Abstract
Background
Anorexia nervosa is characterized by high levels of perseveration and inflexibility, which interfere with successful treatments. Dopamine (DA) signalling seems to play a key role in modulating the prefrontal cortex, since both DA deficiency and excess negatively influence the efficiency of cognitive functions. The present study explores the effect of a functional polymorphism (Val158Met) in the catechol-O-methyltransferase (COMT) gene on the set-shifting abilities and prefrontal functional connectivity of patients with anorexia nervosa.
Methods
All participants performed the Wisconsin Card Sorting Task, and a subsample underwent resting-state functional magnetic resonance imaging.
Results
We included 166 patients with DSM-IV lifetime anorexia nervosa and 140 healthy women in our study. Both underweight and weight-recovered patients with anorexia nervosa showed high levels of perseveration, but only in the underweight group did the Val158Met polymorphism affect cognitive performance, showing the U-shaped curve characteristic of increased DA signalling in the prefrontal cortex. Underweight patients with anorexia nervosa who are Met homozygotes had significantly higher levels of perseveration and increased prefrontal functional connectivity than underweight patients in the other genotype groups, indicating abnormal regional cortical processing.
Limitations
Although our data show that grey matter reduction in starving patients with anorexia nervosa did not explain our findings, the cross-sectional design of the present study did not allow us to distinguish between the effects of starvation and those of low estrogen levels.
Conclusion
Starvation affects DA release in the prefrontal cortex of patients with anorexia nervosa with different effects on executive functioning and prefrontal functional connectivity according to the COMT genotype. This observation has several therapeutic implications that need to be addressed by future studies.
Introduction
Anorexia nervosa is a severe psychiatric illness characterized by a relentless pursuit of thinness and food avoidance, distortion of body image, and high levels of perseveration and inflexibility.1,2 Cognitive inflexibility and set-shifting impairment have been found in women with current and past anorexia nervosa and also among unaffected sisters of patients with anorexia nervosa, suggesting that these cognitive features are putative endophenotypes.3–5
A dysregulation in dopaminergic function, particularly in frontostriatal circuits, has been considered a key factor in explaining the affective and cognitive alterations observed in patients with anorexia nervosa.1,6 However, few studies have explored the role of genes regulating dopaminergic function on the cognitive characteristics of patients with eating disorders,7 and to our knowledge none have investigated starving patients with anorexia nervosa as a separate group. The Val158Met polymorphism of the catechol-O-methyltransferase enzyme (COMT) is particularly interesting, because of the critical role it plays in dopamine (DA) metabolism in the prefrontal cortex (PFC).8 It is an S-adenosylmethionine-dependent methyltransferase enzyme that methylates catecholamines and catechol estrogens. The Val158Met polymorphism influences the thermal stability and activity of COMT,9 with DA signalling probably being enhanced in Met158-carrying compared with Val158-carrying individuals. Various studies of both healthy and psychotic patients have shown that the Val158Met allele has a small but significant influence on pre-frontal cognitive performance, and Met158-carrying individuals perform better than Val158-carrying individuals.8,10
However, DA does not always enhance cognitive performance.11 An inverted U-shaped curve characterizes the relationship between DA levels and cognitive performance, and both reduced and excessive DA activity impair prefrontal functions. These effects may be mediated by the differential effects of D1 and D2 receptor binding. Tonic stimulation of D1 receptors is associated with stabilization of mental representation in active memory, whereas phasic D2 receptor binding allows flexible adjustment of processing and updating of active memory.11 Decreased COMT activity in Met carriers leads to increased DA in the PFC, which diffuses from the synaptic cleft to activate extrasynaptic D1 receptors (high D1:D2 ratio).8,10,11 In contrast, increased COMT activity in Val carriers limits DA diffusion from the synapse, favouring activation of intrasynaptic D2 receptors (low D1:D2 ratio). In the former case, the effects include not only enhanced maintenance of information, but also great inflexibility; in the latter, participants are more flexible, but are also more easily distracted.10,11
Another reason why the COMT polymorphism is particularly interesting in anorexia nervosa is that it allows us to explore its effects in women in various conditions of exposure to estrogens.12 Estrogens have an inhibitory effect on COMT mRNA expression, reducing the effect of COMT enzyme activity and minimizing the repercussions of the COMT genotype in women compared with men.13 Since estrogen levels decrease greatly in starvation,14 underweight patients with anorexia nervosa represent a unique opportunity to analyze the convergence between estrogen levels and the COMT genotype.
