1±1.6% (E); native alloimmune OAD group = 14.3±2.7% (F); CD8 transfer along with single dose MHC class I administration group = 7.3±1.9% (G); and CD4 transfer along with single dose MHC class I Abs administration group = 22.4±3.6% (H). There was a statistically significant difference between the native alloimmune OAD group and CD8 group (p-value <0.05); CD8 and CD4 (p-value <0.01), and native alloimmune OAD group and CD8 (p-value <0.05) groups. (I) MPO assay on the infiltrating cells, representing neutrophil infiltration. The absorbance value (ΔOD) of the infiltrating cells among various groups include: suboptimal single dose MHC class I Abs administration group = 0.11±0.03; native alloimmune OAD group = 0.42±0.09; CD8 transfer along with single dose MHC class I Abs administration group = 0.83±0.12; and CD4 transfer along with single dose MHC class I Abs administration group = 0.27±0.07. There was a statistically significant difference between the native alloimmune OAD and CD8 (p-value <0.05); CD8 and CD4 (p-value <0.01), and native alloimmune OAD and CD8 (p-value <0.05) groups. Data were collected from 5 different animals and presented as mean ± SEM. The significance (p-value <0.05) was determined by student t-test; and represented by asterick (*).
Adoptive transfer of CD4 T cells along with suboptimal MHC class I Abs causes augmented humoral immune and Th17 cellular responses
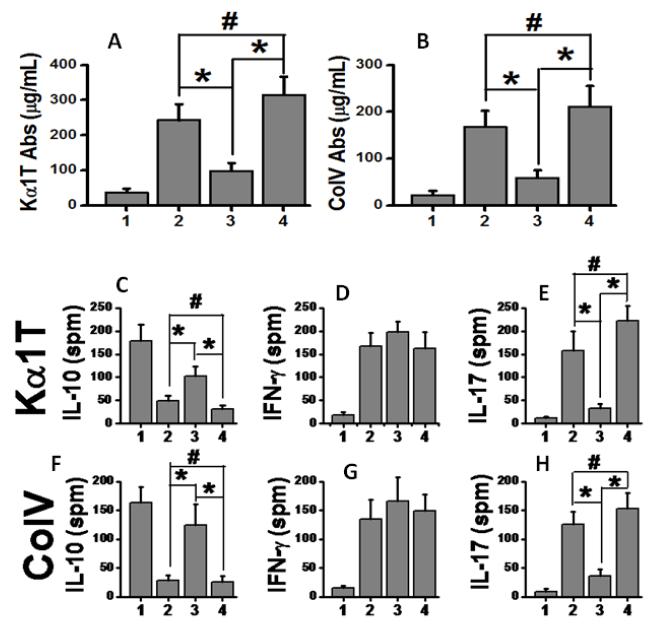
Abs to MHC class I molecule has been shown to induce the development of immune responses to lung associated self-Ags, ColV and Kα1T, in the murine model of OAD and Abs to donor mismatched HLA are shown to precede clinical evidence of BOS following human lung transplantation.5, 10 To determine if adoptive transfer of T cell subsets were able to induce humoral immune responses, we analyzed the serum concentrations of Abs to Kα1T and ColV on 30 days following passive transfer of T cell subsets. Significant increase in the development of Abs to Kα1T and ColV were noticed following adoptive transfer of CD4+ T along with suboptimal MHC class I Abs (figure 6 A and 4B) cells in comparison to the animals given CD8+ T cells along with suboptimal MHC class I Abs (p<0.01). There was no difference in humoral immune response between CD4 transfer and native alloimmune OAD groups. These results demonstrate that passive transfer of CD4+ T cells from anti-MHC administered animals induced efficient immune responses to self-Ags.
Figure 6.
