Abstract
TBX2 and TBX3, closely related members of the T-box family of transcription factor genes, are expressed in mammary tissue in both humans and mice. Ulnar mammary syndrome (UMS), an autosomal dominant disorder caused by mutations in TBX3, underscores the importance of TBX3 in human breast development, while abnormal mammary gland development in Tbx2 or Tbx3 mutant mice provides models for experimental investigation. In addition to their roles in mammary development, aberrant expression of TBX2 and TBX3 is associated with breast cancer. TBX2 is preferentially amplified in BRCA1/2-associated breast cancers and TBX3 overexpression has been associated with advanced stage disease and estrogen-receptor-positive breast tumors. The regulation of Tbx2 and Tbx3 and the downstream targets of these genes in development and disease are not as yet fully elucidated. However, it is clear that the two genes play unique, context-dependent roles both in mammary gland development and in mammary tumorigenesis.
Keywords: Tbx2, Tbx3, mammary development, breast cancer, transcription factor, T-box
Introduction
The first connection of the T-box gene family with mammary gland development came with the discovery that ulnar mammary syndrome (UMS, MIM 181450) is caused by mutations in TBX3. UMS is a rare autosomal dominant disorder so named for defects in the ulna and the mammary glands. Mammary gland abnormalities affect both sexes and include hypoplastic or aplastic breasts and supernumerary, inverted, or absent nipples with an inability to lactate [1, 2]. Axillary apocrine gland, dental, cardiac and reproductive system abnormalities are additional clinical features of UMS [1–5]. Both TBX3 and the closely related TBX2 are overexpressed in mammary gland neoplasia and breast cancer cell lines, suggesting a role for these genes in tumorigenesis and cancer progression. Studies in mice highlight the distinct roles that Tbx2 and Tbx3 play in mammary gland development and have characterized their roles in mammary gland induction and maintenance [6, 7]. However, the involvement of these genes in postnatal, pregnant, lactating or involuting mammary glands has yet to be explored.
TBX2 and TBX3 are members of the T-box family of transcription factor genes, which are defined by the evolutionarily conserved DNA binding domain, the T-box. TBX2 and TBX3 share 95% identical amino acid residues in their T-box domains and contain a highly conserved, C-terminal transcription-repression domain [2, 8, 9]. Although TBX2 is expressed in a wide variety of tissues including the adult breast [10], there are no human developmental syndromes as yet associated with TBX2. The TBX3 expression profile includes the adult breasts, heart, and uterus [1, 11]. UMS is caused by mutations throughout the TBX3 gene [1, 2, 5]. Characterization of the crystal structure of TBX3 bound to DNA supported the supposition that point mutations affecting the T-box domain impair DNA binding [8]. C-terminal TBX3 mutants associated with UMS have decreased ability to repress transcription and accelerated rates of protein decay [9, 12]. Although some mutations disrupting the T-box domain have been associated with more severe mammary gland defects in males [3], mutations usually result in a spectrum of clinical features with no clear correlation between the location of a mutation and the UMS phenotype.
Tbx2 and Tbx3 in mouse mammary development
Tbx2 and Tbx3 are the only members of the T-box family known to be expressed in developing mammary glands. Like their human counterparts, Tbx2 and Tbx3 share over 90% sequence identity in their T-box domains and both contain a functional C-terminal transcription-repression domain [6, 9, 12]. At embryonic day (E) 9.5, neither gene is expressed in the mesenchyme at the ventro-lateral body wall between the forelimb and hindlimb where the mammary line will form within 1–2 days. Expression is first detected at E10.5 as a continuous stripe in the mesenchyme underlying the mammary line, with Tbx3 exhibiting a broader domain of expression than Tbx2 (Fig. 1A, B). While Tbx2 is expressed only in mesenchyme at E10.5 and E11.5, Tbx3 is also expressed in the epithelium of mammary placode (MP) 1 and MP3 at E10.5 and in all 5 pairs of placodes at E11.5 (Fig. 1A–D, arrowheads). At E12.5 and E13.5, all mammary buds express Tbx3 as well as Lef1, a downstream target of Wnt signaling and an early marker of mammary differentiation [6, 7, 13, 14]. At E18.5, both Tbx2 and Tbx3 are expressed in mesenchyme underlying the nipple sheath [7], but only Tbx3 is expressed in the epithelium of the branching mammary ducts. The overlapping, yet distinct expression patterns of Tbx2 and Tbx3 are maintained throughout gestation. Tbx2 expression in adults has not as yet been described, but Tbx3 is expressed in virgin, pregnant, lactating and involuting mammary glands [6, 15].
