Abstract
Herpes simplex virus type 2 (HSV-2) is transmitted through the genital mucosa during sexual encounters. In recent years, HSV-1 has also become commonly associated with primary genital herpes. The mechanism of viral entry of HSV-1 and HSV-2 in the female genital tract is unknown. In order to understand the molecular interactions required for HSV entry into the vaginal epithelium, we examined the expression of herpesvirus entry mediator nectin-1 in the vagina of human and mouse at different stages of their hormonal cycle. Nectin-1 was highly expressed in the epithelium of human vagina throughout the menstrual cycle, whereas the mouse vaginal epithelium expressed nectin-1 only during the stages of the estrous cycle in which mice are susceptible to vaginal HSV infection. Furthermore, the ability of nectin-1 to mediate viral entry following intravaginal inoculation was examined in a mouse model of genital herpes. Vaginal infection with either HSV-1 or HSV-2 was blocked by preincubation of the virus with soluble recombinant nectin-1. Viral entry through the vaginal mucosa was also inhibited by preincubation of HSV-2 with antibody against gD. Together, these results suggest the importance of nectin-1 in mediating viral entry for both HSV-1 and HSV-2 in the genital mucosa in female hosts.
Understanding the entry events of herpes simplex virus type 2 (HSV-2) at the genital mucosa is important in the development of preventative measures, such as topical microbicides, to stop its spread through sexual transmission. Although HSV-1 is classically associated with oral infection, an alarming increase in HSV-1 has been observed in association with primary genital herpes in recent years (14). The in vivo mechanism of viral entry in the female genital tract of HSV-1 and HSV-2 is unknown.
The molecular mechanisms of virus attachment and entry have been a focus of intense investigations, especially for HSV-1 but also for HSV-2 (reviewed in reference 30). Currently, the model for HSV entry begins with the attachment of the virus to a target cell through binding of HSV glycoprotein C (gC) and/or gB to the cell surface heparan sulfate proteoglycans (reviewed in reference 28). Subsequently, the attached virus can begin its entry through interaction between gD and a variety of unrelated cell surface receptors, including the herpesvirus entry mediator A (HveA) (21), a member of the tumor necrosis factor receptor family, HveB (nectin-2) (33) and HveC (nectin-1) (4), both members of the immunoglobulin superfamily, and 3-O-sulfated heparan sulfate (27).
Owing to its limited expression in lymphocytes and monocytes (13), HveA has been shown to mediate HSV entry into human activated T lymphocytes (21). Whether HveA participates in viral entry through genital epithelial cells is unknown. Human HveB mediates the entry of HSV-2 and certain mutant strains of HSV-1 but fails to mediate entry of wild-type HSV-1 (33). Furthermore, evidence indicates that the mouse homologue of HveB fails to mediate entry of either HSV-1 or HSV-2 (16). In contrast, nectin-1 has been known for its potent ability to mediate entry of both HSV-1 and HSV-2 and has been shown to be expressed in a variety of cells, including epithelial cells and neuronal cells (4, 17, 25, 26), making this molecule a prime candidate receptor for HSV-1 and HSV-2 entry at the mucosal epithelium.
Nectin-1 is a member of the nectin family, which localizes at the adherens junctions of epithelial cells and functions as a cell adhesion molecule (31, 32). While in vitro studies support the notion that the binding of gD to a cell surface receptor is necessary for virus entry (4, 8, 15, 21), it is not clear which receptor(s) mediates the entry of HSV-1 and HSV-2 in vivo at the natural sites of virus transmission. Furthermore, although nectin-1 expression has been demonstrated in the rodent epidermis and vaginal epithelium with a rabbit polyclonal antibody (17, 26), the expression of nectin-1 in the vaginal mucosa with respect to the localization and function, particularly with respect to the hormonal cycle, is not clear.
In a mouse model of HSV-2 vaginal infection with the thymidine kinase (TK) mutant strain of HSV-2, susceptibility to the virus occurs primarily during the diestrous and proestrous phases of the estrous cycle (3, 23). However, how susceptibility is influenced by the hormonal changes in the female host is unknown. The susceptible stages are represented by a characteristically thinned epithelium, consisting of five to seven cell layers, which can also be induced and maintained by treatment with progesterone derivatives such as depo-medroxyprogesterone acetate. One potential mechanism relates to the thickness and the permeability of the vaginal epithelial layer. With the increase in serum estrogen levels, the epithelial cell layer thickens during the estrous stage. Following ovulation, with the decrease in the estrogen and increase in the progesterone levels, the superficial layers of the vaginal epithelium are delaminated during the metestrous phase, and by the diestrous phase, the epithelial layer becomes maximally thin and most permeable to luminal proteins (24).
However, this hypothesis was challenged by a recent study in which a dissociation in the correlation between the thickness of the vaginal epithelium and the susceptibility to HSV-2 infection was demonstrated by treatment of mice with different forms of progesterone (10). Kaushic et al. demonstrated that depo-medroxyprogesterone acetate treatment of mice resulted in a 100-fold increase in susceptibility to genital HSV-2 compared to untreated mice at diestrous phase. Furthermore, long-term effects of depo-medroxyprogesterone acetate treatment included reduction in protective immunity to HSV-2 (10). Thus, the thickness and gross morphology of the vaginal epithelium alone cannot account for the susceptibility to HSV-2 infection.