(A-B) Analyses of Abs to self-Ags and cellular responses to self-Ags from day 30 following passive transfer. Serum concentration of Kα1T (A) and ColV (B) Abs among various groups. (C-H) Analyses of cellular immune responses to self-Ags following passive transfer. (C-E). Frequency of IL-10 (C), IFN-γ (D) and IL-17 (E) secreting splenocytes specific to Kα1T; and (F-H) frequency of IL-10 (F), IFN-γ (G) and IL-17 (H) secreting splenocytes specific to ColV. Various groups include: (1) suboptimal single dose MHC class I Abs administration group; (2), native alloimmune OAD group; (3), CD8 transfer along with suboptimal single dose MHC class I Abs administration group; and (4), CD4 transfer along with suboptimal single dose MHC class I Abs administration group. Data were collected from 5 different animals and presented as mean ± SEM. The significance (p-value <0.05) was determined by student t-test; (*) p-value <0.05, statistically significant different between the comparison groups; (#) p-value >0.5, statistically insignificant difference between the comparison groups.
We next investigated the role of memory responses of the auto-reactive T cells associated with the development of OAD. The splenocytes collected on day 30 following passive transfer of T cell subsets were analyzed by ELISpot. As shown in figure 6C and 6F, passive transfer of CD4+ T cells reduced the number of IL-10 specific T cellular response specific to Kα1T and ColV. On the other hand, IL-17 responses to Kα1T (figure 6E) and ColV (figure 6H) significantly increased by day 30 upon passive transfer of CD4+ T cells over CD8 group. These results indicate that passive transfer of CD4 T cells resulted in change in Th phenotype to Th17 phenotype, while the passive transfer of CD8 T cells induced cytotoxic (IFN-γ) responses.
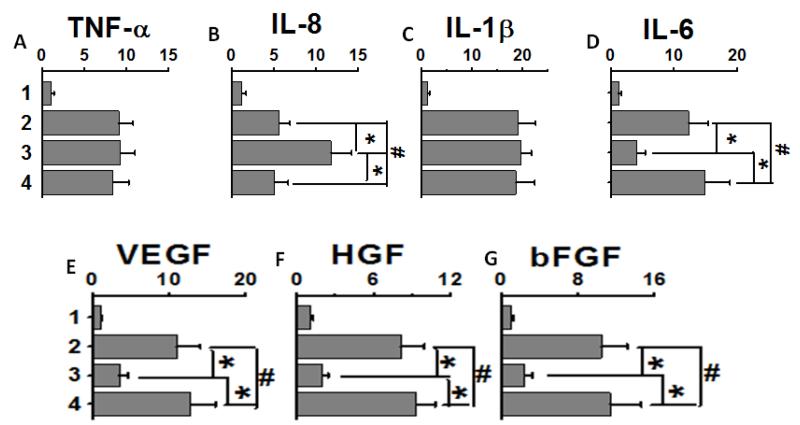
Adoptive transfer of CD8 and CD4 T cells along with suboptimal MHC class I Abs cause pro-inflammatory and pro-fibrotic cytokine and growth factor milieu in the lung
We also analyzed the gene expression profile of the inflammatory cytokines following transfer of CD8+ or CD4+ T cell subsets on day 30. As shown in figure 7A and C, adoptive transfer of CD8 and CD4 T cell subsets induced increased expression of TNF-α and IL-1β, a known pro-inflammatory cytokine inducing both the effector and helper T cell responses.11 While, CD8 group induced higher expression of neutrophil specific IL-8 cytokine (figure 7B), CD4 group induced significantly higher gene expression of IL-6 (figure 7D), which is known to facilitate Th17 signaling and inducing alloimmune induced autoimmune responses.12 Further, CD4 group demonstrated enhanced expression of pro-fibrotic VEGF (2.3 fold), HGF (3.3 fold) and bFGF (3.8 fold) over CD8 group. These results demonstrate that passive transfer of CD4 T cells from animals which developed OAD following intrabronchial MHC class I Abs administration induced an inflammatory local milieu to skew naïve Th-phenotype into Th17 mediated alloimmune induced autoimmune responses along with strong pro-fibrotic signaling resulting in the development of OAD in these animals.
Figure 7.
Analyses of the mRNA level gene expression of pro-inflammatory chemokines (A-D; TNF-α, IL-8, IL-1β and IL-6, respectively) and profibrotic growth factors (E-G; VEGF, HGF, and bFGF, respectively) from day 30 following passive transfer. Various groups include: (1) suboptimal single dose MHC class I Abs administration group; (2), native alloimmune OAD group; (3), CD8 transfer along with suboptimal single dose MHC class I Abs administration group; and (4), CD4 transfer along with suboptimal single dose MHC class I Abs administration group. Data were collected from 5 different animals and presented as mean ± SEM.The significance (p-value <0.05) was determined by student t-test; (*) p-value <0.05, statistically significant different between the comparison groups; (#) p-value >0.5, statistically insignificant difference between the comparison groups.