Figure 1.
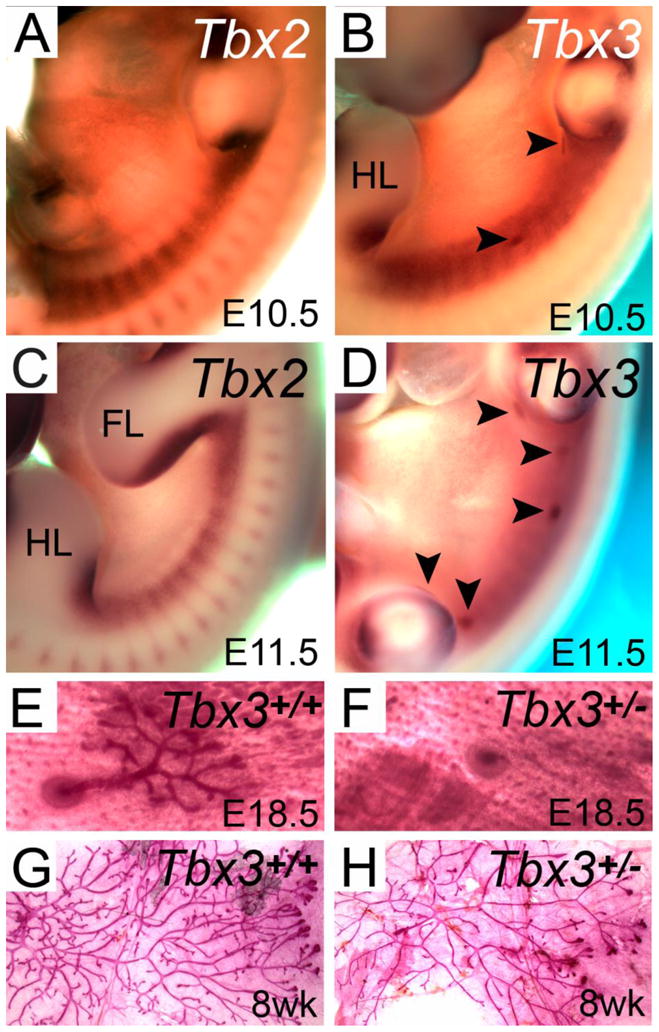
Expression of Tbx2 and Tbx3 in the developing mammary gland and the effect of heterozygosity for a mutation in Tbx3. A–D. Whole mount in situ hybridization for Tbx2 and Tbx3 at embryonic day (E) 10.5 and E11.5 shows expression of both genes in the mammary line between the forelimb and hindlimb and Tbx3 expression in the mammary placodes when they first appear (arrowheads). E, F. Skin and mammary gland preparation from late gestation embryos stained with Carmine’s Alum to show the nipple and ductal tree, which is reduced in Tbx3 heterozygotes. G, H. Mammary gland preparations from 8 week old virgin females stained with Carmine’s Alum showing a reduction in the branching network of Tbx3 heterozygotes. (Modified with permission from [7]).
