Abstract
Objective
To evaluate predictors of persistence of attention deficit/ hyperactivity disorder (ADHD) in a large sample of children with velo-cardio-facial syndrome (VCFS)VCFS with and without ADHD followed prospectively into adolescence.
Study design
Children with VCFS with (N = 37) and without (N = 35) ADHD who were on average 11 years old at the baseline assessment and 15 years old at the follow-up assessment were comprehensively assessed with structured diagnostic interviews and assessments of behavioral, cognitive, social, school, and family functioning. Control participants both with and without ADHD were also followed prospectively.
Results
In adolescence, 65% of children with VCFS continued to have findings consistent with ADHD. Childhood predictors of persistence were higher rates of familial ADHD, having childhood depression, having higher levels of hyperactivity and a larger number of intrusion errors on a verbal list learning test at baseline. Approximately 15% of children with VCFS who did not have ADHD at Time 1 met diagnostic criteria for ADHD at Time 2. All of these children had subthreshold ADHD symptoms at Time 1.
Conclusions
These findings prospectively confirm that persistence of ADHD into adolescence in VCFS is predicted by childhood variables that have been previously documented in the non-VCFS ADHD literature.
Keywords: velocardiofacial syndrome (VCFS), ADHD, 22q11 deletion
Velo-cardio-facial syndrome (VCFS) is caused by an interstitial deletion from chromosome 22 at the 22q11 band. The most common microdeletion syndrome yet identified in humans, VCFS has a reported population prevalence ranging from approximately 1:2000 to 1:6000 (1, 2). In most cases, VCFS is caused by a hemizygous deletion of 3 million base pairs of DNA encompassing 40 genes but approximately 8% have smaller nested deletions of 1.5 million base pairs spanning 34 genes (3). Structural anomalies affect nearly every system of the body and may include congenital heart disease, palatal anomalies, thymic hypoplasia, and endocrine disorders (1).
Attention deficit hyperactivity disorder (ADHD) is one of the most common comorbid disorders associated with VCFS. Approximately 30 – 40 % of individuals with VCFS have an ADHD diagnosis (5, 6). The research examining idiopathic ADHD longitudinally has found that the majority of children diagnosed with ADHD will retain their ADHD diagnosis into late adolescence or adulthood (8, 10, 11). There is little research examining the longitudinal trajectory of ADHD in VCFS. The goal of the present study is to predict which children with VCFS and ADHD are most likely to retain their ADHD status into adolescence.
Similar to the idiopathic ADHD population (14–16), there appears to be a genetic link to ADHD in individuals with VCFS (17). Individuals with VCFS and ADHD had a greater number of first-degree relatives with ADHD(17). However, the utility of having relatives with ADHD in predicting to syndromal persistence of ADHD has not yet been studied in the VCFS population.
The following hypotheses were explored: (1) the majority of children with VCFS and ADHD will maintain their ADHD status as adolescents; and (2) childhood ADHD severity, having relatives with ADHD, and the number of comorbid disorders will be the strongest predictors of ADHD persistence.
Methods
Participants were enrolled in a longitudinal study of risk factors for psychosis in VCFS. At time 1, 80 youth with VCFS (Mean age = 11.9 years, SD = 2.2) and an age- and sex-matched group of 40 non-VCFS youth (community control; Mean age = 12.0 years, SD = 1.9) participated. No age differences existed between the groups at Time 1, F (1, 118) = 0.21, p = .804, η2 = .01.
Children with VCFS were recruited from the Velo-Cardio-Facial Syndrome International Center at the State University of New York Upstate Medical University. Only children with a FISH-confirmed deletion of 22q11.2 were included in the sample. Our control participants were recruited from local public schools. The present longitudinal study is a study of the risk factors for psychosis. ADHD, to our knowledge, does not increase the risk for psychosis. Thus, we included control participants with ADHD to increase our comparability with VCFS (who have a high prevalence of ADHD).
At Time 2, 72 youth with VCFS (Mean age = 15.0 years, SD = 2.1) and 23 community controls (Mean age = 14.7 years, SD = 1.4) were included in the analyses. No age, F (1, 93) = 0.36, p = .687, η2 = .01, or sex differences, χ2 (df = 1) = 0.87, p = .621, existed between the groups at Time 2.
An independent samples t-test found no differences in attrition between our two groups, t (1) = 3.44, p = .222. Furthermore, participants lost to follow-up did not differ from those retained on any relevant Time 1 sociodemographic measures including participant age, sex, and socioeconomic status. In addition, participants lost to follow-up did not differ from those retained on any relevant Time 1 psychiatric or cognitive variables. Thus, those participants who completed Time 2 assessments appear representative of the Time 1 sample. The family moving to a different residence was the most common reason participants were lost to follow-up.
At Time 1, 44% of the control participants with ADHD were prescribed a stimulant medication and 32% of the VCFS participants with ADHD were prescribed a stimulant. There were no statistical differences between the groups in terms of stimulant prescriptions, F (1,51) = 2.03, p = 341. At follow-up, 78% of the control participants with ADHD were receiving stimulant medication. Significantly fewer of the VCFS participants with ADHD (38%) were receiving stimulant medication, F (1.51) = 26.43, p < .001.
At Time 1, in both groups with ADHD, no other medications besides stimulant medications were prescribed to participants. At Time 2, in the control group with ADHD, no medications besides stimulants were prescribed. At Time 2, in the VCFS group with ADHD, atypical antipsychotics (risperidone, aripiprazole; n = 6) and alpha-2 agonists (clonidine, guanfacine; n = 4) were prescribed.