The aim of the present study was to explore the influence of the COMT Val158Met polymorphism on executive functioning and resting-state prefrontal functional connectivity in a sample of patients with lifetime anorexia nervosa, considering the effects of weight and menstrual status. We hypothesized that the starving condition enhances the effects of the COMT genotype on both executive functioning and PFC functional connectivity. The role of the COMT polymorphism on brain function has been further demonstrated by task-related functional magnetic resonance imaging (fMRI) studies, which have consistently shown greater (and less efficient) prefrontal activation during working memory tasks among carriers of the Val allele.15 On the contrary, only 1 study has explored the role of the COMT polymorphism on resting-state functional connectivity;16 this study reported significantly decreased pre-frontal connectivity within the default mode network in homozygous Val compared with heterozygous healthy controls,16 leading to the hypothesis that COMT-related differences in DA levels modulate PFC functional connectivity. Recent discoveries concerning brain function have demonstrated that resting-state spontaneous fluctuations in the blood oxygen level–dependent (BOLD) MRI signals provide unique insights into the organization of intrinsic activity in the human brain.17 Coherent resting-state networks appear to play a fundamental role in brain functional organization, although their correlation with psychopathology remains to be explored. Available studies in the literature show that resting-state functional connectivity predicts the task-response properties of brain regions,18 identifies an individual’s aptitude for cognitive tasks17 and reveals disturbances in their correlation structure in many psychiatric diseases.19
Methods
Participants
We recruited participants with lifetime anorexia nervosa from the Eating Disorders Unit of Padova, Italy. Healthy women within the same age range were recruited from the general population and among volunteers, as described in previous studies.5,20,21 A description of the samples is given in Appendix 1, available at cma.ca/jpn. All patients with anorexia nervosa had to meet DSM-IV criteria for anorexia nervosa in their lifetime; however, patients could be at different stages of their illness at the time of neuropsychological assessment. To be eligible for participation in the healthy control group, women had to have no history of eating disorders. All participants gave written informed consent for the use of data in an anonymous form, and the Ethical Committee of Padova Hospital approved the study.
A subgroup of participants participated in the resting-state functional connectivity study. All patients with anorexia nervosa in the subsample had to be underweight and amenorrhoic. A description of this subsample is provided in Appendix 1.
Clinical assessment
All patients with anorexia nervosa were medically stable at the time of testing/scanning. Only 5 patients had been admitted to a medical ward at the time of participation, but neuropsychological performance and MRI scanning were performed after stabilization of their medical condition and normalization of electrolyte levels (Appendix 1). In all participants (patients and controls), diagnostic interviews were performed using the eating disorders section of the Structured Clinical Interview for DSM-IV. Handedness was assessed with the Edinburgh Handedness Inventory.22
Genotype analysis
We extracted genomic DNA from peripheral blood using a High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH) or from saliva using Oragene DNA Self-Collection kits (DNA Genotek Inc.). The Val158Met variant was analyzed by High Resolution Melt (HRM) on a Rotor-Gene 6000 (Corbett Life Sciences). Details of primers and procedures are reported in Appendix 1.
Neuropsychological assessment
We assessed set-shifting abilities using the Wisconsin Card Sorting Test23 (WCST), as described in our previous study.5 The global index,5,24 number of perseverative responses and number of nonperseverative errors were adjusted for age, sex and educational level, as reported by Laiacona and colleagues.24
Neuroimaging protocol and image processing
Data were collected on a Philips Achieva 1.5 T scanner equipped for echo-planar imaging. A resting-state fMRI scan entailed 250 continuous functional volumes (repetition time [TR] 2009 ms, echo time [TE] 50 ms, flip angle 90°, 21 slices, matrix 128 × 128, acquisition voxel size 1.8 × 1.8 × 6 mm, acquisition time 8 min, field of view [FOV] 23 cm). We instructed participants to rest with their eyes closed during the scan. For spatial normalization and localization, high-resolution 3-dimensional T1-weighted anatomic images were also acquired in a gradient-echo sequence (TR 20 ms, TE 3.78 ms, flip angle 20°, 160 slices, acquisition voxel size 1 × 0.66 × 0.66 mm, FOV 21–22 cm). The segmented anatomic images of all participants were used to obtain voxel-wise probability maps of grey matter to be used as a nuisance variable in statistical analyses, as described by Oakes and colleagues.25
Resting-state scans were preprocessed with both Analysis of Functional NeuroImages (version AFNI_2010_10_19_1028; http://afni.nimh.nih.gov/afni; National Institute of Mental Health) and FMRIB Software Library (FSL version 4.1.6; http://www.fmrib.ox.ac.uk; FMRIB Centre). Preprocessing was performed as described by Biswal and colleagues26 and at www.nitrc.org/projects/fcon_1000. Details of preprocessing and processing are described in Appendix 1. We used a high-pass filter setting of 200 seconds (< 0.005 Hz) to reduce very low-frequency artifacts, such as scanner draft, and a low-pass filter to remove any components in the high-frequency spectrum (> 0.1 Hz).