Effects of Tbx2 and Tbx3 mutations on mammary gland development
Homozygosity for a null mutation of Tbx2 results in embryonic lethality at midgestation, presumably due to heart abnormalities [16], while homozygosity for a null mutation of Tbx3 results in embryonic lethality from mid to late gestation due to yolk sac and heart abnormalities [6]. Because of UMS, mammary gland development has been studied extensively in Tbx3 heterozygous and homozygous null mutants. At E13.5, expression of Lef1 is similar in Tbx3 heterozygous and wild type embryos, indicating normal mammary bud formation [7]. At E18.5, Tbx3 heterozygotes have a significantly higher incidence of failed nipple and ductal tree development of mammary gland (MG) 1 and MG2 (Fig. 1E, F). When present, ductal trees in all mammary glands exhibit a trend towards fewer branches, with significantly fewer branches in MG1 and MG2 [7]. In Tbx3 null homozygotes between E11.5 and E13.5, neither Wnt10b nor Lef1 is expressed and at E12.5 there was no histological evidence of mammary placode formation in five mutant embryos. By E13.5, however, histological examination revealed two rudimentary mammary buds in one mutant embryo and a single bud in a second embryo, both in the position of MG2 [6]. Adult virgin Tbx3 heterozygous mice have a higher incidence of aplasia of MG1, MG2, and MG3 and reduced branching in ductal trees of all 5 pairs of mammary glands (Fig. 1G, H). Taken together, these data indicate that Tbx3 is essential for normal mammary placode induction and that haploinsufficiency of Tbx3 impairs mammary bud maintenance as well as the extent of ductal tree development.
Tbx2 homozygous mutants have no discernible defects in mammary placode induction [7]. Although all mammary glands are present in adult Tbx2 heterozygous virgin females, the extent of ductal tree development is reduced, with a significant reduction in MG1. In Tbx2; Tbx3 double heterozygous mice, there is a higher incidence of aplasia of MG1, MG2, and MG3 at E18.5 compared with Tbx3 heterozygotes. These data reveal a genetic interaction between Tbx2 and Tbx3 in maintenance of mammary placodes [7].
Association with human mammary neoplasia
TBX2 and TBX3 are overexpressed in a number of cancers, including pancreatic, melanoma, and breast [10]. Amplification of the chromosomal region 17q22-q24, a region containing TBX2, RPS6KB1 and several other candidate oncogenes, is common in breast cancers [17]. TBX2 is preferentially amplified in BRCA1/2-associated tumors compared with sporadic tumors, whereas RPS6KB1 is not [18, 19]. Furthermore, TBX2 was amplified equivalently in ductal carcinoma in situ and invasive tumors, suggesting that TBX2 amplification occurs early in the development of BRCA1/2-associated tumors [18]. In situ hybridization and Northern blot analyses confirmed that TBX2 is overexpressed in primary tumors and breast cancer cell lines in which TBX2 is amplified [20, 17]. TBX3 is also overexpressed in a subset of breast cancer cell lines, with higher levels of TBX3 in those with estrogen-receptor-positive status, such as MCF7, an epithelial adenocarcinoma cell line [11]. Treatment of MCF7 cells with estrogen induces TBX3 expression, suggesting that estrogen increases the number of cancer stem-like cells in in vivo breast tumors via TBX3 signaling [21]. In a cohort of 79 breast cancer patients, TBX3 expression was increased in plasma samples from all patients with advanced disease and in the majority of samples from the entire cohort [22]. TBX3 protein is overexpressed in malignant breast epithelial cells compared with nonmalignant breast tissue [23]. Recent characterization of TBX3 mutations in primary breast tumors strengthens the link between TBX3 and mammary tumorigenesis [24, 25].
Molecular mechanisms in development and neoplasia
Regulation of Tbx2 and Tbx3 in the developing mammary gland
While more is known about the molecular function of Tbx3 than Tbx2 in mammary gland development, very little is known about how either gene is regulated in this tissue. In the MCF7 cell line, TBX3 is upregulated in response to the phorbol ester PMA in a protein kinase C-dependent manner via the AP-1 factors c-Jun and JunB, which bind to an AP-1-response element in the TBX3 promoter [26]. In early mammary gland development in vivo, induction of Tbx3 expression depends on WNT signaling, retinoic acid (RA) signaling, and FGF signaling through Fgfr2. In the absence of Tbx3, Wnt10b and Lef1, RA signaling and FGF signaling are all lost [6, 14, 27], indicating feedback mechanisms in these three signaling pathways for induction and/or maintenance of Tbx3 expression. In addition, overexpression studies have implicated BMP signaling in a reciprocal negative feedback loop with Tbx3, an interaction that may play a role in positioning the mammary placodes during development [28].
