Abstract
Background
Although mood-congruent memory (MCM), or the tendency to recall information consistent with one’s mood, is a robust phenomenon in human depression, to our knowledge, it has never been demonstrated in animals.
Methods
Mice were subjected to social isolation (SI) or crowding for 12 hours and had their depressive-like behaviour (evaluated by the forced swim, tail suspension, sucrose preference and splash tests) or their serum corticosterone concentrations evaluated. In addition, we determined the temporal forgetting curve of the plus-maze discriminative avoidance task (PM-DAT) and examined the effects of SI or crowding on memory retrieval in the PM-DAT. Finally, we verified the effects of metyrapone pretreatment on reinstatement of memory retrieval or on the increase of corticosterone levels induced by SI.
Results
Twelve hours of SI produced depressive-like behaviour, enhanced corticosterone concentration and reinstated retrieval of a forgotten discriminative aversive (i.e., negatively valenced) task. Depressive-like behaviour was critical for this facilitative effect of SI because 12 hours of crowding neither induced depressive-like behaviour nor enhanced retrieval, although it increased corticosterone levels at the same magnitude as SI. However, corticosterone increase was a necessary condition for MCM in mice, in that the corticosterone synthesis inhibitor metyrapone abolished SI-induced retrieval reinstatement.
Limitations
Our study did not investigate the effects of the social manipulations proposed here in a positively valenced task.
Conclusion
To our knowledge, the present paper provides the first evidence of MCM in animal models.
Introduction
Memory has distinct phases: the acquisition of material, followed by consolidation. If those processes are successful, a memory can be retrieved hours, days and years later.1,2 As stated by William James, “the only proof of there being retention is that recall actually takes place.”3 Nevertheless, the past decades have seen relatively little literature approaching the neurobiological mechanisms of retrieval.4 Thus, retrieval seems to be facilitated by mood congruence (i.e., the enhanced retrieval of material for which the affective valence is congruent with one’s ongoing mood).5 Indeed, emotional information is remembered best when the mood at the time of retrieval matches it in valence. An associative memory model predicts that mood-congruent facilitation is due to the mood-related reactivation of emotional responses at retrieval, which were linked to valenced information at encoding.6
Mood-congruent memory (MCM) appears to be a robust phenomenon in depression, being associated with enhanced recall of negative information.5–11 Indeed, some have proposed that MCM might be an important maintenance mechanism in depression.12 To our knowledge, MCM has not been investigated in animals, despite an impressive volume of clinical and psychological support for this phenomenon. Rodent models would further our understanding of MCM, as they enable careful control of the experimental subjects as well as the timing and nature of the induction of depressive-like behaviour.
Because depression seems to be elicited by social rather than physical stress in humans,13 there has been a shift toward social stressors for inducing depression in experimental studies. Indeed, loneliness is a clinically important precursor of depression in humans.14,15 Similarly, social isolation (SI) may be an etiological factor for depression in rodents.16 In fact, separation from group housing is a reliable and valid method for inducing depression-like behaviour in mice.16 Specifically, it has been verified that separation increased duration of immobility in the forced swim test (FST) and the number of bouts in immobility in the tail suspension test (TST), which were reversed by fluoxetine administration.
Recent unpublished data from our laboratory have demonstrated that mice presented depressive-like behaviour in the FST after 12 hours of SI. Our goal was to investigate whether SI-induced depressive-like behaviour would facilitate the retrieval of an aversive (negatively valenced) task: the plus-maze discriminative avoidance task (PM-DAT).17–19 Because our hypothesis presumes that depressive-like behaviour induced by SI would facilitate the retrieval of an aversive task, we first determined a forgetting curve. Then, SI effects were evaluated at a time point at which control mice did not retrieve the task, thereby avoiding a ceiling effect. Social isolation is a stress condition that can increase corticosterone concentration in rodents,20 and it has been shown that stress exposure and/or glucocorticoids have complex effects on cognition.21–24 Therefore, crowding for 12 hours was used as a positive control. This kind of social stress produces the same magnitude of corticosterone increase as 12 hours of SI without inducing depressive-like behaviour. Finally, we investigated the effects of the corticosterone synthesis inhibitor metyrapone on retrieval in socially isolated mice.