We used a seed voxel correlation approach to explore functional connectivity in the PFC. We selected 3 bilateral spherical seeds (diameter 12 mm) representing, first, the dorsolateral PFC27 (DLPFC; Montreal Neurological Institute [MNI] space x, y, z = ±36, 27, 29), second, the ventrolateral PFC28 (vlPFC; MNI space x, y, z = ±47, 26, 15), and third, the ventromedial PFC28 (vmPFC; MNI space x, y, z = –6, 50, –9 and 7, 54, –9). We removed nuisance signals by multiple regression before performing functional connectivity analyses. Time series were averaged across all voxels in the seed region of interest (ROI) and then, for each participant, we determined the correlation between the time series of the seed ROI and that of each voxel in the brain. Last, correlation maps were converted to z value maps. We used the resulting standardized maps for group comparisons and correlations, with age and hand lateralization as nuisance variables. Nonparametric permutation testing (5000 permutations) was used for statistical analysis of spatial maps by means of threshold-free cluster enhancement29 and multiple comparison correction across space, thresholding at p < 0.05. Graph data were obtained by extracting the average z value in the area of interest for any individual map and by managing the data using PASW Statistics version 18.0 (SPSS Inc.).
Statistical analysis
All variables were tested for normality. The performance indices of the WCST were not normally distributed, and we used a natural log-transformation to normalize these variables. To account for multiple comparisons (3 variables, 3 groups, 3 genotypes), we considered results to be significant at p < 0.0018. We used the Spearman rho to test correlations among variables and the χ2 test to analyze genotype differences. The clinical and neuropsychological characteristics of participants were compared by carrying out between-group t tests or 1-way analysis of variance using PASW Statistics version 18.0 (SPSS Inc.).
Results
Participants
We included 166 patients with lifetime anorexia nervosa and 140 healthy controls in our study. All participants were white women. In the anorexia nervosa group, the mean age at assessment was 25.0 (standard deviation [SD] 6.9) years, and the mean level of education was 14.0 (SD 2.8) years. All patients met DSM-IV criteria for lifetime anorexia nervosa; however, at the time of assessment, they were at different stages of illness: 73 were acutely underweight (mean body mass index [BMI] 15.9 [SD 1.4], range 9.9–17; 58 were amenorrhoic, 5 were taking oral contraceptives, 10 had recovered menses) and 93 were weight-recovered (mean BMI 20.2 [SD 2.6], range 18–36; 16 were still amenorrhoic, 12 were taking oral contraceptives, 66 had regular menses). In the control group, the mean age at assessment was 27.2 (SD 4.8) years, and the mean level of education was 16.0 (SD 2.4) years. Forty women in the control group were taking oral contraceptives at the time of assessment.
Our subgroup analysis involved 63 participants (33 patients with anorexia nervosa, 30 healthy controls) participating in the resting-state functional connectivity study. In this subsample, all patients with anorexia nervosa were underweight (mean BMI 15.8 [SD 1.9], range 10.4–17.5) and amenorrhoic.
Genotype distribution
Genotype distribution was very similar in patients and controls (χ22 = 0.50, p = 0.78) and was consistent with expectations based on the Hardy–Weinberg equilibrium (Appendix 1).
Set-shifting impairment
Patients with anorexia nervosa performed significantly worse than healthy controls on the WCST (p < 0.001 for both perseverative and nonperseverative errors; Appendix 1, Table S1). No differences emerged between underweight and weight-recovered patients, patients with amenorrhea and those with regular menses, or patients with a restrictive phenotype and those with bingeing/purging behaviour. Correlations between WCST performance and clinical variables are described in Appendix 1, Table S2. In women with anorexia nervosa, no correlation emerged between WCST performance and age at onset of illness, illness duration, hand lateralization or BMI. In both groups, WCST scores showed a negative correlation with educational levels (better performance correlated with higher education). In healthy women, there was a trend toward a positive correlation between WCST and BMI (better performance correlated with lower BMI).
Set-shifting impairment according to the COMT genotype
Given the effects of estrogens on COMT expression, we first explored the differences in WCST performance between participants who were taking oral contraceptives and those who were not in the different genotype groups (Appendix 1, Fig. S1). We found significant differences in the anorexia nervosa, but not in the control group. For this reason, we did not include patients with anorexia nervosa taking oral contraceptives in subsequent analyses. No significant differences on the WCST were found between patients with anorexia nervosa with and without amenorrhea in any of the genotype groups (Appendix 1, Fig. S2).
No differences in the performance on the WCST emerged between the 3 COMT genotypes in the anorexia nervosa sample or among healthy controls (Table 1). However, the difference between patients and controls increased with the number of Met alleles; the difference was not significant in Val-Val carriers (global WCST p = 0.05), showed a trend toward significance in heterozygotes (global WCST p = 0.021) and was highly significant in Met-Met carriers (global WCST p = 0.001).
Table 1.