Downstream targets and mode of action
Both Tbx2 and Tbx3 are transcriptional repressors and, by virtue of their highly similar DNA binding domains, can bind similar promoter sequences. However, their distinctive mutant phenotypes indicate that they do not function redundantly. In the mouse, overexpression of human TBX3 in mammary epithelium causes hyperplasia by increasing proliferation and is associated with an increase in mammary stem-like cells. TBX3 was shown to directly bind and repress NFκBIB, an inhibitor of the NFκB pathway known to play a role in regulating cell proliferation [29]. Combined with the hypoplastic phenotype seen in Tbx3 mutants [6, 7], this indicates a proliferation-promoting role.
An important discovery was the direct transcriptional repression by both TBX2 and TBX3 of the cyclin dependent kinase inhibitors, Cdkn2a (p19ARF in mouse; p14ARF in humans), p16INK4a, and p21CIP, which are involved in proliferation control. TBX2 was identified as a potent immortalizing gene in primary fibroblasts and TBX3 was found to inhibit senescence in mouse immortalized neuronal cells [20, 30]. The senescence pathway is a protective mechanism against cancer and is mainly mediated by p21CIP, p16INK4a and p19ARF, which stabilizes p53 levels. TBX2 and TBX3 regulate p19/p14 through a variant T-box binding element (TBE) [31] and bind a consensus TBE in the p21CIP promoter [32, 33]. There is evidence that Tbx3 can promote the growth of mammary epithelial cells in vitro, with no effect on differentiation or apoptosis, through its ability to repress p19ARF and p21CIP, independently of the p53/Mdm2 pathway [15]. However, there is evidence that the hypoplastic mammary gland phenotype in heterozygous Tbx3 mutants is independent of both p53 and p19ARF [7].
The association of both TBX2 and TBX3 with mammary and other types of neoplasia has been a driver of discovery and in some cases mechanisms relevant to normal development have been uncovered in breast cancer cell lines. For example, interactions between Fgf and Tbx3 signaling are involved in mammary gland initiation [14] and FGF9 expands the stem cell population in breast cancer cells and cell lines through FGFR signaling and TBX3 [21]. However, whether other mechanisms identified in breast cancer cells apply to normal mammary gland development has yet to be determined. For example, TBX3 physically interacts with histone deacetylases (HDACs) in MCF-7 cells and its repressive function on p14ARF is dependent on HDACs [23]. In the same cell line, it was found that in response to stress induced by UV irradiation, Tbx2 protein is phosphorylated by p38 mitogen-activated protein (MAP) kinase, which leads to increased Tbx2 protein levels, nuclear localization and an increase in the ability of Tbx2 to repress the p21 promoter [34]. Whether TBX3 functions by recruiting HDACs to the TBE in the p14ARF promoter and whether Tbx2 repression of p21 is enhanced in response to a stress-induced senescence pathway in development needs to be further explored.
Same or different functions?
A distinction between TBX2 and TBX3 function was highlighted using shRNA to knock down either TBX2 or TBX3 in MCF-7 cells, which overexpress both genes. Knockdown of TBX2 decreased proliferation without affecting migratory ability whereas knockdown of TBX3 slightly increased proliferation and reduced migratory ability. At least in this context, TBX2 acts as a powerful growth promoter, whereas TBX3 is mainly required for cell migration [35]. The effect of TBX3 on migratory behavior may underlie the increased invasiveness observed in response to the phorbol ester PMA since knockdown of TBX3 abrogates PMA-induced cell motility in MCF-7 cells [26]. The demonstration that E-cadherin is a downstream target of TBX3 in invasive melanoma cells provides a possible mechanism for the effect on migratory behavior [36].
On the other hand, conflicting results were found with immortalized but otherwise normal mammary epithelial cell lines not expressing TBX2 (mouse HC11 and human MCF10A). Expression of TBX2 was sufficient to induce changes characteristic of epithelial-mesenchymal transition (EMT) with increased cell motility and invasion. TBX2 was also upregulated upon induction of EMT in primary human mammary epithelial cells. Conversely, silencing endogenous TBX2 in malignant human breast carcinoma cell lines MDA-MB-435 and MDA-MB-157 led to gain of epithelial characteristics, the loss of mesenchymal markers and the abolition of tumor cell invasion and metastatic potential. Like TBX3, TBX2 was found to directly repress E-cadherin [37]. These conflicting results on whether or not TBX2 promotes migratory cell behavior could be the result of cell type-dependent differences and highlight the need to consider the functions of these genes in specific contexts.