Another potential basis for hormonal regulation of susceptibly to HSV-2 genital infection is the expression of receptor on the mucosal epithelial cell surface. Because vaginal epithelium is continually undergoing regenerative changes, cellular expression of the viral entry molecules may also vary depending on the hormonal status of the host. The difference in location and/or intensity of receptor expression may account for the variability in incidence of intravaginal HSV-2 infection throughout the sex hormone cycle. Although correlation between the phases of the menstrual cycle and susceptibility to HSV-2 infection has not been examined in humans, cervical shedding of HSV was significantly associated with oral contraception and depo-medroxyprogesterone acetate use (22).
In this study, we examined the expression of nectin-1 in human and murine vaginal tissues at various stages of the menstrual and estrous cycles, respectively. Nectin-1 is conserved between humans and mice with 95% sequence identity at the amino acid level (20, 26), and both species' forms have the broadest range of herpesviruses for they mediate entry of HSV-1, HSV-2, porcine pseudorabies virus, and bovine herpesvirus-1 in vitro (19, 26). Here, we demonstrate that human vaginal epithelium expresses nectin-1 at all stages of the menstrual cycle. In contrast, nectin-1 is expressed on the superficial epithelial cells of the mouse vagina at the susceptible diestrous and proestrous phases of the estrous cycle. Furthermore, using soluble nectin-1 to block viral binding to its host receptor(s), we demonstrate the requirement for the availability of the nectin-1 binding site in establishing in vivo infection through the vaginal mucosa. The in vivo importance of gD in the establishment of replication in the vaginal tract is revealed through blockage of gD with a highly specific antibody. The results presented in this study demonstrate both the phenotypic and functional relevance of nectin-1 in mediating entry of both HSV-1 and HSV-2 into the vaginal epithelium in vivo and suggest the importance of nectin-1 in genital transmission of HSV in women.
MATERIALS AND METHODS
Animals.
Six- to eight-week-old female BALB/c mice were obtained from the National Cancer Institute (Frederick, Md.). For virus infection studies, mice were injected subcutaneously in the neck ruff with depo-medroxyprogesterone acetate (Depo-Provera; Pharmacia & Upjohn Company, Kalamazoo, Mich.) at 2 mg/mouse in a 100-μl volume 5 to 7 days prior to infection, swabbed with calcium-alginate, and inoculated intravaginally with the indicated dose of HSV-2 or HSV-1 in 10-μl volumes with a blunt-ended micropipette tip. All procedures used in this study complied with federal guidelines and institutional policies of the Yale animal care and use committee.
Tissue collection.
Vaginas from normal mice were collected and embedded into optimum-cutting-temperature compound (OCT; Sakura Finetek USA, Inc., Torrance, Calif.) for frozen sectioning. The estrous stages of mice were determined from analysis of vaginal smears taken prior to sacrifice by a calcium alginate swab (Fisher Scientific, Houston, Tex.) and stained with Diff-Quik Stain (Dade Behring, Dudingen, Switzerland) according to the manufacturer's instructions. Stained cells were carefully examined and the estrous stage of each mouse was identified as diestrus, proestrus, estrus, metestrus-1, or metestrus-2 according to a previously established protocol (5).
Human vaginal tissues resulting from surgical procedures were obtained through the Department of Obstetrics and Gynecology at the Yale University School of Medicine under an institutionally approved Human Investigation Committee protocol and frozen in OCT several hours post-surgical removal. All human specimens used in this study were of premenopausal adult origin and their stage in the menstrual cycle was identified by their maturation indices. Tissues from a total of 18 subjects who ranged from 24 to 44 years of age were collected and examined for the expression of nectin-1 as well as for the presence of dendritic cells and lymphocytes to exclude tissues that had obvious inflammatory conditions. All procedures used in this study complied with federal guidelines and institutional policies of the Yale Human Investigation Committee.
Antibodies.
Monoclonal antibodies CK6 and CK8 (11), which are specific for overlapping linear epitopes on the V domain of nectin-1 between amino acids 83 and 104, were used to detect nectin-1 on tissue sections. The rabbit polyclonal antibody specific for the HSV-2 glycoprotein gD (R8) was generated as previously described (6). The concentrations of all antibodies used in this study were determined by enzyme-linked immunosorbent assay prior to use.
Immunofluorescence staining.
To examine the distribution of nectin-1 in vaginal tissue at different stages of the hormonal cycle, frozen sections of vagina were stained with the described antibodies in a procedure similar to that described previously (7) with minor modifications. Briefly, 7-μm frozen sections were fixed in acetone and blocked with TNB buffer (0.1 M Tris-HCl−0.15 M NaCl−0.5% blocking reagent) (NEN Life Science Products Inc., Boston, Mass.) containing 5% normal donkey serum and an avidin-biotin solution (Vector Laboratories Inc., Burlingame, Calif.) to block endogenous biotin. Endogenous peroxidase activity was quenched with 1% H2O2. To stain mouse tissues with mouse monoclonal antibodies, the sections were further blocked with Fab goat anti-mouse immunoglobulin G (IgG; Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.) for 1 h prior to the addition of the primary antibodies.