Wisconsin Card Sorting Task performance in patients with lifetime anorexia nervosa and in healthy control women according to weight status and COMT genotype
| Group; mean (SD) raw score* | |||||
|---|---|---|---|---|---|
|
|
|||||
| Genotype; WCST measure | Underweight† | Weight-restored† | Control | F2,62‡ | p value‡ |
| Met-Met | |||||
| No. | 16 | 15 | 34 | ||
| Global score | 70.0 (39.0) | 48.6 (39.3) | 34.2 (30.9) | 7.91 | 0.001§ |
| Perseverative responses | 27.6 (19.1) | 17.6 (17.1) | 11.5 (10.0) | 8.59 | 0.001§ |
| Nonperseverative responses | 20.8 (16.5) | 14.1 (9.6) | 11.1 (10.0) | 4.84 | 0.011§ |
| Val-Met | |||||
| No. | 30 | 47 | 68 | ||
| Global score | 42.2 (34.9) | 50.7 (32.1) | 37.2 (29.6) | 3.19 | 0.04 |
| Perseverative responses | 12.7 (9.2) | 17.4 (12.6) | 13.1 (11.3) | 3.08 | 0.05 |
| Nonperseverative responses | 14.3 (15.2) | 15.2 (11.0) | 11.0 (8.8) | 2.23 | 0.11 |
| Val-Val | |||||
| No. | 22 | 19 | 38 | ||
| Global score | 51.1 (35.2) | 53.6 (34.9) | 40.7 (31.8) | 1.90 | 0.16 |
| Perseverative responses | 17.4 (15.4) | 17.2 (14.9) | 13.9 (11.8) | 1.123 | 0.33 |
| Nonperseverative responses | 16.1 (12.0) | 16.0 (11.4) | 12.7 (12.3) | 1.512 | 0.23 |
COMT = catechol-O-methyltransferase; SD = standard deviation; WCST = Wisconsin Card Sorting Task.23
Unless otherwise indicated.
Patients with anorexia nervosa who were taking oral contraceptives were excluded.
WCST scores adjusted for age and education were used for statistical analysis after logarithmic transformation. To account for multiple comparisons, results were considered to be significant at p < 0.0018.
Post hoc underweight anorexia nervosa > control.
Set-shifting impairment according to the COMT genotype and weight status
Table 1 shows the number of perseverative responses and nonperseverative errors on the WCST in participants with low BMI (< 18), weight-recovered patients and controls. In post hoc analyses, no significant differences were found between underweight and weight-recovered patients with anorexia nervosa in any genotype group. However, in the underweight anorexia nervosa sample, but not in weight-recovered patients, heterozygotes showed a trend toward better performance than both homozygotes in the mean number of perseverative responses (12.7 [SD 9.2] v. 21.7 [SD 17.5], t = 2.16, p = 0.034), with the results showing a typical U-shaped curve. Similar performances, though nonsignificant, have been found for nonperseverative errors (p = 0.16).
Exclusion of left-handed participants and of those who were taking antidepressants did not affect any of the significant findings.
Prefrontal functional connectivity
Patients with anorexia nervosa and controls who also participated in the fMRI study did not differ in genotype distribution (healthy women χ22 = 3.45, p = 0.18; women with anorexia nervosa χ22 = 1.58, p = 0.45) or WCST performance (global WCST healthy women t = 0.59, p = 0.56; women with anorexia nervosa t = 0.82, p = 0.41) compared with the other participants. Analyses of the functional connectivity of the 3 pairs of seeds (DLPFC, vmPFC and vlPFC) did not reveal any significant differences between patients with anorexia nervosa and healthy women. In both patients and controls, we then performed group comparisons between Val allele carriers and Met-Met carriers, and between Val-Val carriers and Met allele carriers. No significant differences were found between genotypes in healthy women. In the anorexia nervosa group, several significant differences in the Met-Met group were found in comparison with Val carriers. The Met-Met group displayed significantly higher positive coactivation for the DLPFC and vmPFC seeds (Figs. 1 and 2) and significantly lower negative coactivation for the DLPFC, vmPFC and vlPFC (Appendix 1, Figs. S1–S4). Exploring how much the COMT effects differ between groups (i.e., testing the effect × group interaction), we found a significant interaction only for the functional connectivity of the vmPFC seed (Fig. 2 for positive connectivity and Appendix 1, Figs. S1–S4 for negative connectivity). No significant difference emerged in the comparison between Met carriers and the Val-Val group.
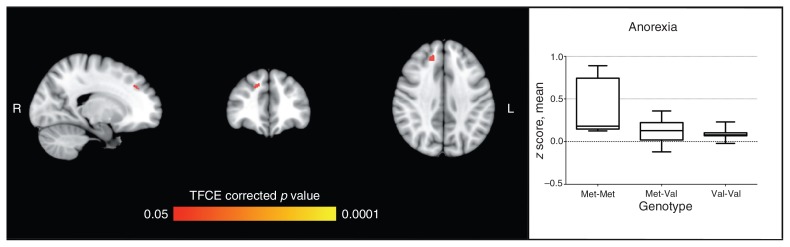
Fig. 1.