Conclusions
UMS highlights the role of TBX3 in human mammary gland development, while studies in the mouse have shown that both Tbx2 and Tbx3 play important roles in the induction and maintenance of mammary placodes and in the growth of the mammary ductal tree. An important unexplored area is the role of these genes in the adult mammary gland during pregnancy, lactation and involution. TBX2 and TBX3 are overexpressed in primary breast cancers and breast cancer cell lines. The association of aberrant expression of both genes with human breast cancer has led to the idea that TBX2 and/or TBX3 could be used as prognostic biomarkers for breast cancer treatment [22].
Acknowledgments
This work was supported by grant number R37HD033082 (V. E. P.) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, grant number K12HD000849 (N.C.D.) from the Reproductive Scientist Development Program and a grant from the Amos Medical Faculty Development Program/Robert Wood Johnson Foundation (RWJF) (N.C.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD, NIH or RWJF. We thank Sharon Prince, Daniel Concepcion and Andrew J. Washkowitz for critical reading of the manuscript.
Abbreviations
- E
embryonic day
- EMT
epithelial-mesenchymal transition
- HDAC
histone deacetylase
- MAP
mitogen-activated protein
- MG
mammary gland
- MP
mammary placode
- RA
retinoic acid
- TBE
T-box binding element
- UMS
ulnar mammary syndrome
References
- 1.Bamshad M, Le T, Watkins WS, Dixon ME, Kramer BE, Roeder AD, et al. The spectrum of mutations in TBX3: genotype/phenotype relationship in ulnar-mammary syndrome. American Journal of Human Genetics. 1999;64:1550–62. doi: 10.1086/302417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamshad M, Lin RC, Law DJ, Watkins WS, Krakowiak PA, Moore ME, et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nature Genetics. 1997;16:311–5. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- 3.Meneghini V, Odent S, Platonova N, Egeo A, Merlo GR. Novel TBX3 mutation data in families with Ulnar-Mammary syndrome indicate a genotype-phenotype relationship: mutations that do not disrupt the T-domain are associated with less severe limb defects. Eur J Med Genet. 2006;49(2):151–8. doi: 10.1016/j.ejmg.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Washkowitz AJ, Gavrilov S, Begum S, Papaioannou VE. Diverse functional networks of Tbx3 in development and disease. WIREs Syst Biol Med. 2012;4:273–83. doi: 10.1002/wsbm.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wollnik B, Kayserili H, Uyguner O, Tukel T, Yuksel-Apak M. Haploinsufficiency of TBX3 causes ulnar-mammary syndrome in a large Turkish family. Annales de Genetique. 2002;45:213–7. doi: 10.1016/s0003-3995(02)01144-9. [DOI] [PubMed] [Google Scholar]
- 6.Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb, and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–73. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- 7.Jerome-Majewska LA, Jenkins GP, Ernstoff E, Zindy F, Sherr CJ, Papaioannou VE. Tbx3, the ulnar-mammary syndrome gene, and Tbx2 interact in mammary gland development through a p19Arf/p53-independent pathway. Developmental Dynamics. 2005;234:922–33. doi: 10.1002/dvdy.20575. [DOI] [PubMed] [Google Scholar]
- 8.Coll M, Seidman JG, Muller CW. Structure of the DNA-bound T-box domain of human TBX3, a transcription factor responsible for ulnar-mammary syndrome. Structure. 2002;10:343–56. doi: 10.1016/s0969-2126(02)00722-0. [DOI] [PubMed] [Google Scholar]