A previous study with rabbit polyclonal antisera R165 and R166 against nectin-1 demonstrated generalized expression of nectin-1 in the mouse vagina during metestrous-1 phase (26). Due to the differences in the staining protocols used, we did not observe specific staining with these rabbit polyclonal antisera, i.e., both preimmune and immune sera bound nonspecifically to most epithelial cells, including skin epidermis (data not shown). Thus, only purified mouse monoclonal antibodies were used in our study. Primary monoclonal antibody or isotype control was applied at 1 to 5 μg/ml for 1.5 h at room temperature. Slides were washed and incubated with biotin-conjugated donkey F(ab′)2 anti-mouse IgG (Jackson Immunoresearch Laboratories, Inc.), followed by incubation with streptavidin-horseradish peroxidase conjugate (Zymed Laboratories, South San Francisco, Calif.). The antigenic signal was amplified with tyramide-cyanine 3 (NEN Life Science Products, Inc.) according to manufacturer's instructions. In the case of double labeling on the same section, the sections were treated with 2% H2O2 for 10 min, followed by an avidin/biotin block. The second primary rat antibody against mouse E-cadherin (Zymed Laboratories) was then added at 5 μg/ml to the sections for 1.5 h. Slides were washed and incubated with biotin-conjugated donkey F(ab′)2 anti-rat IgG (Jackson Immunoresearch Laboratories, Inc.), followed by incubation with streptavidin-horseradish peroxidase conjugate (Zymed Laboratories.) The antigenic signal was amplified with tyramide-fluorescein isothiocyanate (NEN Life Science Products, Inc.) according to the manufacturer's instructions.
To detect HSV viral antigens in vaginal tissues, fluorescein isothiocyanate-conjugated antibody against HSV-1 and HSV-2 (ViroStat, Portland, Maine) was used at a concentration of 5 μg/ml. Slides were washed and incubated with 4′,6′-diamidino-2-phenylindole (DAPI) (Molecular Probes) to visualize nuclei and mounted with Fluoromount G (Southern Biotechnology Associates, Inc., Birmingham, Ala.). The sections were analyzed by a fluorescence microscope (Leitz Orthoplan 2) with either the 40× or 10× objective lenses or by confocal microscopy with the Zeiss LSM510 confocal microscope.
Viruses.
HSV-2 strains 186TKΔKpn (9) and 186syn+ (29) and HSV-1 strain KOS were kindly provided by David Knipe (Harvard Medical School). All viruses were propagated and quantitated by a plaque assay on Vero cells as previously described (9, 29).
HSV-2 blocking and infection.
Purified recombinant HveC(346t) was prepared as previously described (12). Each virus strain was incubated with 10 pg of HveC(346t) or phosphate-buffered saline (PBS)/PFU on ice for 2 h according to a previously described procedure (25). In the experiments described in the legend to Fig. 5, HSV-2 strain 186 was incubated on ice for 2 h with either a polyclonal rabbit antiserum specific for the HSV-2 glycoprotein D (R8) or with nonimmune rabbit serum at 10 μg/105 PFU. Mice were swabbed with calcium-alginate and then inoculated intravaginally with pretreated 186TKΔKpn (106 PFU/mouse), KOS (106 PFU/mouse), or 186 (105 PFU/mouse) virus in a 10-μl volume with a blunt-ended micropipette tip. At 24 h postinfection, mice were euthanized and vaginal washes were collected in 50 μl of PBS, followed by swabbing twice with the calcium-alginate tip applicator. Both the vaginal wash and the applicators were combined with 950 μl of 1% heat-inactivated fetal bovine serum-1% glucose-PBS and stored at −80°C. Viral titers of vaginal washes were determined by performing a plaque assay as previously described (9, 29). In all cases, viral infection was also confirmed by immunofluorescence staining of vaginal tissues with an anti-HSV antibody.
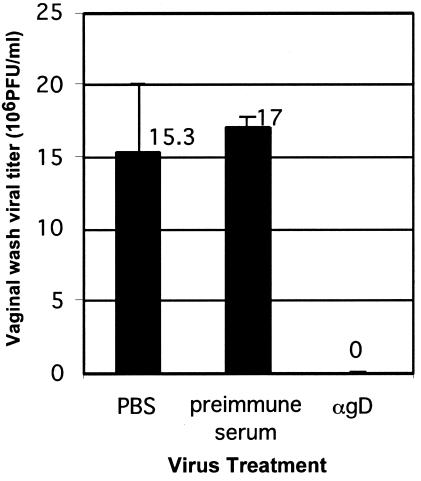
FIG. 5.
In vivo viral entry blockade by antibody against gD. Progesterone-treated female mice were inoculated intravaginally with HSV-2 wild-type strain 186 (105 PFU/mouse) that was incubated with either PBS, preimmune rabbit serum, or anti-gD rabbit antiserum. Viral load in the vaginal washes at 24 h postinfection was determined by plaque-forming assay. These results are expressed as the viral titer ± standard deviation and are representative of two similar experiments with five mice in each group.
RESULTS
Expression of nectin-1 in the human vaginal epithelium during various stages of the menstrual cycle.