At Time 1, of the 37 children with VCFS+ADHD, roughly half (n = 19) met criteria for ADHD-Combined type and the remaining (n = 18) met criteria for the Inattentive subtype. Among the 16 control participants with ADHD, the majority (n = 12) met criteria for ADHD-Combined type and the remaining met criteria for ADHD-Inattentive type (n = 4). Differences emerged between groups at Time 1 in terms of ADHD subtype prevalence rates, χ2 = 5.32, d f =1, p = .009. Unlike Time 1, there were no differences between groups at Time 2 in terms of ADHD subtype prevalence rates. At Time 2, 59% (n = 22) of VCFS participants with ADHD met criteria for the Inattentive subtype and 69% of the control participants with ADHD met criteria for the Inattentive subtype.
Participants were assessed at two time points, with approximately three years between time points. At Time 2, all involved research personnel were blinded to Time 1 findings. Informed consent/assent was obtained from parents and children under protocols approved by the institutional review board.
Each child enrolled in the study was administered a neuropsychological test battery that included tests of all major domains of cognition. Psychological testing was followed by a structured psychiatric interview, administered by a clinical psychologist or a board-certified child psychiatrist. Parents completed behavioral rating inventories and completed forms assessing functional parameters.
Our choice of cognitive measures was influenced by the schizophrenia literature and was selected based upon sensitivity to prodromal psychosis (18–31). Most of the psychological tests, however, are also sensitive to an ADHD diagnosis (32) and are commonly employed in longitudinal ADHD research (33–35).
Measures of general intellectual functioning were the Wechsler Intelligence Scale for Children —Third edition (WISC-III) (36) or Wechsler Adult Intelligence Scale – Third edition (WAIS-III) (37). The WISC-III was administered to all participants at Time 1, and to participants at or under the age of 16 years, 11 months at Time 2. The WAIS-III was administered to all participants over the age of 16–11 at Time 2.
Academic achievement was assessed using the Wechsler Individual Achievement Test-Second edition (WIAT-II) (38). Attention was assessed using the Gordon Diagnostic System - Continuous Performance Test (CPT) (39). Executive functioning was assessed with the Wisconsin Card Sorting Test (WCST) (40) and Tower of London (TOL). Learning and memory was assessed with the California Verbal Learning Test-Children’s version (CVLT-C) (41) and the Visual Span Test (42). All psychological test scores were converted to standard scores or z-scores using published norms. Furthermore, all psychological tests utilized in the current study are commonly employed tests which have adequate psychometric properties (for complete details, see (43)).
The Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) (44) was utilized to make DSM-IV (45) psychiatric diagnoses. The child’s primary caregiver (almost always his/her mother) was interviewed with the K-SADS-PL. Every attempt was made to interview the child, but in many cases the child had difficulty responding, often due to difficulties comprehending the questions; in these cases, the K-SADS-PL data was based on the parent’s response. A child and adolescent psychiatrist or clinical child psychologist administered the KSADS assessment. Inter-rater reliability, which was calculated for 10 interviews, and assessed with the Kappa coefficient, was .91.
The Premorbid Adjustment Scale (PAS) (46) evaluates the achievement of developmental goals from childhood into adulthood in persons who eventually develop schizophrenia. Although the scale was originally designed as a retrospective instrument to assess premorbid functioning up to six months prior to a psychiatric hospitalization, we used the scale prospectively, rating each participant on the items corresponding to his or her current age. The scale focuses on five areas of functioning: social accessibility-isolation; peer relationships; school functioning; ability to function outside the nuclear family; and the capacity to form intimate socio-sexual ties. Ratings for each item were anchored to descriptive phrases, ranging from 0 (representing “healthiest” functioning) to 6 (representing most impaired functioning). Inter-rater reliability, for ten participants between two doctoral level raters, calculated using an intraclass correlation coefficient of item ratings, ranged from .85 to .90.
The Behavior Assessment Scale for Children (BASC) – Parent report version (47) was administered to provide a continuous measure of adaptive and problem behaviors. Each of the 130 items of the child version of the BASC is rated on a 4-point frequency scale, ranging from never to almost always.
Family Interview for Genetic Studies (FIGS) (48) is a semi-structured interview designed for psychiatric genetic studies. It screens for diagnostic information about the relatives of study probands. The interview probes for the history and presence of depression, bipolar disorder, substance abuse, schizophrenia, and personality disorders in first- and second-degree relatives. The interview is open-ended, allowing the interviewer to probe for additional disorders such as anxiety and ADHD. Respondents for this study were limited to the parent(s) (and grandparents, if present) that brought the child for the assessments.
Data Analyses
McNemar non-parametric tests for related samples were computed to compare KSADS diagnostic consistency across time. Separate tests were computed for each sample. Repeated-measures multivariate analyses of covariance (MANCOVA) models, with diagnostic group as the main effect, Time 1 variables as the covariate, and psychological test scores or behavioral variables and time as repeated factors were computed. Group and time effects and group-by-time interaction were examined. Finally, to predict ADHD status at Time 2, all Time 1 demographic, cognitive, behavioral and psychiatric variables were entered into a logistic regression using ADHD status (Yes/No) as the outcome variable. Separate tests were computed for both samples.
