Abstract
Background
Considering the pivotal role of negative emotional experiences in the development and persistence of mental disorders, interfering with the consolidation/reconsolidation of such experiences would open the door to a novel treatment approach in psychiatry. We conducted a meta-analysis on the experimental evidence regarding the capacity of the β-blocker propranolol to block the consolidation/reconsolidation of emotional memories in healthy adults.
Methods
Selected studies consisted of randomized, double-blind experiments assessing long-term memory for emotional material in healthy adults and involved at least 1 propranolol and 1 placebo condition. We searched PsycInfo, PubMed, Web of Science, Cochrane Central, PILOTS, Google Scholar and clinicaltrials.org for eligible studies from the period 1995–2012. Ten consolidation (n = 259) and 8 reconsolidation (n = 308) experiments met the inclusion criteria. We calculated effect sizes (Hedges g) using a random effects model.
Results
Compared with placebo, propranolol given before memory consolidation reduced subsequent recall for negatively valenced stories, pictures and word lists (Hedges g = 0.44, 95% confidence interval [CI] 0.14–0.74). Propranolol before reconsolidation also reduced subsequent recall for negatively valenced emotional words and the expression of cue-elicited fear responses (Hedges g = 0.56, 95% CI 0.13–1.00).
Limitations
Limitations include the moderate number of studies examining the influence of propranolol on emotional memory consolidation and reconsolidation in healthy adults and the fact that most samples consisted entirely of young adults, which may limit the ecological validity of results.
Conclusion
Propranolol shows promise in reducing subsequent memory for new or recalled emotional material in healthy adults. However, future studies will need to investigate whether more powerful idiosyncratic emotional memories can also be weakened and whether this weakening can bring about long-lasting symptomatic relief in clinical populations, such as patients with posttraumatic stress or other event-related disorders.
Introduction
It is well demonstrated that emotion enhances memory encoding and facilitates later recall.1 Such observations have important implications in the realm of psychopathology because many disorders have at their core an overly powerful emotional memory, often stemming from a negative life event. For instance, in order for posttraumatic stress disorder (PTSD) to develop, one must experience a life threat accompanied by peritraumatic distress.2 During trauma exposure, endogenous stress hormones (i.e., noradrenaline) overconsolidate the traumatic memory.3 This memory is subsequently reactivated too easily by contextual cues, thereby eliciting strong conditioned emotional responses4 as well as hyper-vigilance and avoidance of trauma reminders.
Numerous psychiatric disorders also have at their core a negative and sometimes traumatic emotional memory. Trauma exposure is known to increase the risk for other disorders, such as phobias, addiction, depression, panic disorder and obsessive–compulsive disorder.5–7 Negative life events of lesser magnitude that occur during development or later are also believed to increase the risk for psychopathology.8 Furthermore, addicted individuals feel unable to resist emotional memories of their past abuse, which manifest in the form of cue-elicited cravings and can contribute to relapse.9 Thus, decreasing the grip of such powerful emotional memories would seem to have obvious therapeutic value for a whole class of disorders in psychiatry.
One way to decrease the influence of an emotional memory on behaviour would be to interfere with its consolidation, thereby leading to a degraded memory trace. Memory consolidation refers to the time-dependent process of transferring new learning from short- to long-term memory storage where it is reputed to be permanent.10 In a landmark study, Cahill and colleagues11 found that, compared with placebo, propranolol taken before viewing a set of emotionally disturbing slides prevented the heightened recall of those slides. Since then, many studies have replicated this finding. The robustness of this finding has been explored in a qualitative review paper,12 but has yet to be investigated by means of meta-analytic review.
In addition to replicating the results of Cahill and colleagues,11 several researchers have tried to extend them to memory reconsolidation. Reconsolidation theory13 disputes the permanence of consolidated memories and posits that, in order to persist, a retrieved (i.e., recalled) memory needs to be saved again to long-term memory storage, thereby recapitulating, at least in part, the process of memory consolidation.14–16 From a therapeutic point of view, the advantages of blocking reconsolidation rather than consolidation are substantial, since it could allow the otherwise narrow window of opportunity for modifying unwanted memories to be opened at will.17
The substance most frequently used in humans to block memory consolidation and reconsolidation is propranolol. Propranolol is a synthetic β-adrenergic receptor blocker that crosses the blood–brain barrier and exerts peripheral effects on the noradrenergic system as well as central (inhibitory) effects on protein synthesis.15,18,19 Protein synthesis is necessary to consolidate new learning to long-term memory storage. Animal studies have shown that infusing a protein synthesis inhibitor in the amygdala within the time-limited consolidation window leads to a subsequent memory impairment in a fear conditioning task.20
Protein synthesis is also required de novo for memory reconsolidation; postretrieval infusions of a protein synthesis inhibitor led to memory impairment of long-term memory, leaving short-term memory intact.14 Propranolol is one of several protein synthesis inhibitors that have been used in animal studies to reduce the saliency of emotional memories.14,15,18,21 A recent neuroimaging study revealed altered amygdala and hippocampus activity associated with propranolol-induced emotional memory impairment in healthy individuals.22
Propranolol is commonly used to treat migraine,23 tachycardia24 and performance anxiety.25 It is also indicated as a second-line therapy for anxious states because of its effects on the noradrenergic system.
To help determine whether consolidation and reconsolidation blockade using propranolol has any potential as a psychotherapeutic approach for treating mental disorders that have at their core an emotional memory, we conducted a meta-analysis of the experimental protocols involving healthy individuals. We predicted that, compared with placebo, propranolol taken before (or ideally, immediately after) memory consolidation would reduce subsequent recall for negatively valenced material. We made a similar prediction for reconsolidation.
