Abstract
DC-SIGN, a specific C-type lectin expressed on dendritic cells, binds and transmits multiple strains of primate immunodeficiency viruses to susceptible cells. Here, we report that human DC-SIGN also captures feline immunodeficiency virus via high-affinity (1 nM), Ca2+-dependent, d-mannose-inhibited binding to the major envelope glycoprotein, gp95.
DC-SIGN, a cell surface C-type lectin expressed on dendritic cells (DCs), is thought to play key roles in the interaction of DCs with T cells as well as in human immunodeficiency virus (HIV) pathogenesis (reviewed in reference 4). By binding to intracellular adhesion molecule 3 (ICAM-3), DC-SIGN facilitates the initial interaction between DCs and resting T cells, which leads to antigen recognition and initiation of the immune response (6). DC-SIGN is also a unique type of attachment factor for HIV type 1 (HIV-1) in that it binds the envelope glycoprotein gp120 of HIV-1 and promotes infection in trans of target CD4+ T cells (5). It is believed that HIV may exploit DC-SIGN for its transport from mucosal sites of infection to permissive T cells in secondary lymphoid organs. DC-SIGN also binds HIV-2 and simian immunodeficiency virus surface glycoproteins (10) and can enhance infection in cis, especially when CD4 and coreceptor levels are limiting (7). We were thus interested to study potential DC-SIGN interaction with feline immunodeficiency virus (FIV), the causative agent of feline AIDS in the domestic cat (8). Feline DC-SIGN has not been cloned, and reagents and protocols for the preparation of feline DCs are in development. However, based on a previous demonstration of FIV binding to human CXCR4 (13), we initiated studies utilizing human DC-SIGN.
DC-SIGN interactions with FIV.
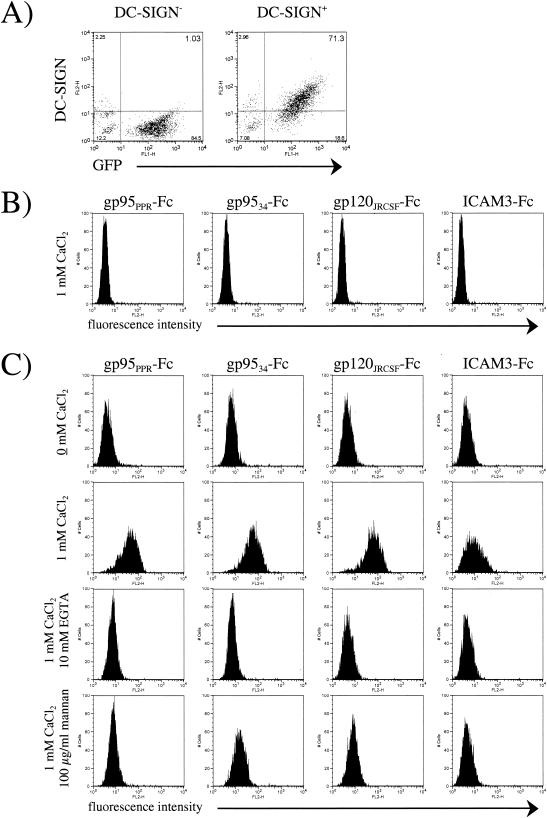
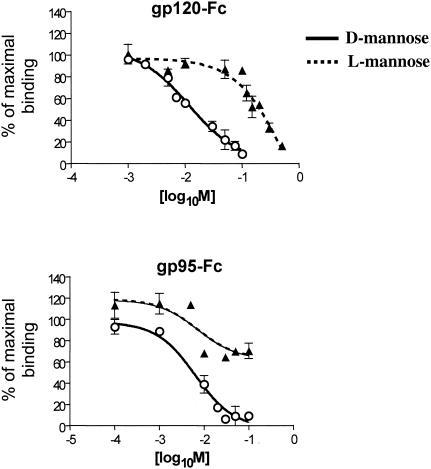
CHO pgsA745 cells were transduced with a murine stem cell retrovirus vector (MIGR1-DC-SIGN-GFP) expressing human DC-SIGN in tandem with green fluorescent protein (GFP) via an internal ribosome entry site linker in which DC-SIGN is tagged with the AU-1 sequence (7) at the extracellular C terminus. The pgsA745 cell line is a CHO mutant cell line defective in the biosynthesis of glycosaminoglycans and thus does not express cell surface heparan sulfate proteoglycans (3), which also act as binding receptors for certain HIV isolates (11) and the 34TF10 (12) molecular clone of FIV (2). GFP-positive cells were sorted by cytofluorimetry, and the expression of DC-SIGN on GFP-positive cells was confirmed with an anti-AU-1 antibody (Fig. 1A). We first analyzed the binding of FIV surface glycoprotein gp95 in comparison with that of HIV gp120 and ICAM-3 by using the immunoadhesins gp95-Fc (FIV), gp120-Fc (HIV-1), and ICAM-3-Fc. The gp95-Fc immunoadhesin has been previously reported (2), and ICAM-3-Fc was purchased from R&D Systems. For gp120-Fc, gp120 from the JR-CSF clone of HIV-1 was subcloned in frame with the Fc domain of immunoglobulin G1 (IgG1) and produced in CHO cells by using the glutamate synthetase amplification system as previously reported for FIV gp95 (2). DC-SIGN interaction with gp95-Fc, gp120-Fc, and ICAM-3-Fc was assessed by fluorescence-activated cell sorter (FACS) analysis with DC-SIGN-positive and -negative pgsA745 cells. Cells (105) were incubated for 1 h at 4°C with 1 μg of immunoadhesin per ml, washed, and then incubated for another 45 min with an anti-Fc phycoerythrin-conjugated antibody (Cappel, Durham, N.C.). As shown in Fig. 1B, binding of FIV gp95-Fc to DC-SIGN-negative cells was not observed. However, we observed a strong binding of gp95-Fc to DC-SIGN-positive cells, but only in the presence of CaCl2 (Fig. 1C). The binding of gp95-Fc was specific in that no binding was observed with Fc alone (data not shown). Furthermore, the presence of 10 mM EGTA abolished the binding of gp95-Fc, as did the preincubation of the cells with mannan at 100 μg/ml (Fig. 1C). Binding of both gp120-Fc and ICAM-3-Fc also required calcium and was inhibited by EGTA and mannan (Fig. 1C). Inhibition by mannan was stereospecific in that d-mannose competed for gp95-Fc and gp120-Fc binding to human DC-SIGN much more efficiently than did l-mannose (Fig. 2).
FIG. 1.
Binding of FIV, HIV-1, and ICAM-3 immunoadhesins to CHO cells as a function of DC-SIGN expression. (A) FACS analysis of pgsA745 cells transduced with the MIGR1 vector either expressing GFP (left panel) or coexpressing GFP and human DC-SIGN (right panel). (B) Control binding of immunoadhesins to pgs745 cells transduced with GFP alone, gp95PPR-Fc (envelope from FIV-PPR), gp9534-Fc (envelope from FIV-34TF10), gp120JRCSF-Fc (envelope from HIV-1 JRCSF strain), and ICAM-3-Fc. (C) Calcium-dependent binding of immunoadhesins to pgs745 cells transduced with human DC-SIGN. The top four panels show a lack of binding in the absence of CaCl2, the second four panels show binding in the presence of 1 mM CaCl2, the third four panels show a lack of binding in the presence of 1 mM CaCl2 and 10 mM EGTA, and the bottom four panels show a lack of binding in the presence of 1 mM CaCl2 and 100 μg of mannans per ml.
FIG. 2.