To determine the role of nectin-1 in viral entry through the vaginal tract in vivo, its relative expression was analyzed by immunofluorescence staining of human vaginal tissues with highly specific monoclonal antibodies, CK6 and CK8. The nectin-1 molecule was found to be highly expressed within the vaginal epithelium during all representative stages of the menstrual cycle (Fig. 1). The expression pattern was consistently most intense in the stratum spinosum (layer above the basal cells) but was minimal in the basal proliferative cell layer (stratum germinativum). Nectin-1 was expressed closest to the luminal edge of the vaginal epithelium during the secretory phase (Fig. 1D). The staining patterns obtained with CK6 and CK8 antibodies were indistinguishable.
FIG. 1.
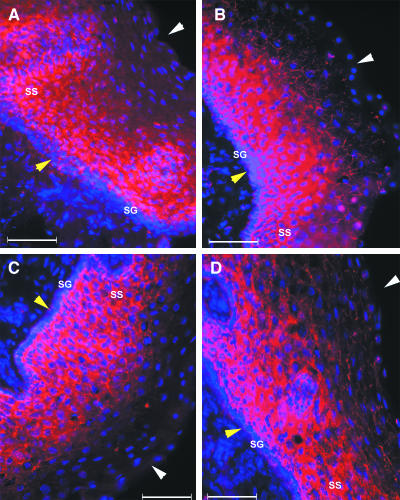
Expression of nectin-1 in human vaginal epithelium during menstrual cycle. Frozen sections of vaginal tissues from humans during menses (A) and early proliferative (B), proliferative (C), and secretory (D) phases were stained with monoclonal antibody CK6 specific for nectin-1 (red), and the nuclei were visualized by staining with DAPI (blue). Epithelial layers are indicated by the white arrowheads (luminal edge) and yellow arrowheads (basement membrane). The stratum germinativum (SG) and stratum spinosum (SS) are indicated. The bar indicates 100 μm in this and all subsequent figures. This figure is representative of several similar experiments involving between two and four subjects in each menstrual phase.
Expression of nectin-1 in murine vaginal epithelium during the estrous cycle.
Since the stages of the estrous cycle are known to influence the susceptibility of mice to intravaginal HSV-2 challenge (3, 23), vaginal tissues representing each stage of the estrous cycle were included in this analysis. In the mouse vaginal tissue, expression of nectin-1 with monoclonal antibody CK6 varied depending on the phase of the estrous cycle in both location and intensity. In the diestrous stage, nectin-1 expression was predominantly concentrated in the outermost layer of the epithelial cells (Fig. 2A). The distribution pattern of nectin-1 molecules at diestrous stage thus could provide a ready access to viruses that enter through the lumen of the vagina.
FIG. 2.
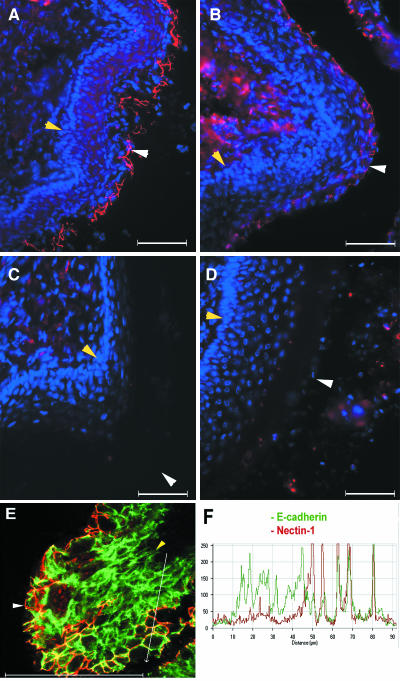
Expression of nectin-1 in mouse vaginal epithelium during estrous cycle. Frozen sections of vaginal tissues from mice at diestrous (A), proestrous (B), estrous (C), and metestrous-2 (D) phases were stained with monoclonal antibody CK6 specific for nectin-1 (red), and the nuclei were visualized by staining with DAPI (blue). Epithelial layers are indicated by the white arrowheads (luminal edge) and yellow arrowheads (basement membrane). (E) A diestrus vaginal epithelial tissue was doubly labeled with an antibody to nectin-1 (CK6; red) and to E-cadherin (green) and analyzed by confocal microscopy (F) An intensity profile for the region along the white line arrow in panel E for both E-cadherin (green) and nectin-1 (red) is depicted. These figures are representative of several similar experiments involving three or four mice in each group.
As the estrogen levels rise, mice enter the proestrous phase, characterized by increased thickness of the epithelial layer. The expression of nectin-1 was still present near the surface of the epithelium, but reduced in terms of its intensity (Fig. 2B) compared to that of the diestrous stage (Fig. 2A). During the estrous stage, in which the epithelial layer is maximally thick and covered with a cornified superficial layer, minimal expression of the nectin-1 molecule was detected (Fig. 2C). The ensuing catabolic metestrous-1 phase gives way to delamination of the cornified epithelial layer, at which point nectin-1 expression was essentially undetectable (data not shown). The following metestrous-2 phase was characterized by minimal expression of nectin-1 in the epithelium (Fig. 2D). Similar expression patterns were observed with another monoclonal antibody, CK8, which recognizes a distinct but overlapping epitope detected by CK6 within nectin-1 (11) (data not shown).