Discussion
It has been proposed that de novo development of immune responses to mismatched donor HLA Ags as well as other inflammatory events can lead to immune responses to lung associated self-Ags resulting in tissue remodeling, fibrosis and obliteration of small airways that is hallmark of BOS following human lung transplantation.2 A temporal correlation between the development of donor specific antibodies (DSA) and the development of BOS have been demonstrated suggesting a pathogenic role for the DSA.4 This is further supported by our findings that preemptive Ab directed treatment and removal of DSA significantly improves freedom from BOS following human lung transplantation.13 Various animal models14 have been proposed to define the immunopathogenesis of this obliterative airway disease (OAD). However, most of these models either do not fully represent the lesions seen in BOS following human LTx or are technically complex and difficult to perform routinely in most of the research facilities. Another complexity stems from the fact that multiple etiologies have been considered as independent risk factors for the development of BOS.15 Therefore, with a goal to specifically address the role of alloimmune responses in the development of OAD, we developed a murine model for OAD, wherein MHC class I Abs were intrabronchially administered into mice.5 In this model, the animals developed classic OAD lesions as manifested by epithelial hyperplasia, cellular infiltration around the bronchiole, luminal occlusion and fibrosis around the smaller bronchioles. However, there are limitations for this model too. Direct intrabronchial administration of MHC class I Abs is considered as non physiological and there is lack of persistent histologic evidence for airway epithelial damage at day 30 even though one can notice epithelial cell damage at the early periods following administration of antibodies to MHC.
To determine the mechanisms by which Abs to MHC may induce infiltration of cells into the lungs and the specific role of various infiltrating cell subpopulations in the pathogenesis of OAD, we performed passive transfer experiments. Administration of either suboptimal dose of the anti-MHC class I or passive transfer of T cells did not elicit OAD lesions. However, when administered together the animals developed OAD, thus strongly suggesting that the initial alloimmune insult exerted by the suboptimal anti-MHC was necessary for subsequent insult by the T cells. Passive transfer of CD8+ T cells resulted in enhanced neutrophil accumulation. Therefore, CD8 cells played a key role in accentuation of a series of initial inflammatory cascades of innate immune responses which is also obligatory for the development of OAD. In contrast, passive transfer of CD4+ T cells caused 4-5 fold higher fibrosis (figure 4) and humoral immune responses (figure 6) over CD8 transfer. Further, increased Th17 responses to ColV and Kα1T were also noticed in CD4 treated animals (figure 6). Previous studies from our laboratory have demonstrated that targeted ablation of Treg in the anti-MHC induced murine OAD model accentuated Th17 response.6 Further, in our current studies using either CD8 and CD4 cohorts demonstrated that there was decreased CD4+Foxp3+ T reg cells (less than 1.5%) infiltrating by day 30 (data not shown). These results are in good agreement with those reported by Hayward et al16 in a murine autoimmune myocarditis model, in which they demonstrated that upon passive transfer of memory T cell subsets, CD8 cell exerted an initiator role while CD4 cells induced an autoimmune effector response. Passive transfer of CD4+ T cells also induced pro-inflammatory cytokine, IL-6, response (Figure 7). The roles of IL-6 in the induction of Th17 cells leading to autoimmune responses are well recognized. In vitro studies as well as knock-out models of rheumatoid arthritis have established that IL-6 can stimulate the production of IL-17 resulting in autoimmunity.17 IL-17 is a potent pro-inflammatory and pro-fibrotic cytokine leading to enhanced autoimmune response and fibrosis.18, 19 IL-17 has also been shown to play a crucial role in the induction of humoral responses to self-Ags.20 Hence, we propose that the IL-17/Th17 polarization of the memory CD4 T cells is a critical mediator in the alloimmune mediated autoimmune response following administration of the MHC class I Abs, resulting in the development of OAD.