Results
Control participants both with (n = 16) and without ADHD (n = 7) had a lower socioeconomic status than VCFS participants with (n = 37) and without ADHD (n = 35; Table I). No differences emerged in socioeconomic status as a function of ADHD status. Sex differences approached significance, χ2 (3) = 2.40, p = .087; in all groups except the VCFS group, males were more prevalent.
Table 1.
Characteristics of Adolescents with and without ADHD at Time 2 (3-year Follow-Up)
| Time 1 ADHD Status | ||||
|---|---|---|---|---|
| VCFS | VCFS+ADHD | Control | Control+ADHD | |
| N | 35 | 37 | 7 | 16 |
| Age | 15.2 (2.0) | 14.9 (1.8) | 14.7 (1.4) | 14.8 (1.9) |
| Sex (% male) | 40 | 62 | 72 | 56 |
| Hollingshead SES | 49.2 (12.2) | 48.3 (12.2) | 43.1 (14.7) c d * | 44.3 (13.0) c,d* |
| Number continuing to have ADHD at Time 2 (%) | 5 (14.3) a* | 24 (64.9) a b c *** | 0 (0) b c d *** | 9 (56.3) a b d*** |
| Treated with Stimulant (%) | 0 b d*** | 38 a b c*** | 0 b d*** | 78 a b d*** |
| Comorbid ODD (%) | 11.4 a* | 24.3 a c*** | 0 b c d *** | 25 a c*** |
| Comorbid Anxiety disorder (%) | 51.4 a b *** | 54.0 a b*** | 0 b c d *** | 18.8 a cd*** |
| Comorbid Mood disorder (%) | 62.9 a b *** | 64.9 a b *** | 0 b c d *** | 6.3 a cd*** |
| Relatives with ADHD (%) | 3.7 b d*** | 27.0 a c*** | 0 b c d *** | 37.5 a c*** |
Note. VCFS = Velocardiofacial syndrome (VCFS). ADHD = Attention deficit hyperactivity disorder. ODD = Oppositional Defiant Disorder. For pairwise comparisons:
vs. Controls;
vs. Controls + ADHD;
vs. VCFS;
vs. VCFS + ADHD
p ≤0.05;
p ≤0.01;
p≤0.001.
No controls gained an ADHD diagnosis (p = .094), 7 of 16 control participants who had a Time 1 ADHD diagnosis did not have a Time 2 ADHD diagnosis (Table I). In the VCFS sample, 13 of the 37 participants who had a Time 1 ADHD diagnosis did not have a Time 2 ADHD diagnosis (p = .096). Control participants were more likely to lose their ADHD diagnosis relative to VCFS participants (44% vs. 35%, p = .046). Likewise, unlike the control sample, 5 youth with VCFS had a Time 2 ADHD diagnosis yet not a Time 1 ADHD diagnosis. Thus, more participants with VCFS than control participants gained an ADHD diagnosis (p < .001).
Parent ratings of hyperactivity increase as a function of age in the VCFS + ADHD sample relative to the other three samples, F (3, 92) = 22.65, p < .001 (Figure). Conversely, parent ratings of inattention do not change as a function of time, F (3, 92) = 2.03, p =.234. At follow-up, 78% of the control participants with ADHD were receiving stimulant medication. Less of the VCFS participants with ADHD (38%) were receiving stimulant medication, F (1.51) = 26.43, p < .001.
Figure 1.
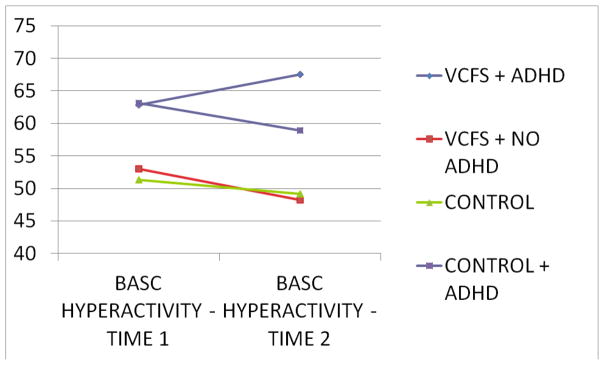
BASC Parent T-Scores at Times 1 and 2
Both ADHD groups were more likely to have comorbid oppositional defiant disorder (ODD) diagnoses, p < .001 (Table I). Both VCFS groups (VCFS, VCFS+ADHD) were more likely to have comorbid anxiety and mood disorders relative to controls with and without ADHD, p < .001. Finally, a higher percentage of the participants with VCFS and ADHD (27.0%) and controls with ADHD (37.5%) had a first degree relative with ADHD than both non-ADHD groups, p < .001.
After controlling for Time 1 variables, no significant differences emerged over time on any PAS or BASC Adaptive Behavioral variables. Although differences exist between the groups at Time 2, after controlling for Time 1 differences, no time X group interactions emerged.
Several behavioral differences existed between groups at Time 1 on BASC Parent Report (Table II; available at www.jpeds.com). Across all BASC domains, the two ADHD groups (with and without VCFS) were rated by parents as having more behavioral symptoms; only the BASC Attention scales, however, reached “clinical importance.” Several cognitive differences existed between the two VCFS groups on Time 1 psychological test performance (Table III). However, only one Time 1 BASC Parent variable predicted Time 2 ADHD status (Table IV). In addition to BASC Hyperactivity, other Time 1 variables which significantly predict ADHD status in the VCFS cohort include CVLT-C Intrusions, CVLT-C List B performance, a Time 1 major depressive disorder diagnosis and having a first degree family relative with ADHD. Of those variables, having a first degree family relative with ADHD was the strongest predictor.