At diestrus, nectin-1 expression formed polygonal networks of staple-like structures encircling the apical regions of the cell and appeared to be at the junctions of the superficial epithelial cells (Fig. 2A). This observation is consistent with previous studies of other epithelial layers (2, 18, 31, 32). To determine whether nectin-1 expression occurred at the adherens junction, a confocal analysis of diestrous vaginal epithelium was conducted in conjunction with an antibody against E-cadherin to illuminate adherens junctions. This analysis revealed that nectin-1 expression coincided with E-cadherin, but that only the adherens junctions of the superficial epithelial layer contained nectin-1 (Fig. 2E). To demonstrate the relative expression levels of nectin-1 and E-cadherin on vaginal epithelial cells, the intensities of the staining by both of these antibodies were measured across the image along the white line-arrow drawn from the submucosa towards the lumen of the vagina (Fig. 2E). The comparison of the green (E-cadherin) and the red (nectin-1) intensity profiles clearly demonstrated that although the majority of nectin-1 expression coincided with E-cadherin, some apical nectin-1 did not colocalize with E-cadherin (Fig. 2F).
HSV-2 entry in vivo is blocked by soluble recombinant nectin-1.
The expression pattern of nectin-1 in the mouse vaginal epithelium throughout the estrous cycle indicated that nectin-1 remains readily accessible during the diestrous and early proestrous stages (Fig. 2). In an effort to examine whether nectin-1 could serve as an entry mediator for HSV-2 in vivo, mice were infected with the wild-type 186 strain of HSV-2 that had been incubated with a recombinant soluble truncated form of nectin-1 [HveC(346t)] prior to intravaginal inoculation. Preincubation of HSV-1 with HveC(346t) has been used to demonstrate that viral entry into neurons and fibroblasts is largely mediated through nectin-1 in vitro (4, 25). Pretreatment of the wild-type HSV-2 with soluble nectin-1 consistently resulted in a complete reduction in the PFU present in vaginal washes at 24 h postinfection compared to that in untreated virus (Fig. 3A).
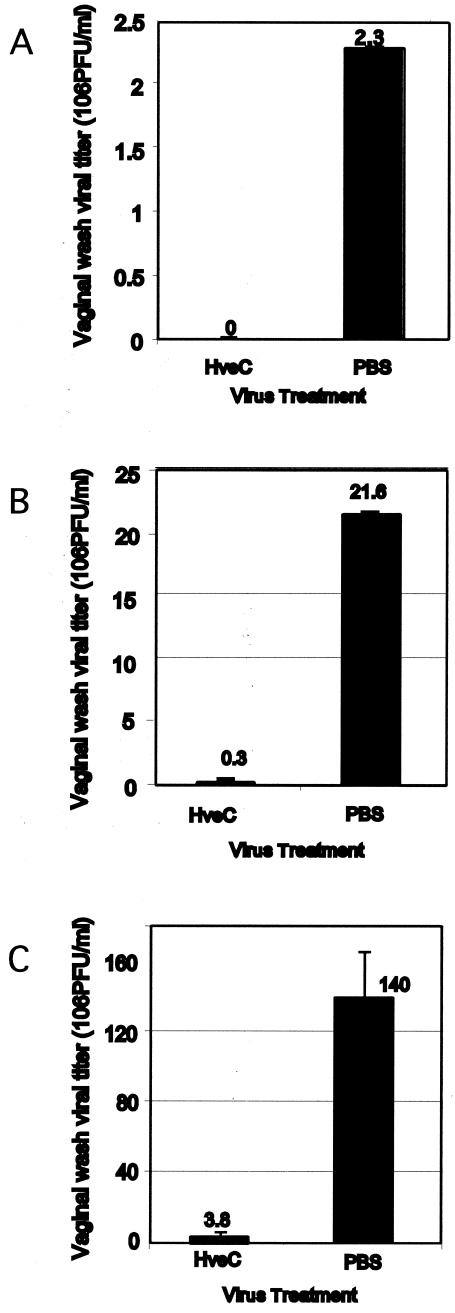
FIG. 3.
In vivo viral entry inhibition by soluble nectin-1 as measured by viral load. Progesterone-treated female mice were inoculated intravaginally with HSV-2 wild-type 186 strain (105 PFU/mouse) (A), 186TKΔΚpn (106 PFU/mouse) (B), or HSV-1 wild-type KOS strain (106 PFU/mouse) (C). Prior to inoculation, viruses were treated with soluble recombinant nectin-1 or PBS for 2 h on ice. Vaginal washes were collected at 24 h postinfection, and viral titers were measured by plaque-forming assay. These results are expressed as the viral titer ± standard deviation and are representative of five similar experiments with five mice in each group.
A widely used murine model for genital herpes uses the TK− HSV-2 that has been attenuated for neurovirulence. To examine whether a similar requirement exists for TK− HSV-2, the virus 186TKΔKpn was incubated with soluble nectin-1 prior to intravaginal inoculation. Similar to the wild-type 186 virus, blocking of strain 186TKΔKpn with HveC(346t) resulted in a 92% reduction in viral load (Fig. 3B). The reduced viral load in the vaginal lumen by HveC(346t) correlated with the undetectable virus infection in the vaginal tissues as examined by immunofluorescence analysis (Fig. 4A, B, D, and E).