Seed-based analysis for the dorsolateral prefrontal cortex, comparing effects of the catechol-O-methyltransferase enzyme (COMT) genotype in patients with anorexia nervosa and healthy controls. The figure shows area of significant difference (p < 0.05, TFCE-corrected) between the Met-Met genotype and Val carriers in the anorexia nervosa group (Met-Met > Val); peak: 15, 39, 36 (Brodmann area 32). The graph shows average coactivation (z score) according to the COMT genotype in patients with anorexia nervosa. No significant effect was found in healthy controls. Analyses were by nonparametric permutation test, with age, education and hand lateralization as covariates. TFCE = threshold-free cluster enhancement.
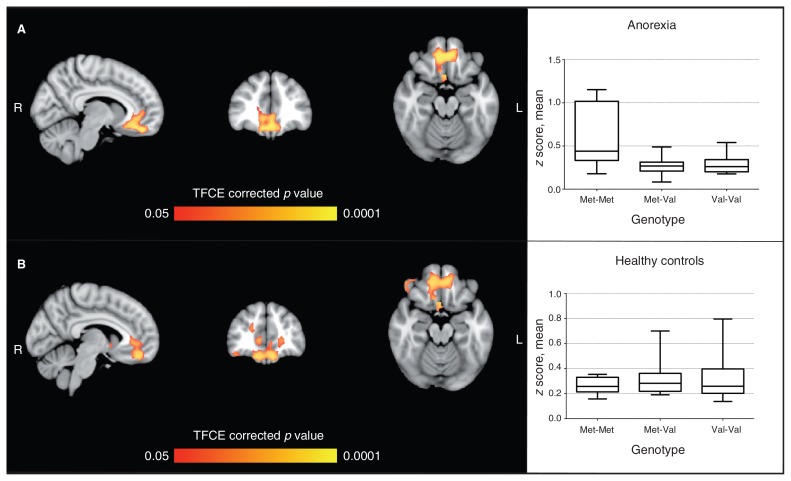
Fig. 2.
Seed-based analysis for the ventromedial prefrontal cortex comparing effects of the catechol-O-methyltransferase enzyme (COMT) genotype in patients with anorexia nervosa and healthy controls. (A) Area of significant genotype × group interaction; peaks: 9, 39, –18 (Brodmann area [BA] 11) and 36, 0, –42 (BA 36). (B) Area of significant difference (p < 0.05, TFCE-corrected) between Met-Met genotype and Val carriers in the anorexia nervosa group (Met-Met > Val); peaks: –6, 45, –18 (BA 11) and 33, –3, –48 (BA 20). Graphs show average coactivation (z score) according to the COMT genotype in patients with anorexia nervosa and healthy controls for the brain areas shown in panel B. No significant effect was found in healthy controls. Analyses were by nonparametric permutation test, with age, education and hand lateralization as covariates. TFCE = threshold-free cluster enhancement.
All group differences remained statistically significant even after including grey matter probability maps as a nuisance variable to control for brain atrophy. In addition, no significant correlations emerged between functional connectivity of the 3 pairs of seeds and grey matter probabilities, antidepressant treatment, depressive or anxiety symptoms, duration of illness, BMI at the time of scanning, or the hydration index obtained by bioelectrical impedence analysis in a subgroup of patients with anorexia nervosa. Exclusion of left-handed participants or those who were taking antidepressants did not affect any of the significant findings.
Discussion
We found evidence that the COMT genotype affects both executive performance, in terms of inflexibility, and PFC functional connectivity in starving patients with anorexia nervosa. Underweight Met homozygous patients with anorexia nervosa displayed the highest levels of perseveration, whereas among healthy women in our study and in the literature,8,10 this genotype was associated with the best performance on the WCST (Table 1). Cognitive performance measured by the WCST in the underweight women with anorexia nervosa was consistent with the U-shaped curve as the effect of the COMT polymorphism, as previously observed in situations characterized by increased PFC dopaminergic activity.30,31 There is growing evidence of a dysfunction of dopaminergic systems in patients with anorexia nervosa,1,32 although published data mainly refer to subcortical functioning. Patients with anorexia nervosa display increased D2/D3 receptor binding in the ventral striatum, alteration of reward-related processes, reduced cerebrospinal fluid concentrations of the DA metabolite homovanillic acid, and altered growth hormone response to apomorphine stimulation.32 However, the effects of the COMT genotype are usually appreciable only on PFC function, probably because of the presence of more efficient DA transporters in subcortical areas.33 Decreased motivation, compulsive exercising and loss of appetitive responses are considered to be a consequence of dopaminergic dysfunction, although the biological mechanisms underlying these symptoms are not clear. Some form of DA dysregulation occurs in both the acute stage of anorexia nervosa32 and in patients who have recovered from the illness,34,35 but clinical characteristics, such as low motivation and compulsive exercise, typically tend to exacerbate with weight loss.