Methods
Inclusion criteria
Studies involving the recall of negatively valenced material in healthy adults published in any language were included if they randomly assigned participants to at least 1 propranolol and 1 placebo group. We limited our search to articles published after that of Cahill and colleagues11 (i.e., between January 1995 and February 2012). We searched for unpublished studies by combing through abstracts from the conferences of the following organizations: Society of Biological Psychiatry, International Society for Traumatic Stress Studies, American College of Neuropsychopharmacology and Society for Neuroscience. We also contacted authors of included studies, other experts in the field, and investigators with studies registered on www.clinicaltrials.gov.
We found 10 studies using propranolol in PTSD populations: 4 examined consolidation blockade,26–29 2 examined reconsolidation blockade,17,30 and the remaining 4 used propranolol for other unrelated purposes. Owing to insufficient numbers and clinical heterogeneity, we could not include studies focusing on consolidation or reconsolidation blockade as a treatment for PTSD in this meta-analysis.
Included experimental paradigms
Memory consolidation
To assess memory consolidation, studies have largely replicated the paradigm of Cahill and colleagues.11 In this protocol, participants watch a series of slides accompanied by either an emotionally upsetting or neutral verbal narrative for the middle section of the story (slides 5–8). In the emotionally upsetting version, a young boy is hit by a car and rushed in critical condition to the hospital, where doctors frantically operate to reattach his severed legs. In the neutral version, the young boy witnesses a routine hospital drill performed on a dummy at his father’s workplace. Propranolol (or placebo) is typically administered 60–90 minutes before viewing the slides so that when memory consolidation begins (i.e., immediately after viewing the slides), propranolol is at its peak plasma concentration.31 Memory for the viewed material is tested in a surprise forced-choice quiz after a washout period of 1–7 days.
Memory reconsolidation
The protocols used in reconsolidation studies consist of fear conditioning, script-driven imagery and/or declarative memory tasks. Although fear conditioning and declarative memory tasks involve different underlying neural mechanisms, a literature review suggests that both are referred to as emotional memories and that both have been subjected to reconsolidation blockade.32 The present study uses the same convention, and the term emotional memory refers to both phenomena. In the fear conditioning paradigm, a neutral stimulus, such as a tone (i.e., conditioned stimulus [CS]), is paired with a fear stimulus until presentation of the CS alone elicits the fear response. One day after initial learning, propranolol is administered orally 60–90 minutes before the retrieval of the fear memory. Reactivation of the fear memory is achieved by a single presentation of the CS, (i.e., the reactivation cue) and memory is tested after a drug washout period of 1–7 days. It should be noted that pre- rather than postretrieval propranolol represents a slight departure from the typical reconsolidation protocol; however, in humans this time is required for pharmacological reconsolidation blockers ingested orally to reach their peak bioavailability31 (for a discussion, see Schiller and Phelps32 and Brunet and colleagues33).
A second method of manipulating memory reconsolidation involves script-driven imagery tasks.34 In one version of this paradigm, participants are asked to write a script detailing an emotionally negative memory. Seven days later, participants receive propranolol or placebo 60–90 minutes before listening to an audiotaped recording of their script, which serves as the reactivation cue. Psychophysiological responses are recorded during this session. After a washout period of 1–7 days, the participants listen to their script while their physiologic responses are recorded, this time without receiving any medication. This final session serves as the test for reconsolidation blockade (see Brunet and colleagues17 for a variation on this method).
In declarative memory tasks, participants are instructed to learn a list of emotionally valenced and neutral words. At least 24 hours later, they are given propranolol or placebo 60–90 minutes before a cued recall task. A second cued recall task, which serves as the test for reconsolidation blockade, occurs after a washout period of at least 24 hours.
Outcome measures
In the paradigm by Cahill and colleagues,11 the outcome of interest was the between-group mean difference in long-term memory performance, as measured by free recall of slides or percent correct on a recognition task for the emotional section of the story. In studies using materials other than the slide story (i.e., pictures from the International Affective Pictures System35 or emotionally valenced word lists), the outcome of interest was between-group difference for recognition memory of negatively valenced pictures or words. In studies whose methods consisted of fear conditioning and script-driven imagery paradigms, physiologic responses (i.e., startle, skin conductance, heart rate) were considered to be measures of fear memory. Neuroimaging studies looking solely at brain activity during memory consolidation and/or reconsolidation were considered to deviate too far from the conventional measurement of memory performance and were thus excluded. For consolidation and reconsolidation studies, we recorded medication dosage, timing, delay before memory testing and sex as possible moderator variables.
Search strategy and data extraction
We searched for articles in PsycInfo, PubMed, ISI Web of Science, Cochrane Central, PILOTS, Google Scholar and www.clinicaltrials.org using the following key words: “propranolol,” “emotion,” “emotional,” “memory,” “consolidation” and “reconsolidation.” The results were exported to a database, and duplicates were removed. Two investigators independently screened the titles and abstracts to exclude irrelevant articles, and they completed independent assessments of all potentially relevant full-text articles. Next, the investigators met to compare results. Discrepancies were resolved by consensus. When consensus could not be achieved, the senior author resolved the disagreement. The reference sections of the included articles were also systematically screened.
Data were extracted by 2 independent reviewers and double-checked by a third party; all disagreements were resolved by consensus. When studies did not report means and standard deviations for standardized between-group differences (i.e., Hedges g), the following sequence36 was applied: first, the t test was used to calculate the effect size; second, the data were requested from the authors; and third, for 2 studies37,38 effects were estimated from the published figures.
Two investigators independently assessed each study using a quality assessment tool.39 One point each was given for randomization, double-blind design and description of withdrawals/dropouts. Fourth and fifth points were given if the randomization and blinding methods, respectively, were well described and considered adequate. Studies were required to be double-blind randomized trials and meet at least 1 other quality criterion to be included. Thus, a score of at least 3 out of a possible 5 was deemed acceptable for inclusion. Tables 1 and 2 list the included consolidation and recon-solodation studies, respectively.