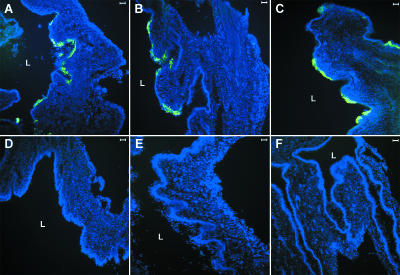
FIG. 4.
Soluble nectin-1 blocks infection by HSV-1 and HSV-2 in the vaginal mucosa. Progesterone-treated female mice were inoculated intravaginally with HSV-2 wild-type 186 strain (105 PFU/mouse) (A and D), 186TKΔΚpn (106 PFU/mouse) (B and E), or HSV-1 wild-type KOS strain (106 PFU/mouse) (C and F). Prior to inoculation, viruses were treated with soluble recombinant nectin-1 (D to F) or PBS (A to C) for 2 h on ice. Vaginal tissues were collected at 24 h postinfection, and viral antigens were detected by immunofluorescence microscopy. Sections were stained with fluorescein isothiocyanate-conjugated anti-HSV antibody (green), and the nuclei were visualized by staining with DAPI (blue). These results are representative of five similar experiments with five mice in each group. L, lumen.
Effects of soluble recombinant nectin-1 on HSV-1 entry in vivo.
To examine whether similar viral entry mechanisms operate for HSV-1, progesterone-treated mice were challenged intravaginally with wild-type HSV-1 KOS strain that had been blocked with soluble nectin-1. Similar to the observation for HSV-2 strains, HSV-1 entry was also dramatically reduced by pretreatment with recombinant nectin-1 as measured by both the viral titer in vaginal washes (97% reduction) (Fig. 3C) and immunofluorescence staining for viral antigens within the vaginal tissues (Fig. 4C and F). Thus, both HSV-1 and HSV-2 entry was significantly blocked with soluble recombinant nectin-1 in vivo.
Effects of HSV-2 treatment with anti-gD antibody.
These results suggested that entry of HSV-1 and HSV-2 through the vaginal mucosa is mediated by viral entry through nectin-1 expressed on the superficial epithelial cells of the vaginal tract. Although the importance of gD in viral entry has been well established in vitro, a requirement for gD in viral entry in vivo has not been demonstrated. Thus, we examined the importance of gD in establishing infection through the vaginal mucosa with a polyclonal antibody. Treatment of HSV-2 with anti-gD antibody completely blocked its ability to establish infection in mice, demonstrated by the lack of detectable virus in the vaginal washes (Fig. 5) as well as the lack of virus infection detectable by immunofluorescence analysis of the vaginal tissues from these mice (data not shown).
DISCUSSION
HSV-2 is transmitted via sexual contact through the genital mucosa. Recent epidemiological evidence revealed that HSV-1 is also transmitted through sexual contact in the United States (14). The mechanism of entry of these viruses has been examined in great detail in tissue culture to be mediated through the binding of the gD of HSV to one of several identified entry mediators, HveA, nectin-2, and nectin-1, or through the 3-O-sulfated heparan sulfate (1, 30). However, little is known regarding the molecular mechanism of virus entry through the genital mucosa in vivo. In particular, understanding the expression of the relevant viral entry mediators during the menstrual cycle in the female genital tract and the functional relevance of the expression of these receptors in mediating viral entry is critical in designing rational interventions to prevent the spread of these viruses.
In this study, we demonstrated that human vaginal epithelium expressed significant levels of nectin-1 at all stages of the menstrual cycle. Nectin-1 expression was observed strongly in the stratum spinosum but was minimal in the stratum germinativum. In some cases, nectin-1 expression extended through the stratum granulosum at the junction of the epithelial cells. This expression pattern is consistent with nectin-1 being located at the adherens junctions (2, 18, 31, 32). The most superficial layers of the human vaginal epithelium exhibited minimal nectin-1 expression, suggesting that terminally differentiated keratinocytes lose the expression of this receptor. The biological significance of the human alphaherpesviruses utilizing an adhesion molecule such as nectin-1 to mediate entry and cell-to-cell spread is of great interest. Recent evidence indicates that the disruption of the adherens junctions is required to liberate nectin-1 to serve as an entry mediator for HSV-1 (34). Regeneration of the epithelial cells during the hormonal cycle and/or the physical injuries sustained during sexual intercourse may cause disruption of adherens junctions in the vaginal epithelium, allowing for the entry of HSV in women.
In the mouse vaginal epithelium, striking changes in the pattern of the expression of nectin-1 were observed. During the estrous stage in which the epithelium is the thickest and covered with a cornified layer, nectin-1 expression was minimal. Upon shedding of the cornified layer and degradation of the delaminated epithelium during metestrous stages, nectin-1 expression was not observed. However, during the diestrus and early proestrus that followed, nectin-1 was specifically highly expressed at the junction of the epithelial cells on the superficial layer of the stratified epithelium which formed polygonal networks encircling the apical regions of the cells. Since some of the apical nectin-1 expression did not colocalize with the adherens junction marker E-cadherin, these structures might represent a special type of junction. A very similar pattern of expression has also been noted for another adherens junction molecule, testin, in murine vaginal epithelial cells (35).