Table 2.
Parent BASC T-Scores by Diagnosis and ADHD at Time 1
| VCFS + ADHD (N=37) | VCFS + No ADHD (N=35) | Control + ADHD (N=16) | Control + No ADHD (N=7) | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Hyperactivity | 63.0 ± 17.0 | 53.1 ± 9.0 | 62.9 ± 14.7 | 52.0 ± 8.6 |
| Aggression | 52.4 ± 10.9 | 48.1 ± 9.4 | 52.9 ± 8.7 | 47.4 ± 7.3 |
| Conduct Problems | 52.3 ± 9.2 | 48.5 ± 7.7 | 53.1 ± 8.5 | 46.2 ± 8.5 |
| Anxiety | 58.0 ± 13.2 | 56.7 ± 12.8 | 56.1 ± 10.9 | 46.5 ± 9.5 |
| Depression | 60.5 ± 13.1 | 53.4 ± 12.4 | 51.4 ± 8.0 | 48.0 ± 12.3 |
| Somatization | 60.2 ± 18.5 | 58.0 ± 10.8 | 52.0 ± 10.0 | 48.2 ± 9.9 |
| Atypicality | 60.1 ± 18.1 | 52.1 ± 12.1 | 55.8 ± 12.7 | 49.6 ± 9.9 |
| Withdrawal | 63.8 ± 14.8 | 62.0 ± 17.2 | 55.8 ± 12.7 | 52.2 ± 17.0 |
| Attention | 69.2 ± 18.7 | 62.8 ± 9.9 | 66.1 ± 10.6 | 55.8 ± 9.3 |
| Social Skills | 39.5 ± 7.9 | 41.1 ± 9.1 | 46.0 ± 11.4 | 45.3 ± 10.0 |
| Leadership Skills | 34.8 ± 6.9 | 38.1 ± 8.9 | 42.0 ± 5.1 | 44.7 ± 9.7 |
| Externalizing Composite | 58.6 ± 11.9 | 48.6 ± 8.5 | 55.9 ± 9.0 | 47.1 ± 7.1 |
| Internalizing Composite | 61.4 ± 13.8 | 57.1 ± 11.6 | 54.3 ± 8.8 | 47.1 ± 10.8 |
| Behavioral Symptom Index | 65.3 ± 14.6 | 55.3 ± 10.9 | 59.0 ± 9.2 | 49.2 ± 9.3 |
| Adaptive Skills Index | 36.0 ± 7.4 | 38.7 ± 9.2 | 43.4 ± 8.8 | 44.2 ± 10.0 |
Table 3.
Time 1 Psychological Test Standard Scores
| Psychological Test | VCFS | VCFS+ADHD | Control | Control+ADHD | Main Effects |
|---|---|---|---|---|---|
| WISC-III | |||||
| Full Scale IQ | 70.4 (13.9) *** | 71.8 (13.3) *** | 95.1 (14.2) | 93.8 (14.8) | C, CA > VCFS, VCFSA |
| Verbal IQ | 72.4 (15.2) *** | 75.5 (13.2) *** | 95.3 (13.7) | 91.1 (12.8) | C, CA > VCFS, VCFSA |
| Performance IQ | 71.2 (11.8) *** | 70.8 (14.9) *** | 96.3 (13.9) | 97.7 (14.7) | C, CA > VCFS, VCFSA |
| FD IQ | 80.4 (14.6) *** | 80.4 (12.6) *** | 96.0 (14.9) | 94.0 (17.0) | C, CA > VCFS, VCFSA |
| Processing Speed IQ | 85.6 (16.9) *** | 81.5 (15.4) *** | 99.8 (16.3) | 94.2 (13.9) | C, CA > VCFS, VCFSA |
| WIAT-II | |||||
| Reading | 78.2 (16.3) *** | 77.5 (13.3) *** | 98.6 (11.2) | 94.6 (14.3) | C, CA > VCFS, VCFSA |
| Math | 71.3 (18.4) *** | 71.6 (13.6) *** | 95.7 (14.5) | 93.4 (12.8) | C, CA > VCFS, VCFSA |
| Written Expression | 73.2 (16.3) *** | 74.7 (14.2) *** | 94.8 (11.3) | 94.0 (13.3) | C, CA > VCFS, VCFSA |
| CVLT-C | |||||
| List A Total T-Score | 38.4 (11.7) ** | 35.6 (12.4) *** | 45.7 (12.2) | 41.5 (13.5) | C, CA > VCFS, VCFSA |
| List A Trial 1 | − 0.7 (1.0) ** | − 0.9 (0.7) ** | − 0.2 (0.7) | − 0.6 (0.7) * | C > CA, VCFS, VCFSA |
| List A Trial 5 | − 0.9 (1.3) ** | − 1.1 (1.0) *** | − 0.3 (1.2) | − 0.8 (0.6) ** | C > CA, VCFS, VCFSA |
| List B | − 0.7 (1.0) ** | − 1.6 (1.2) *** | − 0.4 (0.6) * | 0.0 (0.9) | C > CA, VCFS > VCFSA |
| List A Short Delay | − 0.8 (0.8) *** | − 1.2 (0.8) *** | − 0.2 (0.8) | − 0.2 (0.5) | C, CA > VCFS, VCFSA |
| List A Long Delay | − 0.8 (0.9) *** | − 1.3 (0.9) *** | − 0.1 (0.8) | − 0.2 (0.9) | C, CA > VCFS, VCFSA |
| Total Intrusions | 0.0 (0.8) | 1.7 (0.8) *** | 0.2 (0.5) | 1.1 (0.8) *** | VCFS, C > VCFSA, CA |
| Total Perseverations | − 0.2 (0.7) | − 0.2 (0.6) | − 0.2 (0.3) | − 0.1 (0.4) | ----- ----- |
| Visual Span | |||||
| Forward Z-Score | − 0.7 (0.6) | − 0.9 (0.6) | − 0.4 (0.7) | − 0.7 (0.4) | ----- ----- |
| Backward Z-Score | − 1.2 (0.9) ** | − 1.4 (0.6) ** | − 0.7 (1.0) | − 0.7 (0.4) | C, CA > VCFS, VCFSA |
| CPT – GDS Vigilance Task | |||||
| Omission Z-Score | − 1.1 (1.5) *** | − 1.4 (1.0) *** | − 0.1 (0.8) | − 0.6 (0.9) * | C > CA > VCFS, VCFSA |
| Commission Z-Score | − 1.1 (0.9) ** | − 3.0 (0.9) *** | − 0.1 (0.9) | − 2.4 (0.5) *** | C > VCFS > CA, VCFSA |
| WCST | |||||