Table 1.
Characteristics of included consolidation studies
| Study | Materials | Participants, propranolol:placebo | Sex, % | Age, mean (SD) [range] yr | Study protocol | Outcome measures | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Male | Female | ||||||
| Cahill et al.11 | Emotional slide story | 11:8 | 47 | 53 | 27.4 (27.6) | 40 mg 60 min before encoding | % recognition story phase 2 |
| van Stegeren et al.40 | Emotional slide story | 14:13 | 31 | 69 | Undergraduates | 40 mg 60 min before encoding | % recognition story phase 2 |
| O’Carroll et al.19 | Emotional slide story | 12:12 | 17 | 83 | 21.4 (2.45) | 40 mg 60 min before encoding | % recognition story phase 2 |
| Reist et al.27 | Emotional slide story | 5:5 | 100 | 44.45 (8.08) | 40 mg 60–90 min before encoding | % recognition phase 2 | |
| Reist et al.27* | Emotional slide story | 5:4 | 100 | 50.53 (8.5) | 40 mg 60–90 min before encoding | % recognition story phase 2 | |
| van Stegeren et al.37 | Emotional slide story | 15:15 | 23 | 77 | 22.65 | 40 mg immediately before encoding | % recognition story phase 2 |
| Maheu et al.41 experiment 1 | Emotional slide story | 11:13 | 100 | [19–36] | 40 mg 65 min before encoding | % free recall story phase 2 | |
| Maheu et al.41 experiment 2 | Emotional slide story | 14:13 | 100 | [20–34] | 80 mg 90 min before encoding | % free recall story phase 2 | |
| Strange and Dolan42 | Emotionally valenced words | 12:12 | 50 | 50 | [20–39] | 40 mg 90 min before encoding; memory test 10 h later | % recognition word list |
| Grillon et al.43† | Fear conditioning | 15:15 | 46 | 53 | 29 (2.8) | 40 mg 60 min before conditioning | Retention fear conditioning |
| van Stegeren et al.38 | Emotionally valenced pictures; crossover design | 28:28 | 50 | 50 | 20.93 (2.38) | 80 mg 90 min before encoding; memory test 2 wk later | % recognition pictures |
| Weymar et al.44 | Emotionally valenced pictures | 23:23 | 100 | [19–31] | 80 mg 90 min before encoding | % recognition pictures | |
SD = standard deviation.
Posttraumatic stress disorder population; data excluded from statistical analysis.
Data excluded from statistical analysis.
Table 2.
Characteristics of included reconsolidation studies
| Study | Materials | Participants, propranolol:placebo | Sex, % | Age, mean (SD) [range] yr | Study protocol | Outcome measures | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Male | Female | ||||||
| Miller et al., unpublished* | Differential fear conditioning | 42:25 | 50 | 50 | 25 (4.2) | Day 1: learning Day 2: propanolol 5 min after reactivation Day 3: LTM test |
Skin conductance response to conditioned stimulus during extinction day 3 |
| de Quervain et al.45 | Emotionally valenced word list crossover design | 14:14 | 50 | 50 | 23.9 (2.9) | Day 1: learning Day 2: 40 mg 60 min before LTM test, 2nd LTM test 2 wk later |
Free recall number of words |
| Kindt et al.46* | Differential fear conditioning | 40:20 | 28 | 72 | 20.7 (2.4) | Day 1: learning; Day 2: 40 mg 90 min before memory reactivation Day 3: LTM test |
Fear potentiated startle to conditioned stimulus during extinction day 3 |
| Tollenaar et al.47 | Emotionally valenced word list | 27:26 | 100 | 20.6 (2.1) | Wk 1: learning Wk 2: 80 mg 75 min before memory task Wk 3: memory task |
% recognition nouns week 3 | |
| Tollenaar et al.34 | Script-driven imagery | 27:26 | 100 | 20.7 (2.2) | Wk 1: script preparation Wk 2: 80 mg 90 min before memory reactivation Wk 3: Heart rate and skin conductance response to emotional script |
Heart rate and skin conductance response week 3 | |
| Soeter et al.48* | Differential fear conditioning | 40:20 | 25 | 75 | 20.4 (3.8) | Day 1: learning Day 2: 40 mg 90 min before reactivation Day 3: LTM test |
Fear potentiated startle to conditioned stimulus during extinction day 3 |
| Kroes et al.49 | Emotionally valenced word list | 12:12 | 58 | 42 | 24.4 | Day 1: learning Day 2: 40 mg 90 min before reactivation Day 3: LTM test |
% free recall day 3 |
| Schwabe et al.22 | Emotionally valenced pictures | 13:13 | 50 | 50 | [18–30] | Day 1: learning Day 2: 40 mg 90 min before reactivation Day 3: LTM test |
% recognition pictures day 3 |
Statistical analysis
To examine the between-group difference on memory performance, we opted to use Hedges g,36,50 which produces an adjusted effect size estimate, rather than Cohen d,51 because the latter is upwardly biased with small samples.52 In behavioural studies, Hedges g < 0.2 represents a small, 0.2–0.5 a moderate and 0.6–0.8 a large effect size.52 For a few reconsolidation studies, Hedges g was averaged across outcomes to control for outcome selection bias.