The temporal expression pattern of nectin-1 was remarkably consistent with the timing of the susceptibility of mice to intravaginal HSV-2 infection, as diestrous and early proestrous mice become readily infected with HSV-2, whereas those at the estrous and metestrous-1 phases are resistant to HSV-2 infection (3, 23). The explanation for the selective susceptibility of mice to intravaginal challenges with HSV-2 had been attributed to the thickness and permeability of the vaginal epithelial cells during the diestrous stage or in progesterone-treated mice (3, 23). The diestrous and progesterone-induced stages are also accompanied by leukocyte invasion into the vaginal epithelium (23), which may in some way facilitate the entry of HSV-2 into the underlying mucosa. Our demonstration of the selective expression of the nectin-1 at the diestrous stage suggested a potential molecular explanation for the dependency on hormonal stages in the susceptibility of mice.
To test whether nectin-1 may mediate the entry of HSV through the vaginal epithelium, we examined the ability of HSV that had been blocked with recombinant nectin-1 to establish infection upon intravaginal inoculation in progesterone-treated mice. These studies revealed that nectin-1 binding sites of HSV-1 and HSV-2 are critical in mediating viral entry and replication in the vaginal epithelium. Furthermore, preincubation of HSV-2 with antibody to gD completely blocked virus infection in vivo. We hypothesize that the ability of rHveC to block viral entry in vivo is related to its ability to bind to the region of gD that is required for entry into host epithelial cells through nectin-1.
Although we have demonstrated the expression of nectin-1 and the dependency of HSV-1 and HSV-2 on gD and nectin-1 binding sites for entry and infection, the use of another entry mediator by these viruses remains a distinct possibility. For instance, soluble nectin-1 may block regions of gD critical for entry of HSV through HveA or 3-O-sulfated heparan sulfate. In addition, gD is responsible for mediating viral entry involving all known entry mediators (28, 30). Thus, a definitive proof of the requirement of nectin-1 in viral entry in vivo will require characterization of the expression of all other entry mediators in the vaginal epithelium and ultimately the use of various knockout mice for one or a combination of these receptors.
Taking into consideration the expression patterns of nectin-1 on vaginal epithelial cells and the ability of the soluble nectin-1 and anti-gD antibody to block viral entry in vivo, nectin-1 remains a strong candidate for facilitating the entry of both HSV-1 and HSV-2 into the female genital tract. These findings are highly relevant to designing preventative measures against sexual transmission of HSV-1 and HSV-2. Future efforts should be placed on identifying inhibitory molecules that can bind to either the viral gD region that is critical in nectin-1 binding or to the region of nectin-1 that is required for binding to gD (11).
Acknowledgments
We thank David Knipe for providing the viruses used in this study. We are grateful to Steffen Mueller for insightful discussions.
This investigation was supported by Public Health Service grants NS-30606 and NS-36731 from the National Institute of Neurological Disorders and Stroke (to R.J.E. and G.H.C.), AI-18289 from the National Institute of Allergy and Infectious Diseases (to R.J.E. and G.H.C.), and the Ethel F. Donaghue Women's Health Investigator Program at Yale (A.I.).
REFERENCES
- 1.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. [DOI] [PubMed] [Google Scholar]
- 2.Fukuhara, A., K. Irie, A. Yamada, T. Katata, T. Honda, K. Shimizu, H. Nakanishi, and Y. Takai. 2002. Role of nectin in organization of tight junctions in epithelial cells. Genes Cells 7:1059-1072. [DOI] [PubMed] [Google Scholar]
- 3.Gallichan, W. S., and K. L. Rosenthal. 1996. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology 224:487-497. [DOI] [PubMed] [Google Scholar]
- 4.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 5.Green, E. L. 1989. Biology of the laboratory mouse, 2nd ed. Dover Publications, New York, N.Y.