- 9.He M-L, Wen L, Campbell CE, Wu JY, Rao Y. Transcription repression by Xenopus ET and its human ortholog TBX3, a gene involved in ulnar-mammary syndrome. Proceedings of the National Academy of Sciences, USA. 1999;96:10212–7. doi: 10.1073/pnas.96.18.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrahams A, Parker MI, Prince S. The T-box transcription factor Tbx2: Its role in development and possible implication in cancer. IUBMB Life. 2010;62(2):92–102. doi: 10.1002/iub.275. [DOI] [PubMed] [Google Scholar]
- 11.Fan W, Huang X, Chen C, Gray J, Huang T. TBX3 and its isoform TBX3+2a are functionally distinctive in inhibition of senescence and are overexpressed in a subset of breast cancer cell lines. Cancer Research. 2004;64:5132–9. doi: 10.1158/0008-5472.CAN-04-0615. [DOI] [PubMed] [Google Scholar]
- 12.Carlson H, Ota S, Campbell CE, Hurlin PJ. A dominant repression domain in Tbx3 mediates transcriptional repression and cell immortalization: relevance to mutations in Tbx3 that cause ulnar-mammary syndrome. Human Molecular Genetics. 2001;10:2403–13. doi: 10.1093/hmg/10.21.2403. [DOI] [PubMed] [Google Scholar]
- 13.Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik S, Gibson Brown JJ, et al. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Developmental Dynamics. 1996;206:379–90. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Eblaghie MC, Song S-J, Kim J-Y, Akita K, Tickle C, Jung H-S. Interactions between FGF and Wnt signals and Tbx3 gene expression in mammary gland initiation in mouse embryos. Journal of Anatomy. 2004;205:1–13. doi: 10.1111/j.0021-8782.2004.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platonova N, Scotti M, Babich P, Bertoli G, Mento E, Meneghini V, et al. TBX3, the gene mutated in ulnar-mammary syndrome, promotes growth of mammary epithelial cells via repression of p19ARF, independently of p53. Cell Tissue Res. 2007;328:2, 301–16. doi: 10.1007/s00441-006-0364-4. [DOI] [PubMed] [Google Scholar]
- 16.Harrelson Z, Kelly RG, Goldin SN, Gibson-Brown JJ, Bollag RJ, Silver LM, et al. Tbx2 is essential for patterning the atrioventricular canal and for morphogenesis of the outflow tract during heart development. Development. 2004;131:5041–52. doi: 10.1242/dev.01378. [DOI] [PubMed] [Google Scholar]
- 17.Bärlund M, Monni O, Kononen J, Cornelison R, Torhorst J, Sauter G, et al. Multiple genes at 17q23 undergo amplification and overexpression in breast cancer. Cancer Res. 2000;60:5340–4. [PubMed] [Google Scholar]
- 18.Sinclair CS, Adem C, Naderi A, Soderberg CL, Johnson M, Wu K, et al. TBX2 is preferentially amplified in BRCA1- and BRCA2-related breast tumors. Cancer Research. 2002;62:3587–91. [PubMed] [Google Scholar]
- 19.Adem C, Soderberg CL, Hafner K, Reynolds C, Slezak JM, Sinclair CS, et al. ERBB2, TBX2, RPS6KB1, and MYC alterations in breast tissues of BRCA1 and BRCA2 mutation carriers. Genes, Chromosomes & Cancer. 2004;41:1–11. doi: 10.1002/gcc.20057. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs JJL, Keblusek P, Robanus-Maandag E, Kristel P, Lingbeek M, Nederlof PM, et al. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19ARF) and is amplified in a subset of human breast cancers. Nature Genetics. 2000;26:291–9. doi: 10.1038/81583. [DOI] [PubMed] [Google Scholar]
- 21.Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, et al. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Nat Acad Sci. 2010;107:21737–42. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lomnytska M, Dubrovska A, Hellman U, Volodko N, Souchelnytskyi S. Increased expression of cSHMT, Tbx3 and utrophin in plasma of ovarian and breast cancer patients. International Journal of Cancer. 2006;118:412–21. doi: 10.1002/ijc.21332. [DOI] [PubMed] [Google Scholar]