In contrast with previous studies that investigated mainly subcortical functions, our findings on WCST performance are consistent with excessive DA signalling in the PFC of underweight patients with anorexia nervosa. Generally, exposure to both acute and chronic stress increases DA release in the PFC36,37 together with activation of the hypothalamic–pituitary–adrenal axis.37 In these situations, interruption of top–down control mechanisms by the PFC and a shift to bottom–up control by the sensory cortices and subcortical structures have been observed.37 This results in impairment on tasks that require PFC operations (flexible thinking, error monitoring), whereas mental functions relying on basal ganglia circuits are spared or enhanced (fear conditioning and habits). Starving patients with anorexia nervosa show alterations of the hypothalamic–pituitary–adrenal axis that are characteristic of stress,38 and their clinical symptoms clearly reflect a dysfunction of top–down control, showing impaired executive functioning, high levels of inflexibility, harm avoidance and compulsive behaviour. In the starving condition, it is impossible to distinguish the effects of starvation from those of estrogen deficiency, but in our patients, both factors probably contributed to our findings. Low levels of estrogens, like those observed in underweight women with anorexia nervosa, fail to exert their well-known inhibitory effects on COMT activity and, for this reason, patients with anorexia nervosa who were taking oral contraceptives showed important differences in the effects of the COMT genotype on WCST performance (Appendix 1, Fig. S1).
In comparisons between patients with anorexia nervosa and healthy women, we also observed an effect of the COMT polymorphism on PFC functional connectivity at rest. We found significantly greater coactivation in Met-homozygous patients than in Val carriers with anorexia nervosa in the connectivity of the dorsolateral and ventromedial PFC, together with a significant group interaction with respect to the functional connectivity of ventromedial prefrontal seeds, indicating effects in the opposite direction in patients and controls. Brain areas with significantly different functional connectivity in homozygous Met patients are involved in important aspects of cognitive functioning, such as conflict and error monitoring, reward-based learning and decision-making.
Previous literature on the resting-state in healthy participants reports increased prefrontal connectivity within the default mode network in Met carriers compared with homozygous Val carriers.16 Liu and colleagues16 hypothesized that decreased prefrontal functional connectivity was linked to lower DA levels associated with the Val variant, which is also the cause of poor cognitive performance in this group. Our data match those of Liu and colleagues in that the Met variant, which is associated with greater DA availability and greater tonic stimulation of D1 receptors, is linked to higher levels of functional connectivity within the PFC. However, in our study, this was true only in the anorexia nervosa sample, and the effect was only evident in the homozygous Met group. Differences between our study and that by Liu and colleagues may explain the discrepancies, since the latter sample did not include homozygous Met participants and included both sexes. In addition, the convergent effects of starvation, low estrogen levels and the COMT genotype effects explain both the impairment in executive performance and the increased functional connectivity observed in underweight patients with anorexia nervosa who were homozygous Met carriers. The lack of effect of the COMT genotype in healthy women in our sample is in line both with our neuropsychological findings and those in the literature, which failed to find any effects of this polymorphism on cognitive functioning in healthy women.13 It is possible, however, that a study including information about the timing of the menstrual cycle in healthy women would be more informative on this point.12
In the present study, both types of assessment, cognitive abilities and functional MRI, provide evidence of increased DA signalling in the PFC of starving patients with anorexia nervosa. In these patients, the COMT genotype has a significant influence on both executive functioning and brain functional connectivity. The lack of these COMT effects in weight-recovered patients leads to the hypothesis that starvation affects DA release in the PFC with COMT-mediated consequences that are more evident in the absence of the protective inhibitory effect of estrogens.
Limitations
The present study has important limitations that should be taken into consideration. Patients with anorexia nervosa are malnourished, and their brain atrophy or dehydration may hamper or bias the detection of differences in BOLD signals compared with healthy controls. To check for this possibility, we performed additional analyses with probability maps of grey matter (a measure of brain atrophy) as nuisance variables,25 and we collected data on dehydration in a subgroup of participants (Appendix 1) and studied the effects of BMI and weight loss on the significance of our contrasts. Longitudinal studies are required to verify our hypotheses and to test the effects of weight gain and menses resumption. To enhance the meaning and importance of our results, it could be interesting to compare underweight and weight-recovered patients with anorexia nervosa with a group of women showing constitutional thinness (i.e., underweight participants with regular menses). This further group of probands could be useful to discriminate between the effects of starvation and those related to low estrogen levels. Last, both neuropsychological assessment and resting-state functional connectivity showed high levels of individual variability, leading to the hypothesis that other factors are implicated in the manifestation of these phenotypes.