Administration of anti-MHC class I, resulted in increased serum concentration of Abs to self-Ags, ColV and Kα1T, prior to the development of OAD in animal models 5 and de novo development of Abs to donor mismatched HLA precedes BOS following human lung transplantation.10 In this report we demonstrate that CD4+ T cells play the major role in eliciting humoral immune response to self-Ags. This is not surprising since CD4 T cells are indeed helper cells for B cells towards production of Abs.21 This is also supported by our earlier report demonstrating an obligatory role for B cells towards development of OAD induced by administration of Abs to MHC.22 Taken together we propose that CD4+ T cells induce increased B cell infiltration and production of both humoral and cellular immune responses to lung associated self-Ags resulting in the development of OAD. However, the exact role of the priming by suboptimal dose of MHC class I Abs in this current adoptive transfer study needs further investigation. Previous studies from our group3 and seminal work from Dr. Reed’s laboratory23-25 demonstrate that following ligation HLA class I by its specific Abs can induce activation of epithelial and endothelial cells by mTOR and src-kinase pathways, which we propose may also induce the initial priming effect by the suboptimal dose of MHC class I abs in our current study. Although a possible priming effect of the suboptimal dose of MHC class I Abs could also be in part due to activation of memory T- and B-cells. The mechanisms by which anti-MHC primes needs further study including the assessment of destruction of transferred T cell subsets by the resident macrophages in the naïve animals.
In conclusion, we demonstrate an initiator role for the CD8 T cells and effector role for CD4 T cell subsets including development of both cellular and humoral immune responses to lung associated self -Ags leading to OAD. We propose that ligation of MHC molecules on the airway epithelial or endothelial cells by its specific Abs induce an initial innate (neutrophils, macrophages) response. This leads to exposure of self-Ags and their eventual presentation by the late infiltrating CD4+ T cells and B cells. This late response leads to IL-17 immune responses to tissue restricted self-Ags and activation of pro-fibrotic cascades leading to OAD. Our results provides evidence for a unique role for de novo developed Abs to mismatched donor MHC in activating cellular immune responses which elicits immune responses to self-Ags leading to chronic rejection.
Acknowledgements
This work was supported by NIH/NHLBI/NIAID HL092514 and the BJC Foundation (TM). The authors thank Ms. Billie Glasscock for her help in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors have any conflicts of interest to disclose.
References
- 1.Tiriveedhi V, Sarma N, Mohanakumar T. An important role for autoimmunity in the immunopathogenesis of chronic allograft rejection. Int J Immunogenet. 2012;39:373–80. doi: 10.1111/j.1744-313X.2012.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiriveedhi V, Weber J, Seetharam A, Mohanakumar T. Cross-talk of alloimmune response and autoimmunity: role in pathogenesis of chronic rejection. Discov Med. 2010;9:229–35. [PubMed] [Google Scholar]
- 3.Jaramillo A, Smith CR, Maruyama T, Zhang L, Patterson GA, Mohanakumar T. Anti-HLA class I antibody binding to airway epithelial cells induces production of fibrogenic growth factors and apoptotic cell death: a possible mechanism for bronchiolitis obliterans syndrome. Hum Immunol. 2003;64:521–9. doi: 10.1016/s0198-8859(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 4.Jaramillo A, Smith MA, Phelan D, et al. Temporal relationship between the development of anti-HLA antibodies and the development of bronchiolitis obliterans syndrome after lung transplantation. Transplant Proc. 1999;31:185–6. doi: 10.1016/s0041-1345(98)01495-x. [DOI] [PubMed] [Google Scholar]