- 23.Yarosh W, Barrientos T, Esmailpour T, Lin L, Carpenter PM, Osann K, et al. TBX3 is overexpressed in breast cancer and represses p14ARF by interacting with histone deacetylases. Cancer Res. 2008;68:693–9. doi: 10.1158/0008-5472.CAN-07-5012. [DOI] [PubMed] [Google Scholar]
- 24.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486(7403):400–4. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Network TCGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mowla S, Pinnock R, Learner VD, Goding CR, Prince S. PMA-induced up-regulation of TBX3 is mediated by AP-1 and contributes to breast cancer cell migration. Biochem J. 2011;433:145–53. doi: 10.1042/BJ20100886. [DOI] [PubMed] [Google Scholar]
- 27.Cho K-W, Kwon H-J, Shin J-O, Lee J-M, Cho S-W, Tickle C, et al. Retinoic acid signaling and the initiation of mammary gland development. Dev Biol. 2012;365:259–66. doi: 10.1016/j.ydbio.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Cho K-W, Kim J-Y, Song S-J, Farrell E, Eblaghie MC, Kim H-J, et al. Molecular interactions between Tbx3 and Bmp4 and a model for dorsoventral positioning of mammary gland development. Proc Natl Acad Sci U S A. 2006;103(45):16788–93. doi: 10.1073/pnas.0604645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Esmailpour T, Shang X, Gulsen G, Liu A, Huang T. TBX3 over-expression causes mammary gland hyperplasia and increases mammary stem-like cells in an inducible transgenic mouse model. BMC Developmental Biology. 2011;11:65. doi: 10.1186/1471-213X-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brummelkamp TR, Kortlever RM, Lingbeek M, Trettel F, MacDonald ME, van Lohuizen M, et al. TBX-3, the gene mutated in ulnar-mammary syndrome, is a negative regulator of p19ARF and inhibits senescence. Journal of Biological Chemistry. 2002;277:6567–72. doi: 10.1074/jbc.M110492200. [DOI] [PubMed] [Google Scholar]
- 31.Lingbeek ME, Jacobs JJL, van Lohuizen M. The T-box repressors TBX2 and TBX3 specifically regulate the tumor-suppressor p14ARF via a variant T-site in the initiator. Journal of Biological Chemistry. 2002;277:26120–7. doi: 10.1074/jbc.M200403200. [DOI] [PubMed] [Google Scholar]
- 32.Hoogaars WMH, Barnett P, Rodriguez M, Clout DE, Moorman AFM, Goding CR, et al. TBX3 and its splice variant TBX3 + exon 2a are functionally similar. Pigment Cell Melanoma Res. 2008;21:379–87. doi: 10.1111/j.1755-148X.2008.00461.x. [DOI] [PubMed] [Google Scholar]
- 33.Prince S, Carreira S, Vance KW, Abrahams A, Goding CR. Tbx2 directly represses the expression of the p21WAF1 cyclin-dependent kinase inhibitor. Cancer Research. 2004;64:1669–74. doi: 10.1158/0008-5472.can-03-3286. [DOI] [PubMed] [Google Scholar]
- 34.Abrahams A, Mowla S, Parker MI, Goding CR, Prince S. UV-mediated regulation of the anti-senescence factor Tbx2. J Biol Chem. 2007;283:2223–30. doi: 10.1074/jbc.M705651200. [DOI] [PubMed] [Google Scholar]
- 35.Peres J, Davis E, Mowla S, Bennett DC, Li JA, Wansleben S, et al. The highly homologous T-box transcription factors, TBX2 an TBX3, have distinct roles in the oncogenic process. Genes & Cancer. 2010;1(3):272–82. doi: 10.1177/1947601910365160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez M, Aladowicz E, Lanfrancone L, Goding CR. Tbx3 represses E-cadherin expression and enhances melanoma invasiveness. Cancer Res. 2008;68(19):7872–81. doi: 10.1158/0008-5472.CAN-08-0301. [DOI] [PubMed] [Google Scholar]
- 37.Wang B, Lindley LE, Fernandez-Vega V, Rieger ME, Sims AH, Briegel KJ. The T box transcription factor TBX2 promotes epithelial-mesenchymal transition and invasion of normal and malignant breast epithelial cells. PLoS ONE. 2012;7(7):e41355. doi: 10.1371/journal.pone.0041355. [DOI] [PMC free article] [PubMed] [Google Scholar]