Conclusion
Although they require confirmation, our findings have important implications for treatment. Cognitive inflexibility may influence treatment compliance, and the associated features of aberrant reward mechanisms39,40 and low motivation lead to resistance to available treatment. Our study indicates that the various genotypes might respond in different ways to treatment. Homozygous Met carriers with anorexia nervosa have not only high levels of inflexibility, but also the potential for improving them after weight gain. Conversely, patients with anorexia nervosa who are Val carriers may require additional specific treatment, such as cognitive remediation therapy.41 To our knowledge, the cognitive effect of treatment with estrogens (or tolcapone, another inhibitor of the COMT enzyme) has never been tested in patients with anorexia nervosa, but such treatment may be helpful in improving cognitive performance, especially in Val-Val carriers.12,13 Our observation of an improved performance in Met-Met carriers with anorexia nervosa who were taking oral contraceptives (Appendix 1, Fig. S1) was unexpected and, although based on only 4 patients, needs to be replicated. In addition, observations from the field of schizophrenia research42 indicate that treatment with atypical antipsychotics43 may have different indications or doses in different COMT genotype groups.
By providing evidence of the convergent effect of the COMT genotype, starvation and low estrogen levels on DA signalling in the PFC and executive performance of patients with anorexia nervosa, our study emphasizes the importance of integrating various types of knowledge to explain the complexity of the clinical manifestations of anorexia nervosa.44 The therapeutic implications of our observations need to be tested in future studies.
Acknowledgements
This study was partially supported by Veneto Region Grant BIOVEDA.
Footnotes
Conflict of interest: None declared.
Contributors: A. Favaro, M. Clementi and P. Santonastaso designed the study. R. Manara, R. Bosello, M. Forzan, A. Bruson, E. Tenconi, D. Degortes and F. Titton acquired the data. A. Favaro and R. Di Salle analyzed the data. A. Favaro, R. Bosello, M. Forzan, A. Bruson, E. Tenconi, D. Degortes and F. Titton wrote the article. M. Clementi, R. Manara, R. Di Salle and P. Santonastaso reviewed the article. All authors approved its publication.
References
- 1.Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–84. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 2.Treasure J, Claudino AM, Zucker N. Eating disorders. Lancet. 2010;375:583–93. doi: 10.1016/S0140-6736(09)61748-7. [DOI] [PubMed] [Google Scholar]
- 3.Holliday J, Tchanturia K, Landau S, et al. Is impaired set-shifting an endophenotype of anorexia nervosa? Am J Psychiatry. 2005;162:2269–75. doi: 10.1176/appi.ajp.162.12.2269. [DOI] [PubMed] [Google Scholar]
- 4.Roberts ME, Tchanturia K, Stahl D, et al. A systematic review and meta-analysis of set shifting in eating disorders. Psychol Med. 2007;37:1075–84. doi: 10.1017/S0033291707009877. [DOI] [PubMed] [Google Scholar]
- 5.Tenconi E, Santonastaso P, Degortes D, et al. Set-shifting abilities, central coherence, and handedness in anorexia nervosa patients, their unaffected siblings, and healthy controls: exploring putative endophenotypes. World J Biol Psychiatry. 2010;11:813–23. doi: 10.3109/15622975.2010.483250. [DOI] [PubMed] [Google Scholar]
- 6.Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry. 2009;166:664–74. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim YR, Kim JE, Kim MH. Impaired set-shifting ability in patients with eating disorders, which is not moderated by their catechol-O-methyltransferase Val158Met genotype. Psychiatry Investig. 2010;7:298–301. doi: 10.4306/pi.2010.7.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickinson D, Elvevag B. Genes, cognition and brain through a COMT lens. Neuroscience. 2009;164:72–87. doi: 10.1016/j.neuroscience.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Lipska BK, Halim N, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savitz J, Solms M, Ramesar R. The molecular genetics of cognition: dopamine, COMT and BDNF. Genes Brain Behav. 2006;5:311–28. doi: 10.1111/j.1601-183X.2005.00163.x. [DOI] [PubMed] [Google Scholar]
- 11.Bilder RM, Volavka J, Lachman HM, et al. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–61. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs E, D’Esposito M. Estrogen shapes dopamine-dependent cognitive processes: implications for women’s health. J Neurosci. 2011;31:5286–93. doi: 10.1523/JNEUROSCI.6394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33:3037–45. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- 14.Brambilla F, Monteleone P. Physical complications and physiological aberrations in eating disorders: a review. In: Maj M, Halmi K, Lopez-Ibor JJ, et al., editors. Eating disorders WPA Series: Evidence and Experience in Psychiatry. Hoboken (NJ): John Wiley & Sons; 2003. pp. 139–92. [Google Scholar]