Stereospecificity of mannose competition for gp95-Fc and gp120-Fc binding to soluble DC-SIGN. Data were obtained from an assay based on an enzyme-linked immunosorbent assay in which a high-binding-capacity 96-well plate was coated with 200 nM recombinant His6-tagged extracellular domain of DC-SIGN overnight at 4°C. Either gp95-Fc (1 nM) or gp120-Fc (2 nM) was added with an increasing amount of d-mannose or l-mannose. Bound SU-Fc adhesin was detected with an anti-human Fc IgG1-specific antibody conjugated with horseradish peroxidase.
Binding affinity measurements.
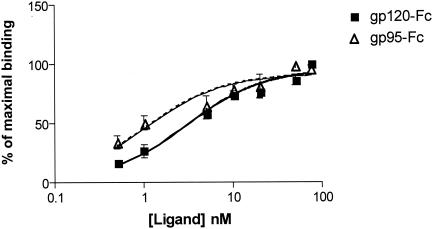
Comparisons were made of the relative binding affinities of FIV gp95-Fc and HIV-1 gp120-Fc (Fig. 3). Interestingly, FIV gp95-Fc had a higher binding affinity for DC-SIGN than did HIV-1 gp120-Fc, with Kd values of 1.03 and 2.99 nM, respectively (Fig. 3). In toto, these binding data suggest that the phenomenon of DC-SIGN binding to the HIV envelope protein is recapitulated in the feline lentivirus model.
FIG. 3.
Relative binding affinities of FIV gp95-Fc and HIV gp120-Fc for DC-SIGN. Inhibition curves were derived from an assay based on an enzyme-linked immunosorbent assay as described in the legend to Fig. 2, except that 125 nM His-tagged extracellular domain was used. Increasing concentrations of ligand were added, and the amount of bound ligand was detected with anti-human Fc IgG1-specific antibody conjugated with horseradish peroxidase. Experimental values were plotted, and the Kd was computed with GraphPad Prism software.
DC-SIGN facilitates FIV infection in trans.
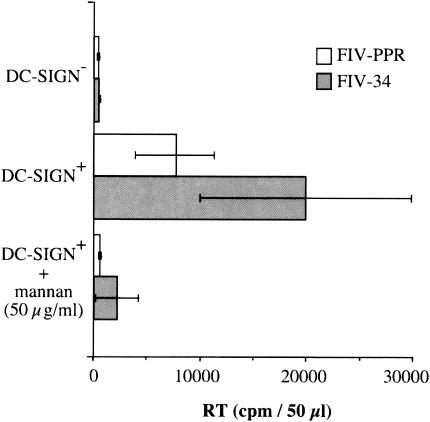
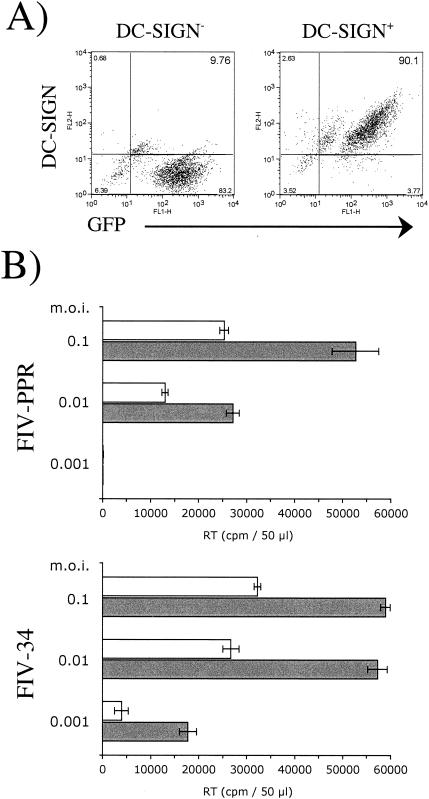
To determine whether DC-SIGN could present FIV to target cells as observed for HIV-1, DC-SIGN-positive pgsA745 cells were seeded in 12-well dishes and incubated with either FIV-PPR (molecularly cloned primary isolate) (9) or FIV-34orf2rep (molecularly cloned laboratory-adapted isolate) (12) at a multiplicity of infection (MOI) of 0.01 for 3 h at room temperature. After extensive washing, cells were cocultured overnight with 2.5 × 104 104-C1 cells. On the following day, the T cells were transferred to fresh wells, and virus production was monitored at 7 days post-coculture by a reverse transcriptase activity assay as previously described (2). As shown in Fig. 4, DC-SIGN-positive pgsA745 cells effectively transmit FIV to the feline 104-C1 T-cell population. FIV capture was DC-SIGN dependent, since preincubation of DC-SIGN-positive cells with mannan at 50 μg/ml blocked binding of FIV and subsequent transfer to target cells. Additionally, DC-SIGN-negative parental pgs745 cells failed to capture and facilitate transmission of FIV (Fig. 4).