- 5.Fukami N, Ramachandran S, Saini D, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–18. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tiriveedhi V, Takenaka M, Ramachandran S, et al. T regulatory cells play a significant role in modulating MHC class I antibody-induced obliterative airway disease. Am J Transplant. 2012;12:2663–74. doi: 10.1111/j.1600-6143.2012.04191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takenaka M, Tiriveedhi V, Subramanian V, Hoshinaga K, Patterson AG, Mohanakumar T. Antibodies to MHC Class II Molecules Induce Autoimmunity: Critical Role for Macrophages in the Immunopathogenesis of Obliterative Airway Disease. PLoS One. 2012;7:e42370. doi: 10.1371/journal.pone.0042370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saini D, Angaswamy N, Tiriveedhi V, et al. Synergistic effect of antibodies to human leukocyte antigens and defensins in pathogenesis of bronchiolitis obliterans syndrome after human lung transplantation. J Heart Lung Transplant. 2010;29:1330–6. doi: 10.1016/j.healun.2010.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nath DS, Tiriveedhi V, Basha HI, et al. A role for antibodies to human leukocyte antigens, collagen-V, and K-alpha1-Tubulin in antibody-mediated rejection and cardiac allograft vasculopathy. Transplantation. 2011;91:1036–43. doi: 10.1097/TP.0b013e318211d2f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saini D, Weber J, Ramachandran S, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624–31. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 12.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–7. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 13.Hachem RR, Yusen RD, Meyers BF, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29:973–80. doi: 10.1016/j.healun.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreisel D, Gelman AE, Palmer SM. In pursuit of new experimental models of obliterative bronchiolitis. Am J Transplant. 2011;11:882–3. doi: 10.1111/j.1600-6143.2011.03483.x. [DOI] [PubMed] [Google Scholar]
- 15.Seetharam A, Tiriveedhi V, Mohanakumar T. Alloimmunity and autoimmunity in chronic rejection. Curr Opin Organ Transplant. 2010;15:531–6. doi: 10.1097/MOT.0b013e32833b31f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayward SL, Bautista-Lopez N, Suzuki K, Atrazhev A, Dickie P, Elliott JF. CD4 T cells play major effector role and CD8 T cells initiating role in spontaneous autoimmune myocarditis of HLA-DQ8 transgenic IAb knockout nonobese diabetic mice. J Immunol. 2006;176:7715–25. doi: 10.4049/jimmunol.176.12.7715. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Ivanov, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 18.Nakano K, Yamaoka K, Hanami K, et al. Dopamine induces IL-6-dependent IL-17 production via D1-like receptor on CD4 naive T cells and D1-like receptor antagonist SCH-23390 inhibits cartilage destruction in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol. 2011;186:3745–52. doi: 10.4049/jimmunol.1002475. [DOI] [PubMed] [Google Scholar]
- 19.Jovanovic DV, Di Battista JA, Martel-Pelletier J, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–21. [PubMed] [Google Scholar]
- 20.Irmler IM, Gajda M, Brauer R. Exacerbation of antigen-induced arthritis in IFN-gamma-deficient mice as a result of unrestricted IL-17 response. J Immunol. 2007;179:6228–36. doi: 10.4049/jimmunol.179.9.6228. [DOI] [PubMed] [Google Scholar]
- 21.Lemoine S, Morva A, Youinou P, Jamin C. Human T cells induce their own regulation through activation of B cells. J Autoimmun. 2011;36:228–38. doi: 10.1016/j.jaut.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Fukami N, Ramachandran S, Takenaka M, Weber J, Subramanian V, Mohanakumar T. An obligatory role for lung infiltrating B cells in the immunopathogenesis of obliterative airway disease induced by antibodies to MHC class I molecules. Am J Transplant. 2012;12:867–76. doi: 10.1111/j.1600-6143.2011.03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin YP, Fishbein MC, Said JW, et al. Anti-HLA class I antibody-mediated activation of the PI3K/Akt signaling pathway and induction of Bcl-2 and Bcl-xL expression in endothelial cells. Hum Immunol. 2004;65:291–302. doi: 10.1016/j.humimm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Jin YP, Korin Y, Zhang X, Jindra PT, Rozengurt E, Reed EF. RNA interference elucidates the role of focal adhesion kinase in HLA class I-mediated focal adhesion complex formation and proliferation in human endothelial cells. J Immunol. 2007;178:7911–22. doi: 10.4049/jimmunol.178.12.7911. [DOI] [PubMed] [Google Scholar]
- 25.Jindra PT, Jin YP, Rozengurt E, Reed EF. HLA class I antibody-mediated endothelial cell proliferation via the mTOR pathway. J Immunol. 2008;180:2357–66. doi: 10.4049/jimmunol.180.4.2357. [DOI] [PubMed] [Google Scholar]