| Perseverative Errors | 73.7 (18.3) *** | 68.7 (20.1) *** | 91.3 (16.4) | 84.5 (13.3) | C, CA > VCFS, VCFSA |
| Nonperseverative Errors | 86.6 (18.0) ** | 81.0 (11.7) *** | 94.3 (18.2) | 87.4 (14.4) ** | C > VCFS, CA > VCFSA |
| Tower of London | |||||
| Total Moves Z-Score | − 0.4 (0.4) *** | − 0.4 (0.8) *** | 0.7 (0.5) | − 0.5 (0.8) *** | C > CA, VCFS, VCFSA |
Note. C = Control group. CA = Control group with ADHD. VCFS = Velocardiofacial syndrome. VCFSA = VCFS + ADHD. WISC-III = Wechsler Intelligence Scale for Children – 3rd edition (Wechsler, 1991). FD = Freedom from Distractibility. WIAT-II = Wechsler Individual Achievement Test-Third edition. CVLT = California Verbal Learning Test – Children’s version (CVLT-C). GDS-CPT = Gordon Diagnostic System - Continuous Performance Test (CPT). WCST = Wisconsin Card Sorting Test. IQ = Intellectual Quotient.
p < .05;
p < .01;
p < .001.
Table 4.
Significant Results from Logistic Regression Predicting Time 2 ADHD status in VCFS and Control Participants from Time 1 Variables
| VCFS | ||
|---|---|---|
| Time 1 Variable | β | p |
| Psychological Tests | ||
| CVLT-C Total Intrusions Standard Score | .486 | .036 |
| CVLT-C List B Standard Score | .468 | .041 |
| Behavioral / Psychiatric | ||
| BASC Parent Hyperactivity T-score | .103 | .050 |
| MDD diagnosis | .163 | .042 |
| First Degree Relative with ADHD | .523 | .010 |
| Control | ||
|---|---|---|
| Time 1 Variable | β | p |
| Psychological Tests | ||
| CPT Errors of Commission Z-score | .442 | .002 |
| Behavioral / Psychiatric | ||
| BASC Parent Hyperactivity T-score | .230 | .034 |
| BASC Parent Attention Problems T-score | .321 | .024 |
| First Degree Relative with ADHD | .445 | .017 |
Note. CVLT-C = California Verbal Learning Test-Children’s version. BASC = Behavioral Assessment Scales for Children. CPT = Continuous Performance Test. MDD = Major Depressive Disorder.
In the control sample, Time 1 CPT errors of commission predicted Time 2 ADHD status. Similar to the VCFS sample, Time 1 parent report of hyperactivity and having first degree relatives with ADHD were predictive of maintaining ADHD status at Time 2. In addition, and unlike the VCFS sample, parent report of attention problems was predictive of Time 2 ADHD status.
Discussion
Our data suggest that the longitudinal persistence of ADHD in VCFS is comparable with the longitudinal trajectory of ADHD in the non-VCFS population. Also consistent with the non-VCFS ADHD literature (50–53), youth with VCFS and comorbid ADHD had significantly increased prevalence of oppositional defiant disorder relative to adolescents with VCFS yet without ADHD. However, unlike the idiopathic ADHD literature (11, 51–53), mood and anxiety disorders were not more prevalent in the VCFS + ADHD cohort relative to the VCFS cohort. This suggests that VCFS, not ADHD, may be driving the increased prevalence of anxiety and mood disorders in VCFS.
An interesting finding is the increase in parent report of hyperactivity / impulsivity in the VCFS+ADHD cohort. This is the opposite of what is typically reported in the idiopathic ADHD literature (49) where inattention is more enduring. Our finding of increased hyperactivity / impulsivity in the VCFS+ADHD cohort may be a function of the relatively low rates of stimulant treatment in this group. Stimulants lessen hyperactivity / impulsivity symptoms in both the idiopathic ADHD (54) as well as the VCFS+ADHD population (55). Although this has not been tested empirically, it may be that concerns regarding possible manic or psychotic symptoms as a function of stimulant treatment are responsible for the lower stimulant treatment rates in our VCFS + ADHD sample.