FIG. 4.
DC-SIGN transmits FIV to susceptible target cells. Control pgsA745 cells (DC-SIGN−) and pgsA745 cells transduced with human DC-SIGN (DC-SIGN+) were exposed to tissue culture supernatants containing either FIV-PPR or FIV-34orf2rep. The cells were thoroughly washed and then cocultured with susceptible feline 104-C1 T cells. The 104-C1 cells were recovered and cultured separately, and virus production was detected at 7 days postinfection by a reverse transcriptase (RT) activity assay. No infectivity was noted in cocultivations with DC-SIGN− cells, but both strains of FIV were efficiently transmitted to 104-C1 cells in cocultures with DC-SIGN+ cells. DC-SIGN+ cells that were pretreated with 50 μg of mannan per ml failed to transmit FIV infection, consistent with dependence on DC-SIGN for virus transmission.
Influence of DC-SIGN on FIV infection in cis.
Identification of GFP- and DC-SIGN-positive or GFP-positive 104-C1 cells was established by retroviral transduction, and cells were then sorted by FACS analysis, with DC-SIGN expression confirmed by staining with an anti-AU-1 antibody (Fig. 5A). DC-SIGN-negative and -positive 104-C1 cells were infected at different MOIs with FIV-PPR and FIV-34orf2rep strains, and virus production was monitored at 7 days postinfection by a reverse transcriptase assay. At MOIs higher than 0.1, substantial levels of cytopathicity for both DC-SIGN-negative and -positive cells were observed, and this effect was more pronounced in the DC-SIGN-positive cell population; i.e., at a high MOI, the enhancing effect of DC-SIGN expression in cis was masked (data not shown). However, at MOIs of 0.1 to 0.001, an enhancing effect of DC-SIGN expression was evident, and viral infectivity was two- to threefold more pronounced, on average, in the DC-SIGN-positive cell population (Fig. 5B). The enhancing effect of DC-SIGN expression in cis is more evident when CD4 and coreceptor levels are limiting (7). Here, the feline 104-C1 T cells that we used expressed relatively high levels of FIV primary receptor and CXCR4 (2), which might explain the moderate enhancing effect in cis of DC-SIGN that we observed.
FIG. 5.
Influence of DC-SIGN expressed in cis on the infection of feline T cells by FIV. (A) FACS analysis of 104-C1 cells transduced with the MIGR1 vector either expressing GFP (left panel) or coexpressing GFP and human DC-SIGN (right panel). (B) Cells were infected with the two virus strains, FIV-PPR and FIV-34orf2rep, at a range of MOIs. Virus expression was monitored by measuring reverse transcriptase (RT) levels in the culture supernatant at 7 days postinfection. A two- to threefold enhancement of FIV infection was noted as a function of DC-SIGN expression.