Owing to the moderate number of included studies and methodological heterogeneity, we used a random-effects model to test our hypotheses. We performed homogeneity analyses to identify outliers and sources of heterogeneity using the Q and I2 statistics.36,50 To account for the limited number of unpublished studies found, we built a funnel plot to examine publication bias for emotional memory consolidation. Furthermore, we calculated the Rosenthal fail-safe N to determine the number of missing negative studies required to nullify our results.50 Publication bias was not assessed for the reconsolidation analysis owing to the limited number of studies included.36 All tests were 2-sided with α < 0.05, unless stated otherwise. We performed our analyses using Comprehensive Meta-Analysis software version 2 (Biostat Inc.).53
Results
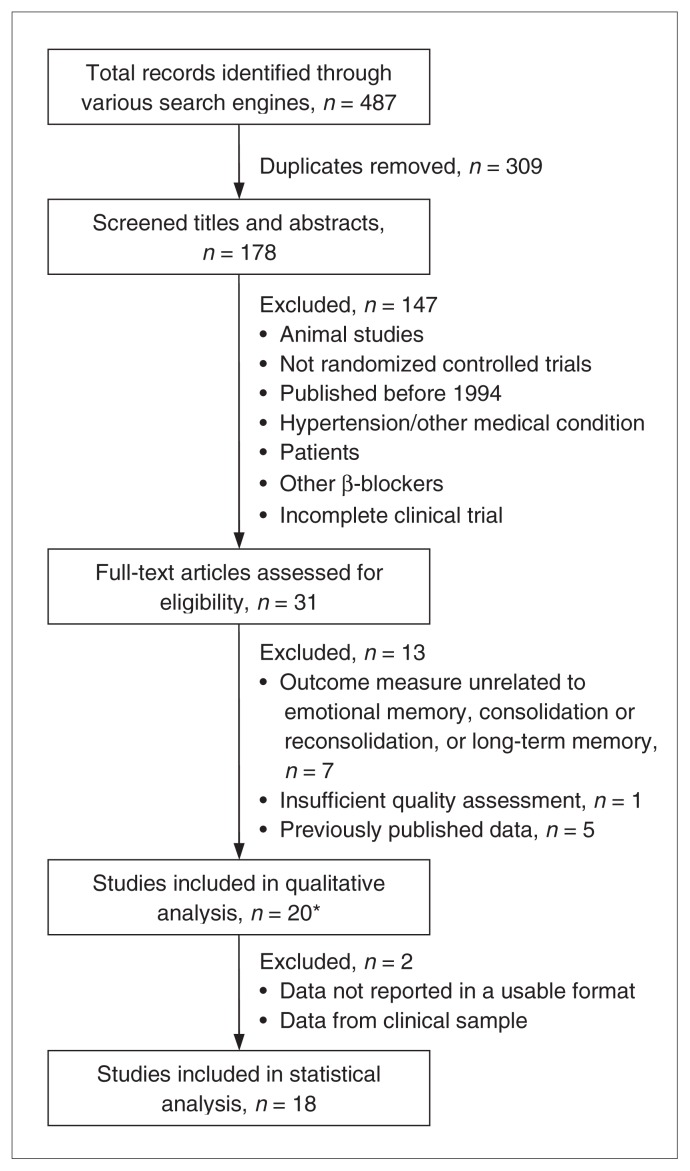
Figure 1 depicts the study selection process. Twenty experiments from 18 articles were included: 12 pertained to memory consolidation and 8 to reconsolidation. Of the 12 consolidation experiments, 2 were excluded from meta-analysis because they did not report data in a usable format43 or because the data pertained to a clinical sample.27
Fig. 1.
Study selection. *The studies by Reist et al.27 and Maheu et al.41 each contain 2 experiments.
Qualitative results
Table 1 summarizes the characteristics of the memory consolidation studies. Overall, 8 of 12 studies closely followed the paradigm of Cahill and colleagues.11 Of these, 5 studies found that participants on propranolol remembered less material than those on placebo,11,27,40,41 and 3 studies19,37,41 failed to find an effect. Of the 4 remaining studies38,42–44 that used different stimuli (emotionally valenced word lists, pictures, fear-conditioning paradigms), 238,43 found an effect for propranolol.
Four of the 8 reconsolidation studies examined cognitive memory performance similarly to the consolidation studies.22,45,47,49 Of these, 2 found a large significant effect22,49 (Table 2). Of the remaining 4 studies, 3 aimed at reducing the expression of cue-elicited fear responses, and 2 of them found an effect for propranolol.46,48 The last study34 used a script-driven imagery task with healthy participants and failed to find an effect for propranolol. In total, 155 participants received propranolol, and 153 received a closely matched placebo.
The average Jadad score for all included studies was 3.5 on a 5-point scale. The most common reason for losing points was failure to report the exact randomization method or failure to report the dropout rate, which may induce a bias.
Quantitative results
Consolidation analysis
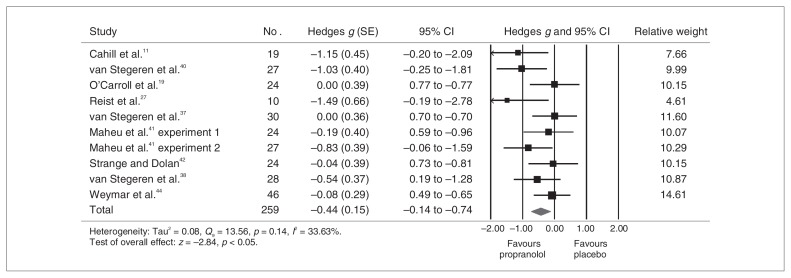
Figure 2 presents the pooled results for the memory consolidation analysis. Overall, participants treated with propranolol (n = 131) remembered less aversive material than those treated with placebo (n = 128; g = 0.44, 95% confidence interval [CI] 0.14–0.74). Statistical heterogeneity was nonsignificant (Q = 13.56, p = 0.14). Between-study variability was considered low (I2 = 33.63%). Sensitivity analyses revealed no outlier. Effect sizes varied between 0 and 1.49. Publication bias analyses revealed a symmetric funnel plot, with only 2 studies missing in order to completely eliminate the publication bias. The Rosenthal fail-safe N analysis indicated that 40 studies with null results would be needed to bring the combined 1-tailed significance to p > 0.05.
Fig. 2.
Propanolol’s effects on emotional memory consolidation. CI = confidence interval; SE = standard error.