- 6.Isola, V. J., R. J. Eisenberg, G. R. Siebert, C. J. Heilman, W. C. Wilcox, and G. H. Cohen. 1989. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J. Virol. 63:2325-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki, A., R. Welker, S. Mueller, M. Linehan, A. Nomoto, and E. Wimmer. 2002. Immunofluorescence analysis of poliovirus receptor expression in Peyer's patches of humans, primates, and CD155 transgenic mice: implications for poliovirus infection. J. Infect. Dis. 186:585-592. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, D. C., and M. W. Ligas. 1988. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J. Virol. 62:4605-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, C. A., T. J. Taylor, and D. M. Knipe. 2000. Biological properties of herpes simplex virus 2 replication-defective mutant strains in a murine nasal infection model. Virology 278:137-150. [DOI] [PubMed] [Google Scholar]
- 10.Kaushic, C., A. A. Ashkar, L. A. Reid, and K. L. Rosenthal. 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 77:4558-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krummenacher, C., I. Baribaud, M. Ponce de Leon, J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2000. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by with antireceptor monoclonal antibodies. J. Virol. 74:10863-10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krummenacher, C., A. V. Nicola, J. C. Whitbeck, H. Lou, W. Hou, J. D. Lambris, R. J. Geraghty, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon, B. S., K. B. Tan, J. Ni, K. O. Oh, Z. H. Lee, K. K. Kim, Y. J. Kim, S. Wang, R. Gentz, G. L. Yu, J. Harrop, S. D. Lyn, C. Silverman, T. G. Porter, A. Truneh, and P. R. Young. 1997. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J. Biol. Chem. 272:14272-14276. [DOI] [PubMed] [Google Scholar]
- 14.Lafferty, W. E. 2002. The changing epidemiology of HSV-1 and HSV-2 and implications for serological testing. Herpes 9:51-55. [PubMed] [Google Scholar]
- 15.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez, W. M., and P. G. Spear. 2001. Structural features of nectin-2 (HveB) required for herpes simplex virus entry. J. Virol. 75:11185-11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mata, M., M. Zhang, X. Hu, and D. J. Fink. 2001. HveC (nectin-1) is expressed at high levels in sensory neurons, but not in motor neurons, of the rat peripheral nervous system. J. Neurovirol. 7:476-480. [DOI] [PubMed] [Google Scholar]
- 18.Matsushima, H., A. Utani, H. Endo, H. Matsuura, M. Kakuta, Y. Nakamura, N. Matsuyoshi, C. Matsui, H. Nakanishi, Y. Takai, and H. Shinkai. 2003. The expression of nectin-1alpha in normal human skin and various skin tumours. Br. J. Dermatol. 148:755-762. [DOI] [PubMed] [Google Scholar]
- 19.Menotti, L., E. Avitabile, P. Dubreuil, M. Lopez, and G. Campadelli-Fiume. 2001. Comparison of murine and human nectin1 binding to herpes simplex virus glycoprotein D (gD) reveals a weak interaction of murine nectin1 to gD and a gD-dependent pathway of entry. Virology 282:256-266. [DOI] [PubMed] [Google Scholar]
- 20.Menotti, L., M. Lopez, E. Avitabile, A. Stefan, F. Cocchi, J. Adelaide, E. Lecocq, P. Dubreuil, and G. Campadelli-Fiume. 2000. The murine homolog of human Nectin1delta serves as a species nonspecific mediator for entry of human and animal alpha herpesviruses in a pathway independent of a detectable binding to gD. Proc. Natl. Acad. Sci. USA 97:4867-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 22.Mostad, S. B., J. K. Kreiss, A. J. Ryncarz, K. Mandaliya, B. Chohan, J. Ndinya-Achola, J. J. Bwayo, and L. Corey. 2000. Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: effects of hormonal contraception, pregnancy, and vitamin A deficiency. J. Infect. Dis. 181:58-63. [DOI] [PubMed] [Google Scholar]
- 23.Parr, M. B., L. Kepple, M. R. McDermott, M. D. Drew, J. J. Bozzola, and E. L. Parr. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Investig. 70:369-380. [PubMed] [Google Scholar]
- 24.Parr, M. B., and E. L. Parr. 1990. Antigen recognition in the female reproductive tract. I. Uptake of intraluminal protein tracers in the mouse vagina. J. Reprod. Immunol. 17:101-114. [DOI] [PubMed] [Google Scholar]
- 25.Richart, S. M., S. A. Simpson, C. Krummenacher, J. C. Whitbeck, L. I. Pizer, G. H. Cohen, R. J. Eisenberg, and C. L. Wilcox. 2003. Entry of herpes simplex virus type 1 into primary sensory neurons in vitro is mediated by nectin-1/HveC. J. Virol. 77:3307-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukla, D., M. C. Dal Canto, C. L. Rowe, and P. G. Spear. 2000. Striking similarity of murine nectin-1α to human nectin-1α (HveC) in sequence and activity as a glycoprotein D receptor for alphaherpesvirus entry. J. Virol. 74:11773-11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 28.Shukla, D., and P. G. Spear. 2001. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Investig. 108:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spang, A. E., P. J. Godowski, and D. M. Knipe. 1983. Characterization of herpes simplex virus 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. J. Virol. 45:332-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi, K., H. Nakanishi, M. Miyahara, K. Mandai, K. Satoh, A. Satoh, H. Nishioka, J. Aoki, A. Nomoto, A. Mizoguchi, and Y. Takai. 1999. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J. Cell Biol. 145:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takai, Y., and H. Nakanishi. 2003. Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 116:17-27. [DOI] [PubMed] [Google Scholar]
- 33.Warner, M. S., R. J. Geraghty, W. M. Martinez, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179-189. [DOI] [PubMed] [Google Scholar]
- 34.Yoon, M., and P. G. Spear. 2002. Disruption of adherens junctions liberates nectin-1 to serve as receptor for herpes simplex virus and pseudorabies virus entry. J. Virol. 76:7203-7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu, L., A. Moo-Young, C. W. Bardin, and C. Y. Cheng. 1997. Immunohistochemical localization of testin in the female reproductive system of the rat is consistent with its involvement in the turnover of specialized junctional complexes. Biol. Reprod. 56:1330-1335. [DOI] [PubMed] [Google Scholar]