Similar to the idiopathic ADHD literature (56–59), genetics appear to play a role in the etiology of those with VCFS who continue to demonstrate clinically significant ADHD across time. If not already doing so, clinicians who assess / treat children with VCFS + ADHD should inquire about first-degree relatives with ADHD.
Childhood hyperactivity appears to be a better predictor of which children with VCFS + ADHD will continue to have ADHD as adolescents. This is in contrast to our control ADHD sample in which both hyperactivity and inattention levels during childhood predicted syndromal ADHD persistence. The low predictive power of inattentive behaviors in the VCFS sample may be a function of the general cognitive delays inherent in this population. Inattentive behaviors are considered relatively common in individuals with lowered general intellectual functioning (60–62).
A MDD diagnosis in childhood also appears to predict the stability of ADHD across time in the VCFS population. This finding, although not present in our control ADHD sample, is consistent with some extant ADHD data (63) and suggests that MDD in the context of ADHD may be predictive power for what is to come in adolescence.
The CVLT-C appears to be the best psychological test for predicting persistence of ADHD in the VCFS population. Having higher levels of errors of intrusions (recalling items that were not on the list) and lower performance on List B (the interference trial) both predicted which children with VCFS + ADHD would become adolescents with VCFS + ADHD. To our knowledge, the CVLT-C has not been used longitudinally in the idiopathic ADHD literature.
Our results must be interpreted in the context of methodological limitations. Our control sample was small. Second, ADHD is a difficult diagnosis to make in the context of intellectual delays (64). Third, because we did not manipulate treatment as an independent variable, we cannot use our data to describe the impact that the relative absence of treatment has on ADHD stability. Fourth, we relied exclusively on parent report of school functioning rather than querying the teachers directly.
These findings confirm that persistence of ADHD into adolescence in VCFS is predictable by childhood variables that are also predictive in the non-VCFS ADHD population.
Acknowledgments
Funded by the National Institute of Mental Health (MH64824 and MH65481 to W. K.). S. F. has received consulting fees and served on advisory boards for several pharmaceutical companies.
Abbreviations
- VCFS
velo-cardio-facial syndrome
- ADHD
attention deficit / hyperactivity disorder
- MDD
major depressive disorder
Footnotes
The other authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robin NH, Shprintzen RJ. Defining the clinical spectrum of deletion 22q11.2. J Pediatr. 2005;147(1):90–6. doi: 10.1016/j.jpeds.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, et al. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112(1 Pt 1):101–7. doi: 10.1542/peds.112.1.101. [DOI] [PubMed] [Google Scholar]
- 3.Morrow B, Goldberg R, Carlson C, Das Gupta R, Sirotkin H, Collins J, et al. Molecular definition of the 22q11 deletions in velo-cardio-facial syndrome. Am J Hum Genet. 1995;56(6):1391–403. [PMC free article] [PubMed] [Google Scholar]
- 4.Shprintzen R, Higgins AM, Antshel K, Fremont W, Roizen N, Kates W. Velo-cardio-facial syndrome. Curr Opin Pediatr. 2005;17(6):725–30. doi: 10.1097/01.mop.0000184465.73833.0b. [DOI] [PubMed] [Google Scholar]
- 5.Antshel KM, Fremont W, Roizen NJ, Shprintzen R, Higgins AM, Dhamoon A, et al. ADHD, major depressive disorder, and simple phobias are prevalent psychiatric conditions in youth with velocardiofacial syndrome. J Am Acad Child Adolesc Psychiatry. 2006;45(5):596–603. doi: 10.1097/01.chi.0000205703.25453.5a. [DOI] [PubMed] [Google Scholar]
- 6.Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009;48(11):1060–8. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- 7.Larsson H, Dilshad R, Lichtenstein P, Barker ED. Developmental trajectories of DSM-IV symptoms of attention-deficit/hyperactivity disorder: genetic effects, family risk and associated psychopathology. J Child Psychol Psychiatry. 2011;52(9):954–63. doi: 10.1111/j.1469-7610.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- 8.Lara C, Fayyad J, de Graaf R, Kessler RC, Aguilar-Gaxiola S, Angermeyer M, et al. Childhood predictors of adult attention-deficit/hyperactivity disorder: results from the World Health Organization World Mental Health Survey Initiative. Biol Psychiatry. 2009;65(1):46–54. doi: 10.1016/j.biopsych.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessler RC, Adler LA, Barkley R, Biederman J, Conners CK, Faraone SV, et al. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: results from the national comorbidity survey replication. Biol Psychiatry. 2005;57(11):1442–51. doi: 10.1016/j.biopsych.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biederman J, Faraone S, Milberger S, Curtis S, Chen L, Marrs A, et al. Predictors of persistence and remission of ADHD into adolescence: results from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1996;35(3):343–51. doi: 10.1097/00004583-199603000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Fischer M, Barkley RA, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: self-reported psychiatric disorders, comorbidity, and the role of childhood conduct problems and teen CD. J Abnorm Child Psychol. 2002;30(5):463–75. doi: 10.1023/a:1019864813776. [DOI] [PubMed] [Google Scholar]