Methods
Subjects
Three-month-old Swiss male mice (outbred, raised and maintained in the Center for Development of Experimental Models in Medicine and Biology of the Universidade Federal de São Paulo – UNIFESP) were used. Animals were housed in polypropylene home cages (41 × 34 × 16.5 cm). Animals weighing 30–35 g were housed under controlled temperature (22–23°C) and lighting (12-hour light–dark cycle; lights on at 6:45 am) conditions. Purina chow for rodents and water were available ad libitum. Animals were maintained in accordance with the National Institute of Health Guide for the care and use of laboratory animals (NIH Publications N° 8023, revised 2011). The Ethical Committee of UNIFESP approved all experiments (#0269/07).
Housing conditions
Animals were housed for 12 hours in cages (30 × 20 × 12.5 cm) alone (SI) or in groups of 15 (crowding). Control mice were housed in a group of 15 in a larger cage (41 × 34 × 16.5 cm), during the same period in the same room. In some experiments, only a subset of the animals submitted to crowding was used.
Drug
Metyrapone (Sigma-Aldrich), a corticosterone synthesizing enzyme 11 β-hydroxylase inhibitor, was dissolved in polyethylene glycol and diluted with 0.9% saline solution to reach concentration. This final concentration was 40%, and the vehicle solution contained the same polyethylene glycol concentration. Metyrapone (100 mg/kg) and vehicle solutions were administered intraperitoneally at a volume of 10 mL/kg of body weight.
Plus-maze discriminative avoidance task
We used the PM-DAT to concomitantly evaluate learning, memory, anxiety-like behaviour and motor activity, as described previously.17–19
The aversive stimuli consisted of a 100 W light and an 80 dB noise. The source of the noise was the horizontal displacement of a piece of metal against wood produced continuously by a small machine placed under the arm. The aversive stimuli were present during the 10-minute training. Testing lasted 3 minutes and was performed in the absence of the aversive stimuli. In all experiments, observations were recorded by an observer blinded to the experimental conditions, and the apparatus was cleaned with a 5% alcohol solution after each session.
Learning and memory were evaluated by the time spent in the aversive versus nonaversive enclosed arms in the training and testing sessions, respectively. Anxiety-like behaviour was evaluated by percentage of time spent in the open arms (time spent in the open arms/time spent in both open and enclosed arms). Locomotion was evaluated by the number of entries into any of the arms of the apparatus. An entry was defined as the entrance of all 4 paws in a given arm.
Forced swim test (FST)
Mice were placed individually in a cylindrical glass container (30 cm height, 16 cm diameter, 11 cm of water depth, 23°C) for 6 minutes. The duration of immobility was manually scored during the last 4 minutes by observers who were blind to the social manipulation applied. A mouse was considered immobile when it floated in an upright position and made only small movements to keep its head above water.
Tail suspension test (TST)
Mice were suspended by their tails at a height of 25 cm using adhesive tape wrapped around the tail 2 cm from the tip. Animals were observed for 5 minutes by an observer blind to the condition. Latency to the first episode of immobility and immobility duration were manually quantified using stopwatches. Animals were considered immobile when hanging completely motionless.
Splash test (ST)
The ST consists of squirting a 10% sucrose solution on the dorsal coat of a mouse placed individually in a clear observation cage (16 × 30 × 19 cm) without food. Mirrors are placed under the floor and behind the cage to permit observation of movements when the animal faces away from the observer. The sucrose solution dirties the mouse fur, eliciting grooming behaviour. Latency to the first episode of grooming and duration of grooming behaviour were measured manually during a 5-minute period by an observer blind to the condition.
Sucrose preference test (SPT)
Mice were given free choice between a tap water bottle and a 2% sucrose solution bottle for 1 hour immediately after the social manipulation. No previous food or water deprivation was applied before testing. The consumption of water and sucrose solution was estimated for each animal individually based on the remaining fluid volume in each bottle. Sucrose preference was calculated as the percentage of consumed sucrose solution out of the total amount of liquid drunk.