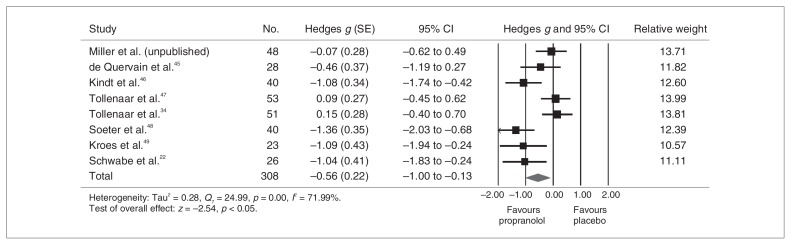
Reconsolidation analysis
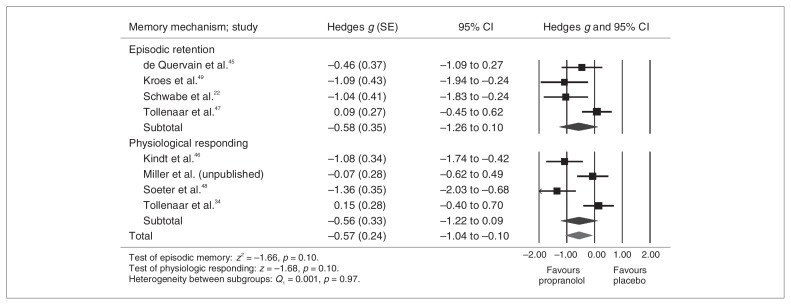
Figure 3 presents the results for the memory reconsolidation analysis. Although heterogeneous (Q = 24.99, p = 0.001, I2 = 71.99%), results were significant, and the effect size was large (Hedges g = 0.56, 95% CI 0.13–1.00). Effect sizes ranged between 0.07 and 1.36. According to the sensitivity analyses, no single study explained the observed heterogeneity. Interestingly, no significant between-group difference emerged when episodic memory retention studies (Hedges g = 0.58) were examined separately from studies measuring physiologic responses to fear conditioning (Hedges g = 0.56; Fig. 4).
Fig. 3.
Propanolol’s effects on emotional memory reconsolidation. CI = confidence interval; SE = standard error.
Fig. 4.
Split-group analysis showing reconsolidation by memory mechanism — fully random effects. CI = confidence interval; SE = standard error.
Owing to pronounced heterogeneity, we conducted a number of post hoc analyses to determine whether medication dosage, the delay before memory test, or sex54,55 moderated the effect size observed in reconsolidation studies. Significant between-group differences were found for all 3 moderators. Studies using 40 mg of propranolol showed a stronger effect that those using 80 mg (Q1= 19.40, p < 0.001), and studies with a 24-hour delay between drug administration and recall demonstrated a larger effect than those with a 1-week period before memory tests (Q1= 6.62, p = 0.010). In addition, studies with male-only samples showed a significantly weaker effect than mixed study samples (Q1= 6.62, p = 0.010). However, these effects should be interpreted with caution since they are all driven by the same 2 studies with null findings.34,47
We conducted a meta-regression to further examine the sex effect. No predictive effect was found within studies examining memory consolidation (z = 0.39, p = 0.70). However, within reconsolidation studies, the greater the proportion of women within a given sample, the larger the effect size (z = 3.35, p = 0.001).
Discussion
Learning under the influence of propranolol led to subsequent recall deficits congruent with consolidation blockade when the stimuli consisted of negatively valenced slides, pictures, word lists and fear-conditioned stimuli. Recall of previously learned material under the influence of propranolol had a similar effect: it led to subsequent recall deficits congruent with reconsolidation theory when the material consisted of negatively valenced emotional words, or it reduced the expression of previously learned cue-elicited fear responses. In the case of reconsolidation, the evidence is considered less robust, despite the larger effect size, because of the heterogeneity across studies. This result should be interpreted with caution until more studies are published. Finally, the observed effects of propranolol on memory apply to moderately emotional material, as tested in an experimental design with healthy adults. It remains to be determined whether more strongly valenced idiosyncratic memories can also be decreased by propranolol (e.g., in clinical populations affected with a mental disorder).
Underlying mechanisms of memory consolidation and reconsolidation
There are a number of issues that the present meta-analysis does not address, including the question of the underlying mechanism of the phenomena observed. Although the results are congruent with a consolidation and reconsolidation blockade explanation, other explanations are plausible. Ideally, the propranolol should be given immediately after the memory task (i.e., postretrieval) rather than 60–90 minutes beforehand to eliminate a possible confounding effect of propranolol on memory encoding or retrieval. Importantly, studies administering propranolol immediately before encoding37 or immediately after (Miller and colleagues, unpublished data, 2004) reactivating the memory failed to find a sustained effect of propranolol. However, this bias is controlled, in principle, by the fact that in studies using the protocol from Cahill and colleagues,11 the propranolol and placebo groups do not differ on memory performance for the neutral material, suggesting that propranolol does not influence memory encoding more than placebo. In addition, recent data indicates that the memory-impairing effects of propranolol are relatively long-lasting and that propranolol has no effect on brain mechanisms activated during reactivation,22,49 ruling out the possibility of an effect solely on retrieval.
Selection of the emotional material
Some stimuli may be more susceptible to propranolol-induced amnesia. Because of the limited number of studies currently available for review, a fine-grained analysis taking into consideration the type of stimuli presented could not be performed. However, among the studies reporting negative findings, especially those that did not use the paradigm of Cahill and colleagues,11 it is possible that the stimuli used were not powerful enough to demonstrate the effect of propranolol in blocking consolidation or reconsolidation of negatively valenced emotional material. In other words, in some cases the so-called “emotional” material could have been quite neutral, thus explaining the lack of results in some studies. If some negative findings could be explained by the emotional stimuli not being powerful enough, the effect size reported in our meta-analysis may be a conservative estimate.