- 12.Fischer M, Barkley RA, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: II. Academic, attentional, and neuropsychological status. J Consult Clin Psychol. 1990;58(5):580–8. doi: 10.1037//0022-006x.58.5.580. [DOI] [PubMed] [Google Scholar]
- 13.Antshel KM, Faraone SV, Fremont W, Monuteaux MC, Kates WR, Doyle A, et al. Comparing ADHD in Velocardiofacial Syndrome to Idiopathic ADHD: A Preliminary Study. J Atten Disord. 2007;11(1):64–73. doi: 10.1177/1087054707299397. [DOI] [PubMed] [Google Scholar]
- 14.Faraone S, Biederman J, Milberger S. An exploratory study of ADHD among second-degree relatives of ADHD children. Biological Psychiatry. 1994;35:398–402. doi: 10.1016/0006-3223(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 15.Manshadi M, Lippmann S, O’Daniel R, Blackman A. Alcohol abuse and attention deficit disorder. Journal of Clinical Psychiatry. 1983;44:379–80. [PubMed] [Google Scholar]
- 16.Biederman J, Faraone SV, Mick E, Spencer T, Wilens T, Keily K, et al. High risk for attention deficit hyperactivity disorder among children of parents with childhood onset of the disorder: A pilot study. American Journal of Psychiatry. 1995;152:431–5. doi: 10.1176/ajp.152.3.431. [DOI] [PubMed] [Google Scholar]
- 17.Gothelf D, Presburger G, Levy D, Nahmani A, Burg M, Berant M, et al. Genetic, developmental, and physical factors associated with attention deficit hyperactivity disorder in patients with velocardiofacial syndrome. Am J Med Genet B Neuropsychiatr Genet. 2004;126B(1):116–21. doi: 10.1002/ajmg.b.20144. [DOI] [PubMed] [Google Scholar]
- 18.Brewer WJ, Francey SM, Wood SJ, Jackson HJ, Pantelis C, Phillips LJ, et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry. 2005;162(1):71–8. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins KA, Addington J, Keefe RS, Christensen B, Perkins DO, Zipurksy R, et al. Neuropsychological status of subjects at high risk for a first episode of psychosis. Schizophr Res. 2004;67(2–3):115–22. doi: 10.1016/j.schres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51(2):124–31. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 21.Byrne M, Hodges A, Grant E, Owens DC, Johnstone EC. Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh High Risk Study (EHRS) Psychol Med. 1999;29(5):1161–73. doi: 10.1017/s0033291799001002. [DOI] [PubMed] [Google Scholar]
- 22.Erlenmeyer-Kimling L, Roberts SA, Rock D, Adamo UH, Shapiro BM, Pape S. Prediction from longitudinal assessments of high-risk children. In: Lenzenweger MFaD, RH, editors. Origins and Development of Schizophrenia: Advances in Experimental Psychopathology. Washington, DC: American Psychological Association; 1998. pp. 427–45. [Google Scholar]
- 23.Seidman LJ, Giuliano AJ, Smith CW, Stone WS, Glatt SJ, Meyer E, et al. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: results from the Harvard and Hillside Adolescent High Risk Studies. Schizophr Bull. 2006;32(3):507–24. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cosway R, Byrne M, Clafferty R, Hodges A, Grant E, Abukmeil SS, et al. Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychol Med. 2000;30(5):1111–21. doi: 10.1017/s0033291799002585. [DOI] [PubMed] [Google Scholar]
- 25.Simon AE, Cattapan-Ludewig K, Zmilacher S, Arbach D, Gruber K, Dvorsky DN, et al. Cognitive functioning in the schizophrenia prodrome. Schizophr Bull. 2007;33(3):761–71. doi: 10.1093/schbul/sbm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hambrecht M, Lammertink M, Klosterkotter J, Matuschek E, Pukrop R. Subjective and objective neuropsychological abnormalities in a psychosis prodrome clinic. Br J Psychiatry Suppl. 2002;43:s30–7. doi: 10.1192/bjp.181.43.s30. [DOI] [PubMed] [Google Scholar]
- 27.Toomey R, Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Tsuang MT. Association of neuropsychological vulnerability markers in relatives of schizophrenic patients. Schizophr Res. 1998;31(2–3):89–98. doi: 10.1016/s0920-9964(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 28.Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20(1):31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- 29.Cornblatt B, Obuchowski M, Schnur D, O’Brien JD. Hillside study of risk and early detection in schizophrenia. Br J Psychiatry Suppl. 1998;172(33):26–32. [PubMed] [Google Scholar]
- 30.Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59(9):863–71. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Wolf LE, Cornblatt BA, Roberts SA, Shapiro BM, Erlenmeyer-Kimling L. Wisconsin Card Sorting deficits in the offspring of schizophrenics in the New York High-Risk Project. Schizophr Res. 2002;57(2–3):173. doi: 10.1016/s0920-9964(01)00301-2. [DOI] [PubMed] [Google Scholar]
- 32.Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–55. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- 33.Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. American Journal of Psychiatry. 2000;157(5):816–8. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- 34.Biederman J, Petty CR, Ball SW, Fried R, Doyle AE, Cohen D, et al. Are cognitive deficits in attention deficit/hyperactivity disorder related to the course of the disorder? A prospective controlled follow-up study of grown up boys with persistent and remitting course. Psychiatry Res. 2009;170(2–3):177–82. doi: 10.1016/j.psychres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biederman J, Petty CR, Doyle AE, Spencer T, Henderson CS, Marion B, et al. Stability of executive function deficits in girls with ADHD: a prospective longitudinal followup study into adolescence. Developmental Neuropsychology. 2008;33(1):44–61. doi: 10.1080/87565640701729755. [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D. Wechsler Intelligence Scale for Children. 3. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 37.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 38.Wechsler D. Wechsler Individual Achievement Test. 2. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]