Serum corticosterone determination
Animals were euthanized by decapitation. Trunk blood was collected in plastic tubes and centrifuged at 3500 rpm for 10 minutes. Serum was frozen at −20°C. Corticosterone concentration was determined in duplicate using a double antibody radioimmunoassay kit specific for rats and mice (ICN Biomedicals). The sensitivity of the assay is 0.25 ng/mL. Intra- and interassay variations are 10.3% and 7.1%, respectively. We euthanized the mice between 8:00 am and 9:00 am to minimize circadian variations on serum glucocorticoid concentrations.25
Experimental design
Experiment 1: Effects of SI or crowding on the FST in mice
Forty-five mice were either subjected to SI for 12 hours (n = 15), transferred to a small cage and maintained there under the crowding condition for 12 hours (n = 15) or transferred to a new home cage (control; n = 15). Immediately after, all of the animals were subjected to the FST.
Experiment 2: Effects of SI or crowding on the TST in mice
Forty-five mice were randomly allocated to groups of 15, as described for experiment 1. Immediately after the social manipulations, all of the animals were subjected to the TST.
Experiment 3: Effects of SI or crowding on the ST in mice
Forty-five mice were randomly allocated to groups of 15, as described for experiment 1. Immediately after the social manipulations, all of the animals were subjected to the ST.
Experiment 4: Effects of SI or crowding on the SPT in mice
Forty-five mice were randomly allocated to groups of 15, as described for experiment 1. Immediately after the social manipulations, all of the animals were subjected to the SPT.
Experiment 5: Effects of SI or crowding on serum corticosterone concentrations
Forty-five mice were randomly allocated to the SI, crowding or control groups for 12 hours. Immediately after, we euthanized 12 animals in each group. Serum was then collected, and we determined the corticosterone concentration.
Experiment 6: Determination of the temporal forgetting curve of the PM-DAT
Mice were allocated to the 7-, 15- and 30-day (n = 10) groups and were trained in the PM-DAT 7, 15 or 30 days before testing, respectively. After the training, all of the animals were kept in their home cages until testing. All groups were tested simultaneously.
Experiment 7: Effects of pretest SI or crowding on the retrieval of the PM-DAT
Mice were trained in the PM-DAT and allocated to 1 of 3 groups (n = 15). They were kept in their home cages from the time of training until testing 30 days later. Mice were subjected to SI or crowding or transferred to a new home cage (control) 12 hours before testing.
Experiment 8: Effects of metyrapone pretreatment on the reinstatement of retrieval induced by 12 hours of SI
Forty-five mice were allocated into 3 groups (n = 15) and trained in the PM-DAT. Thirty days later, 2 of these groups were subjected to SI for 12 hours, forming the MET-SI and VEH-SI groups. Animals in the third group (VEH-CTRL) were transferred to another home cage and kept in the same room. Immediately after, animals were subjected to testing. Forty minutes before testing, mice of the MET-SI group received 100 mg/kg of metyrapone. Animals in the VEH-SI and VEH-CTRL groups received vehicle.
Experiment 9: Effects of metyrapone pretreatment on the increase of corticosterone induced by 12 hours of SI
Twenty-five mice were allocated to the SI condition, and 13 were transferred to another home cage in the same room. Forty minutes before the end of the 12 hour SI procedure, a subset (n = 13) of socially isolated mice received 100 mg/kg of metyrapone (MET-SI), whereas the other subset (n = 12) received metyrapone vehicle (VEH-SI). Animals in the control group also received vehicle (CTRL-VEH). Immediately after, all of the animals were euthanized, and we collected their blood to determine corticosterone concentration.
Statistical analyses
We evaluated data using 1- or 2-way analysis of variance (ANOVA) and Tukey tests when necessary. We considered results to be significant at p < 0.05.