Measurement of recall
In contrast to Cahill and colleagues,11 Strange and Dolan42 examined propranolol’s effects on long-term recognition memory performance for emotionally valenced word lists. Furthermore, Weymar and colleagues44 and van Stegeren and colleagues38 examined emotional memory performance using a set of unrelated pictures. It is unclear whether the non-significant results obtained by Strange and Dolan42 and by Weymar and colleagues44 were due in part to the way they measured recall, their use of a set of stimuli very different from that used by Cahill and colleagues,11 or both.
The way recall is measured may have another type of impact on the results. In the consolidation studies, the most reliable finding seems to be that, compared with the placebo group, participants in the propranolol group failed to recall specific elements of the slides that were accompanied by the emotionally distressing narrative. This is a form of declarative memory. This failure to recall was also reported in 2 of 3 studies looking at reconsolidation,45,49 although results from the study by de Quervain and colleagues45 were not significant. However, in 2 other reconsolidation studies, participants displayed reduced expression of cue-elicited, fear-conditioned physiologic responses (i.e., amygdala-dependent emotional memory), whereas declarative memory remained intact.46,48 Future studies will need to explore whether memory reactivation under propranolol decreases the strength of emotional or declarative memory or both and whether this effect is long-lasting.
Medication dosage and sex effects
Most consolidation studies found an effect for propranolol by using a dose of 40 mg. In contrast, Maheu and colleagues41 did not find significant results using 40 mg of propranolol in a sample of men only. However, 80 mg of propranolol yielded significant results. Using a fixed low dose of propranolol may not work equally well in all populations because of varying body mass, sex or other reasons. For instance, van Stegeren and colleagues38 found a significant effect of 80 mg of propranolol on memory performance, but only in women. In parallel, using 80 mg in a male-only sample, Weymar and colleagues44 did not find an effect of propranolol.
Within the reconsolidation experiments, post hoc analyses revealed a stronger effect for mixed samples using 40 mg of propranolol. In contrast, Tollenaar and colleagues34,47 were the only ones to use 80 mg of propranolol in 2 different reconsolidation protocols; both studies involved male-only samples and failed to find significant effects. A sex difference has been found,54 with women being more influenced by propranolol than men. Three consolidation and 2 reconsolidation studies included in our meta-analysis were conducted with male-only samples; therefore our results potentially underestimate the overall effect size results. Although only observed in the reconsolidation analysis, meta-regression results revealed that the proportion of women significantly predicted the strength of the effect size, lending support to the suggestion that propranolol’s effects may be more pronounced in women than men. Dose effects and sex effects will need to be further explored, also taking into consideration the hormonal cycle of women.56
Can propranolol effectively block memory consolidation or reconsolidation in memory-related mental disorders?
The effect of propranolol on memory observed in this meta-analysis applies to moderately negative emotional material, as tested in an experimental design with healthy adults. Propranolol has also shown promise in animals in reducing avoidance conditioning,16 fear conditioning14 and craving related to cocaine and nicotine dependence.57,58 In humans, Reist and colleagues27 replicated the paradigm by Cahill and colleagues11 in both a healthy population and a clinical (PTSD) sample, finding no between-group differences. Although the study by Reist and colleagues did not involve deeply ingrained traumatic memories, propranolol dampened memory enhancement for an emotionally upsetting slide story in both the healthy and clinical populations.
Thus far, 1 small randomized controlled trial17 used propranolol in a sample of individuals with unremitting PTSD of more than 10 years’ duration in an attempt to decrease the strength of a traumatic memory. In this study, the strength of the trauma memory was lower after a single dose of propranolol than placebo, as measured 1 week later by psycho-physiologic responses while listening to audiotaped personal trauma narratives. This study is important because psycho-physiologic responding to trauma scripts is the most replicated biological finding in PTSD samples, is less prone to demand characteristics and directly tackles the issue of the strength of the emotional memory. Anecdotally, all the participants of that study retained a declarative memory of their traumatic event. Furthermore, in 3 open-label trials, 1 of which included a control group, Brunet and colleagues30 demonstrated that 6 treatment sessions with propranolol administered before trauma memory reactivation led to a significant decrease in PTSD symptoms. Propranolol’s capacity to prevent the development of PTSD immediately after trauma exposure has also been examined in a few studies with conflicting findings and methodological flaws.26,28,29,59,60 Further investigations under improved methodological conditions are underway (see clinical trials.gov) and should help elucidate this question in the near future.
Limitations
This meta-analysis is limited by the moderate number of studies examining the influence of propranolol on emotional memory consolidation and reconsolidation in healthy adults, making it difficult to examine the effects of moderating variables on outcome. Furthermore, it is noteworthy that most samples consisted entirely of young adults, most commonly undergraduate students, which may limit the ecological validity of results. Finally, in 2 studies,37,38 effects had to be estimated from published figures owing to the original data being unavailable. However, removing these studies from our analyses did not change our results.
Conclusion
Pharmacological reconsolidation blockade might have the potential to become a novel treatment in psychiatry. Summarizing the currently available evidence from placebo-controlled experimental studies involving healthy participants, this meta-analysis represents an important step in this direction. In this review, propranolol reduced memory for both new and previously learned emotional material in healthy adults. Future studies will have to test whether more powerful idiosyncratic emotional memories can be durably weakened and whether this weakening can bring about lasting symptomatic relief in various clinical populations that have at their core an emotional memory (e.g., PTSD, phobias, depression, obsessive–compulsive disorder, eating disorders, addictions).
Acknowledgements
A. Brunet holds a salary award from the Fonds de Recherche du Québec - Santé. M.H. Lonergan holds a student award from the Canadian Institutes of Health Research. L.A. Olivera-Figueroa holds a postdoctoral fellowship from the Fernand-Seguin Research Centre.