- 39.Gordon M. The Gordon Diagnostic System. DeWitt, NY: Gordon Systems; 1983. [Google Scholar]
- 40.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 41.Delis D, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Children’s version. San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
- 42.Davis HR. Colorado Assessment Tests - Visual Span Test. Boulder, CO: Colorado Assessment Tests; 1998. [Google Scholar]
- 43.Spreen O, Strauss E. A compendium of neuropsychological tests. 2. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 44.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 45.APA. DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 46.Cannon-Spoor HE, Potkin SG, Wyatt RJ. Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull. 1982;8(3):470–84. doi: 10.1093/schbul/8.3.470. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds CR, Kamphaus RW. Behavior Assessment Scales for Children (BASC) Circle Pines, MN: American Guidance Service; 1992. [Google Scholar]
- 48.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–59. doi: 10.1001/archpsyc.1994.03950110009002. discussion 63–4. [DOI] [PubMed] [Google Scholar]
- 49.Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychological Medicine. 2006;36(2):159–65. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- 50.Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, et al. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psychol Med. 2006;36(2):167–79. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- 51.MTA Collaborative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: changes in effectiveness and growth after the end of treatment. Pediatrics. 2004;113(4):762–9. doi: 10.1542/peds.113.4.762. [DOI] [PubMed] [Google Scholar]
- 52.Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol Psychiatry. 2004;45(2):195–211. doi: 10.1111/j.1469-7610.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 53.Mannuzza S, Klein RG, Bessler A, Malloy P, Hynes ME. Educational and occupational outcome of hyperactive boys grown up. J Am Acad Child Adolesc Psychiatry. 1997;36(9):1222–7. doi: 10.1097/00004583-199709000-00014. [DOI] [PubMed] [Google Scholar]
- 54.Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed. 2006;8(4):4. [PMC free article] [PubMed] [Google Scholar]
- 55.Gothelf D, Gruber R, Presburger G, Dotan I, Brand-Gothelf A, Burg M, et al. Methylphenidate treatment for attention-deficit/hyperactivity disorder in children and adolescents with velocardiofacial syndrome: an open-label study. J Clin Psychiatry. 2003;64(10):1163–9. doi: 10.4088/jcp.v64n1004. [DOI] [PubMed] [Google Scholar]
- 56.Biederman J, Faraone SV, Monuteaux MC, Bober M, Cadogen E. Gender effects on attention-deficit/hyperactivity disorder in adults, revisited. Biol Psychiatry. 2004;55(7):692–700. doi: 10.1016/j.biopsych.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 57.Faraone SV, Biederman J, Monuteaux MC. Toward guidelines for pedigree selection in genetic studies of attention deficit hyperactivity disorder. Genetic Epidemiology. 2000;18(1):1–16. doi: 10.1002/(SICI)1098-2272(200001)18:1<1::AID-GEPI1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 58.Biederman J, Faraone S, Milberger S, Guite J, Mick E, Chen L, et al. A prospective 4-year follow-up study of attention-deficit hyperactivity and related disorders. Arch Gen Psychiatry. 1996;53(5):437–46. doi: 10.1001/archpsyc.1996.01830050073012. [DOI] [PubMed] [Google Scholar]
- 59.Faraone SV, Biederman J, Feighner JA, Monuteaux MC. Assessing symptoms of attention deficit hyperactivity disorder in children and adults: which is more valid? Journal of Consulting and Clinical Psychology. 2000;68(5):830–42. [PubMed] [Google Scholar]
- 60.Einfeld SL, Aman M. Issues in the taxonomy of psychopathology in mental retardation. J Autism Dev Disord. 1995;25(2):143–67. doi: 10.1007/BF02178501. [DOI] [PubMed] [Google Scholar]
- 61.Gjaerum B, Bjornerem H. Psychosocial impairment is significant in young referred children with and without psychiatric diagnoses and cognitive delays--applicability and reliability of diagnoses in face of co-morbidity. Eur Child Adolesc Psychiatry. 2003;12(5):239–48. doi: 10.1007/s00787-003-0329-z. [DOI] [PubMed] [Google Scholar]
- 62.Tonge BJ, Einfeld SL, Krupinski J, Mackenzie A, McLaughlin M, Florio T, et al. The use of factor analysis for ascertaining patterns of psychopathology in children with intellectual disability. J Intellect Disabil Res. 1996;40 (Pt 3):198–207. [PubMed] [Google Scholar]
- 63.Hurtig T, Ebeling H, Taanila A, Miettunen J, Smalley SL, McGough JJ, et al. ADHD symptoms and subtypes: relationship between childhood and adolescent symptoms. J Am Acad Child Adolesc Psychiatry. 2007;46(12):1605–13. doi: 10.1097/chi.0b013e318157517a. [DOI] [PubMed] [Google Scholar]
- 64.Antshel KM, Phillips MH, Gordon M, Barkley R, Faraone SV. Is ADHD a valid disorder in children with intellectual delays? Clin Psychol Rev. 2006;26(5):555–72. doi: 10.1016/j.cpr.2006.03.002. [DOI] [PubMed] [Google Scholar]