- 15.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry. 2010;15:918–27. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- 16.Liu B, Song M, Li J, et al. Prefrontal-related functional connectivities within the default network are modulated by COMT val158met in healthy young adults. J Neurosci. 2010;30:64–9. doi: 10.1523/JNEUROSCI.3941-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favaro A, Tenconi E, Santonastaso P. Perinatal factors and the risk of developing anorexia and bulimia nervosa. Arch Gen Psychiatry. 2006;63:82–8. doi: 10.1001/archpsyc.63.1.82. [DOI] [PubMed] [Google Scholar]
- 21.Favaro A, Tenconi E, Bosello R, et al. Perinatal complications in unaffected sisters of anorexia nervosa patients: testing a covariation model between genetic and environmental factors. Eur Arch Psychiatry Clin Neurosci. 2011;261:391–6. doi: 10.1007/s00406-010-0181-3. [DOI] [PubMed] [Google Scholar]
- 22.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 23.Berg EA. A simple objective treatment for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- 24.Laiacona M, Inzaghi MG, De Tanti A, et al. Wisconsin card sorting test: a new global score, with Italians norms, and its relationship with the Weigl sorting test. Neurol Sci. 2000;21:279–91. doi: 10.1007/s100720070065. [DOI] [PubMed] [Google Scholar]
- 25.Oakes TR, Fox AS, Johnstone T, et al. Integrating VBM into the general linear model with voxelwise anatomical covariates. Neuroimage. 2007;34:500–8. doi: 10.1016/j.neuroimage.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–9. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheline YI, Price JL, Yan Z, et al. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010;107:11020–5. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steffens DC, Taylor WD, Denny KL, et al. Structural integrity of the uncinate fasciculus and resting state functional connectivity of the ventral prefrontal cortex in late life depression. PLoS ONE. 2011;6:e22697. doi: 10.1371/journal.pone.0022697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 30.Mattay VS, Goldberg TE, Fera F, et al. Catechol-O-methyltransferase Val158-Met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A. 2003;100:6186–91. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahlstrom D, White T, Hooper CJ, et al. Variations in the catechol-O-methyltransferase polymorphism and prefrontally guided behaviors in adolescents. Biol Psychiatry. 2007;61:626–32. doi: 10.1016/j.biopsych.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 32.Castro-Fornieles J, Deulofeu R, Baeza I, et al. Psychopathological and nutritional correlates of plasma homovanillic acid in adolescents with anorexia nervosa. J Psychiatr Res. 2008;42:213–20. doi: 10.1016/j.jpsychires.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Krugel LK, Biele G, Mohr PNC, et al. Genetic variation in dopaminergic neuromodulation influences the ability to rapidly and flexibly adapt decisions. Proc Natl Acad Sci U S A. 2009;106:17951–6. doi: 10.1073/pnas.0905191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank GK, Bailer UF, Henry SE, et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry. 2005;58:908–12. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Bailer UF, Narendran R, Frankle WG, et al. Amphetamine induced dopamine release increases anxiety in individuals recovered from anorexia nervosa. Int J Eat Disord. 2012;45:263–71. doi: 10.1002/eat.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci U S A. 2009;106:912–7. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo Sauro C, Ravaldi C, Cabras PL, et al. Stress, hypothalamic-pituitary-adrenal axis and eating disorders. Neuropsychobiology. 2008;57:95–115. doi: 10.1159/000138912. [DOI] [PubMed] [Google Scholar]
- 39.Fladung AK, Gron G, Grammer K, et al. A neural signature of anorexia nervosa in the ventral striatal reward system. Am J Psychiatry. 2010;167:206–12. doi: 10.1176/appi.ajp.2009.09010071. [DOI] [PubMed] [Google Scholar]
- 40.Vocks S, Busch M, Gronemeyer D, et al. Neural correlates of viewing photographs of one’s own body and another woman’s body in anorexia and bulimia nervosa: an fMRI study. J Psychiatry Neurosci. 2010;35:163–76. doi: 10.1503/jpn.090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tchanturia K, Davies H, Lopez C, et al. Neuropsychological task performance before and after cognitive remediation in anorexia nervosa: a pilot case-series. Psychol Med. 2008;38:1371–3. doi: 10.1017/S0033291708003796. [DOI] [PubMed] [Google Scholar]
- 42.Illi A, Kampman O, Hanninen K, et al. Catechol-O-methyltransferase val108/158met genotype and response to antipsychotic medication in schizophrenia. Hum Psychopharmacol. 2007;22:211–5. doi: 10.1002/hup.841. [DOI] [PubMed] [Google Scholar]
- 43.Brambilla F, Garcia CS, Fassino S, et al. Olanzapine therapy in anorexia nervosa: psychobiological effects. Int Clin Psychopharmacol. 2007;22:197–204. doi: 10.1097/YIC.0b013e328080ca31. [DOI] [PubMed] [Google Scholar]
- 44.Favaro A, Santonastaso P, Manara R, et al. Disruption of visuo-spatial and somatosensory functional connectivity in anorexia nervosa. Biol Psychiatry. 2012 May 24; doi: 10.1016/j.biopsych.2012.04.025. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]