Footnotes
An earlier version of this work was presented at the 12th European Conference on Traumatic Stress in Vienna, Austria, June 2–5, 2011.
Competing interests: None declared.
Contributors: All authors contributed to study design, wrote and reviewed the article and approved its publication. M.H. Lonergan, L.A. Olivera-Figueroa and A. Brunet acquired the data, which M.H. Lonergan, R.K. Pitman and A. Brunet analyzed.
References
- 1.Mueller D, Cahill SP. Noradrenergic modulation of extinction learning and exposure therapy. Behav Brain Res. 2010;208:1–11. doi: 10.1016/j.bbr.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 2.Brunet A, Weiss DS, Metzler TJ, et al. The Peritraumatic Distress Inventory: a proposed measure of PTSD criterion A2. Am J Psychiatry. 2001;158:1480–5. doi: 10.1176/appi.ajp.158.9.1480. [DOI] [PubMed] [Google Scholar]
- 3.Pitman RK, Orr SP. The black hole of trauma. Biol Psychiatry. 1990;27:469–71. doi: 10.1016/0006-3223(90)90437-7. [DOI] [PubMed] [Google Scholar]
- 4.Pitman RK. Post-traumatic stress disorder, hormones, and memory. Biol Psychiatry. 1989;26:221–3. doi: 10.1016/0006-3223(89)90033-4. [DOI] [PubMed] [Google Scholar]
- 5.Gershuny BS, Baer L, Parker H, et al. Trauma and posttraumatic stress disorder in treatment-resistant obsessive-compulsive disorder. Depress Anxiety. 2008;25:69–71. doi: 10.1002/da.20284. [DOI] [PubMed] [Google Scholar]
- 6.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 7.Spinhoven P, Elzinga BM, Hovens JG, et al. The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. J Affect Disord. 2010;126:103–12. doi: 10.1016/j.jad.2010.02.132. [DOI] [PubMed] [Google Scholar]
- 8.Dohrenwend BP. The role of adversity and stress in psychopathology: some evidence and its implications for theory and research. J Health Soc Behav. 2000;41:1–19. [PubMed] [Google Scholar]
- 9.Duka T, Crombag HS, Stephens DN. Experimental medicine in drug addiction: towards behavioral, cognitive and neurobiological biomarkers. J Psychopharmacol. 2011;25:1235–55. doi: 10.1177/0269881110388324. [DOI] [PubMed] [Google Scholar]
- 10.Carlson NR. Physiology of behavior. 10th ed. Boston (MA): Allyn & Bacon; 2010. Learning and memory; pp. 440–84. [Google Scholar]
- 11.Cahill L, Prins B, Weber M, et al. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–4. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain SR, Muller U, Blackwell AD, et al. Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacology (Berl) 2006;188:397–407. doi: 10.1007/s00213-006-0391-6. [DOI] [PubMed] [Google Scholar]
- 13.Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–5. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- 14.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–6. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 15.Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19:6623–8. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Przybyslawski J, Sara SJ. Reconsolidation of memory after its reactivation. Behav Brain Res. 1997;84:241–6. doi: 10.1016/s0166-4328(96)00153-2. [DOI] [PubMed] [Google Scholar]
- 17.Brunet A, Orr SP, Tremblay J, et al. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–6. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Cahill L, Pham CA, Setlow B. Impaired memory consolidation in rats produced with β-adrenergic blockade. Neurobiol Learn Mem. 2000;74:259–66. doi: 10.1006/nlme.1999.3950. [DOI] [PubMed] [Google Scholar]
- 19.O’Carroll RE, Drysdale E, Cahill L, et al. Memory for emotional material: a comparison of central versus peripheral beta blockade. J Psychopharmacol. 1999;13:32–9. doi: 10.1177/026988119901300104. [DOI] [PubMed] [Google Scholar]
- 20.Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–59. [PubMed] [Google Scholar]
- 21.Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–72. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Schwabe L, Nader K, Wolf OT, Beaudry T, Pruessner JC. Neural signature of reconsolidation impairments by propranolol in humans. Biol Psychiatry. 2012;71:380–6. doi: 10.1016/j.biopsych.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Holroyd KA, Penzien DB, Cordingley GE. Propranolol in the management of recurrent migraine: a meta-analytic review. Headache. 1991;31:333–40. doi: 10.1111/j.1526-4610.1991.hed3105333.x. [DOI] [PubMed] [Google Scholar]
- 24.Raj SR, Black BK, Biaggioni I, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: Less is more. Circulation. 2009;120:725–34. doi: 10.1161/CIRCULATIONAHA.108.846501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brantigan CO, Brantigan TA, Joseph N. Effect of beta blockade and beta stimulation on stage fright. Am J Med. 1982;72:88–94. doi: 10.1016/0002-9343(82)90592-7. [DOI] [PubMed] [Google Scholar]
- 26.Pitman RK, Sanders KM, Zusman RM, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–92. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 27.Reist C, Duffy JG, Fujimoto K, et al. Beta-adrenergic blockade and emotional memory in PTSD. Int J Neuropsychopharmacol. 2001;4:377–83. doi: 10.1017/S1461145701002607. [DOI] [PubMed] [Google Scholar]
- 28.Vaiva G, Ducrocq F, Jezequel K, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54:947–9. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 29.Hoge EA, Worthington JJ, Nagurney JT, et al. Effect of acute post-trauma propranolol on PTSD outcome and physiological responses during script-driven imagery. CNS Neurosci Ther. 2012;18:21–7. doi: 10.1111/j.1755-5949.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunet A, Poundja J, Tremblay J, et al. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J Clin Psychopharmacol. 2011;31:547–50. doi: 10.1097/JCP.0b013e318222f360. [DOI] [PubMed] [Google Scholar]
- 31.Dey M, Brisson J, Davis G, et al. Relationship between plasma propranolol concentration and dose in young, healthy volunteers. Biopharm Drug Dispos. 1986;7:103–11. doi: 10.1002/bdd.2510070202. [DOI] [PubMed] [Google Scholar]