The cloning and characterization of feline DC-SIGN and consistent successful culture of feline DCs have yet to be achieved. Thus, in vivo relevance of FIV-DC-SIGN interactions must still be demonstrated. However, the findings reported here suggest the existence of an additional parallel between FIV and HIV-1 infection which may lead to the use of the cat model as a tool for the study of the significance of DC-SIGN association to virus spread in a natural infection. The findings from such investigations may be relevant not only to the study of retrovirus infections but also to the treatment of unrelated agents such as Ebola virus, where it has been shown that the surface glycoprotein of that virus also interacts with DC-SIGN (1). As with DC-SIGN-HIV-1 gp120 interaction, the binding of FIV gp95 to DC-SIGN is dependent on Ca2+, is completely inhibited by EGTA, and is blocked by mannan (Fig. 1). The exquisite specificity of DC-SIGN for the particular glycan structures found on the FIV envelope is underscored by the stereospecificity shown in competition for binding by d-mannose, both for FIV gp95 and for HIV gp120 (Fig. 2). Additionally, the binding affinity of FIV gp95 for human DC-SIGN is high, with a Kd of approximately 1 nM (Fig. 3), The findings also demonstrate that human DC-SIGN can, as with HIV, act to present FIV for infection of permissive cells (Fig. 4) and, further, that the expression of human DC-SIGN in cis results in the enhanced infection of permissive feline cells (Fig. 5). Whether DC-SIGN alone can serve to facilitate FIV infection remains to be determined. However, all other observations suggest that ultimate uptake and productive infection by FIV will require entry via CXCR4 binding (2). Rather, the findings suggest that DC-SIGN may serve as an additional binding receptor to increase the effective concentration of viruses at the cell surface and thus increase viral infection via the entry receptor, CXCR4.
Acknowledgments
We thank Stacie Ngo for excellent technical assistance.
This work was supported in part by grants R01 AI25825 (J.H.E.) and R01 AI52021 (B.L.) from the National Institutes of Health. B.L. is also a recipient of the Culpepper Biomedical Scholar award and a Burroughs Wellcome Fund Career Development award.
REFERENCES
- 1.Alvarez, C. P., F. Lasala, J. Carrillo, O. Muñiz, A. L. Corbí, and R. Delgado. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76:6841-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Parseval, A., and J. H. Elder. 2001. Binding of recombinant feline immunodeficiency virus surface glycoprotein to feline cells: role of CXCR4, cell-surface heparans, and an unidentified non-CXCR4 receptor. J. Virol. 75:4528-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geijtenbeek, T. B., A. Engering, and Y. Van Kooyk. 2002. DC-SIGN, a C-type lectin on dendritic cells that unveils many aspects of dendritic cell biology. J. Leukoc. Biol. 71:921-931. [PubMed] [Google Scholar]
- 5.Geijtenbeek, T. B. H., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. F. van Duijnhoven, J. Middel, I. L. M. H. A. Cornelissen, H. S. L. M. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 6.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 7.Lee, B., G. Leslie, E. Soilleux, U. O'Doherty, S. Baik, E. Levroney, K. Flummerfelt, W. Swiggard, N. Coleman, M. Malim, and R. W. Doms. 2001. cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75:12028-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen, N. C., E. W. Ho, M. L. Brown, and J. K. Yamamoto. 1987. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 235:790-793. [DOI] [PubMed] [Google Scholar]
- 9.Phillips, T. R., R. Talbott, C. Lamont, S. Muir, K. Lovelace, and J. H. Elder. 1990. Comparison of two host cell range variants of feline immunodeficiency virus. J. Virol. 64:4605-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pöhlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Münch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saphire, A. C. S., M. D. Bobardt, Z. Zhang, G. David, and P. A. Gallay. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75:9187-9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waters, A. K., A. P. De Parseval, D. L. Lerner, J. C. Neil, F. J. Thompson, and J. H. Elder. 1996. Influence of ORF2 on host cell tropism of feline immunodeficiency virus. Virology 215:10-16. [DOI] [PubMed] [Google Scholar]
- 13.Willett, B. J., L. Picard, M. J. Hosie, J. D. Turner, K. Adema, and P. R. Clapham. 1997. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J. Virol. 71:6407-6415. [DOI] [PMC free article] [PubMed] [Google Scholar]