- 32.Schiller D, Phelps EA. Does reconsolidation occur in humans? Front Behav Neurosci. 2011;5:1–12. doi: 10.3389/fnbeh.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunet A, Ashbaugh AR, Saumier D, et al. Does reconsolidation occur in humans: a reply. Frontiers in behavioral neuroscience. 2011;5:74. doi: 10.3389/fnbeh.2011.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tollenaar MS, Elzinga BM, Spinhoven P, et al. Psychophysiological responding to emotional memories in healthy young men after cortisol and propranolol administration. Psychopharmacology (Berl) 2009;203:793–803. doi: 10.1007/s00213-008-1427-x. [DOI] [PubMed] [Google Scholar]
- 35.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Gainesville (FL): University of Florida; 2008. [Google Scholar]
- 36.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2009. [accessed 2012 Oct. 10]. Version 5.0.2 [updated September 2009]. Available: www.cochrane-handbook.org. [Google Scholar]
- 37.van Stegeren AH, Everaerd W, Gooren LJ. The effect of beta-adrenergic blockade after encoding on memory of an emotional event. Psychopharmacology (Berl) 2002;163:202–12. doi: 10.1007/s00213-002-1163-6. [DOI] [PubMed] [Google Scholar]
- 38.van Stegeren AH, Goekoop R, Everaerd W, et al. Noradrenaline mediates amygdala activation in men and women during encoding of emotional material. Neuroimage. 2005;24:898–909. doi: 10.1016/j.neuroimage.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 40.van Stegeren AH, Everaerd W, Cahill L, et al. Memory for emotional events: differential effects of centrally versus peripherally acting beta-blocking agents. Psychopharmacology (Berl) 1998;138:305–10. doi: 10.1007/s002130050675. [DOI] [PubMed] [Google Scholar]
- 41.Maheu FS, Joober R, Beaulieu S, et al. Differential effects of adrenergic and corticosteroid hormonal systems on human short- and long-term declarative memory for emotionally arousing material. Behav Neurosci. 2004;118:420–8. doi: 10.1037/0735-7044.118.2.420. [DOI] [PubMed] [Google Scholar]
- 42.Strange BA, Dolan RJ. Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc Natl Acad Sci U S A. 2004;101:11454–8. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grillon C, Cordova J, Morgan CA, et al. Effects of the beta-blocker propranolol on cued and contextual fear conditioning in humans. Psychopharmacology (Berl) 2004;175:342–52. doi: 10.1007/s00213-004-1819-5. [DOI] [PubMed] [Google Scholar]
- 44.Weymar M, Low A, Modess C, et al. Propranolol selectively blocks the enhanced parietal old/new effect during long-term recollection of unpleasant pictures: a high density ERP study. Neuroimage. 2010;49:2800–6. doi: 10.1016/j.neuroimage.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 45.de Quervain DJ, Aerni A, Roozendaal B. Preventive effect of beta-adrenoceptor blockade on glucocorticoid-induced memory retrieval deficits. Am J Psychiatry. 2007;164:967–9. doi: 10.1176/ajp.2007.164.6.967. [DOI] [PubMed] [Google Scholar]
- 46.Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–8. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 47.Tollenaar MS, Elzinga BM, Spinhoven P, et al. Immediate and prolonged effects of cortisol, but not propranolol, on memory retrieval in healthy young men. Neurobiol Learn Mem. 2009;91:23–31. doi: 10.1016/j.nlm.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol Learn Mem. 2010;94:30–41. doi: 10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Kroes MC, Strange BA, Dolan RJ. Beta-adrenergic blockade during memory retrieval in humans evokes a sustained reduction of declarative emotional memory enhancement. J Neurosci. 2010;30:3959–63. doi: 10.1523/JNEUROSCI.5469-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis. Chichester: Wiley; 2009. [Google Scholar]
- 51.Cohen J. A power primer. Psychol Bull. 1992;112:155–9. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 52.Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks (CA): Sage Publications; 2001. [Google Scholar]
- 53.Borenstein M, Hedges L, Higgins J, et al. Comprehensive meta-analysis. Version 2. Englewood (NJ): Biostat; 2005. [Google Scholar]
- 54.Cahill L, van Stegeren A. Sex-related impairment of memory for emotional events with beta-adrenergic blockade. Neurobiol Learn Mem. 2003;79:81–8. doi: 10.1016/s1074-7427(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 55.Poundja J, Sanche S, Tremblay J, et al. Trauma reactivation under the influence of propranolol: an examination of clinical predictors. Eur J Psychotraumatol. 2012;3 doi: 10.3402/ejpt.v3i0.15470. [Epub 2012 Feb. 14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen SE, Ertman N, Lakhani YS, et al. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiol Learn Mem. 2011;96:378–84. doi: 10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiamulera C, Tedesco V, Zangrandi L, et al. Propranolol transiently inhibits reinstatement of nicotine-seeking behaviour in rats. J Psychopharmacol. 2010;24:389–95. doi: 10.1177/0269881108097718. [DOI] [PubMed] [Google Scholar]
- 58.Kampman KM, Volpicelli JR, Mulvaney F, et al. Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend. 2001;63:69–78. doi: 10.1016/s0376-8716(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 59.Nugent NR, Christopher NC, Crow JP, et al. The efficacy of early propranolol administration at reducing PTSD symptoms in pediatric injury patients: a pilot study. J Trauma Stress. 2010;23:282–7. doi: 10.1002/jts.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stein MB, Kerridge C, Dimsdale JE, et al. Pharmacotherapy to prevent PTSD: results from a randomized controlled proof-of-concept trial in physically injured patients. J Trauma Stress. 2007;20:923–32. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]