Results
Experiment 1: Effects of SI or crowding on FST behaviour
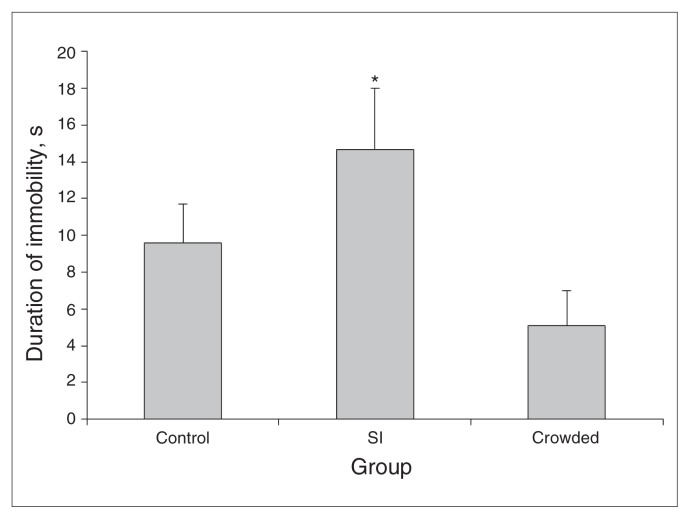
The ANOVA and Tukey tests (F2,42 = 5.38, p = 0.008) revealed that mice in the SI group exhibited a significantly higher immobility time than the crowding group (q = 4.21, p = 0.013) or control groups (q = 3.79, p = 0.028; Fig. 1).
Fig. 1.
Duration of immobility (s) in the forced swim test presented by mice that were socially isolated (SI), crowded or kept in their home cages (control) for 12 hours. Data are reported as means and standard errors. *p < 0.05 compared with the other 2 groups (1-way analysis of variance and Tukey test).
Experiment 2: Effects of SI or crowding on the TST
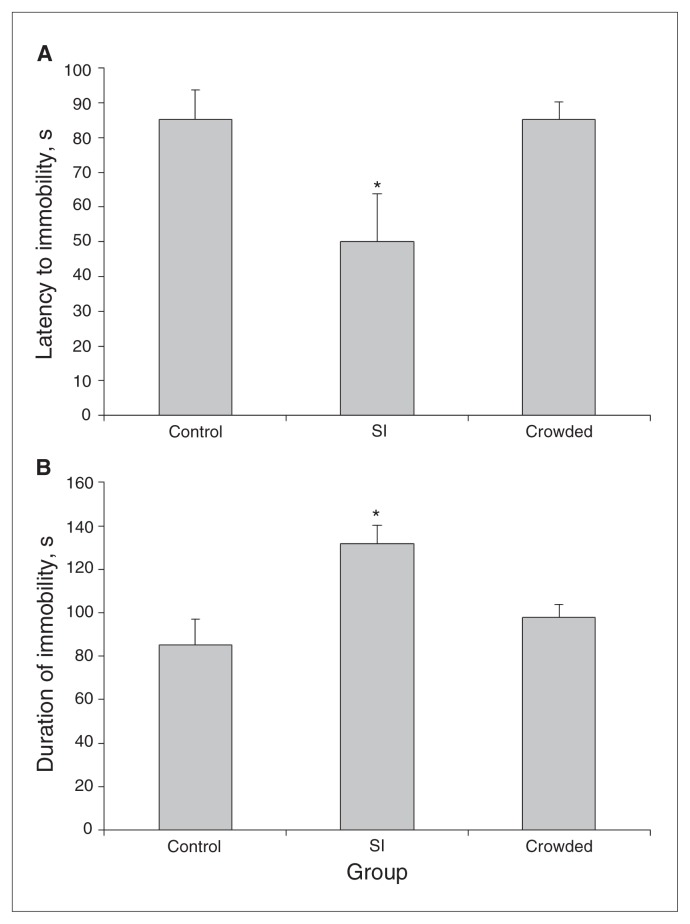
The ANOVA revealed significant differences in the latency to first immobility episode among the groups (F2,42 = 4.39, p = 0.018). The Tukey test showed that the SI group displayed a decreased latency compared with the control and crowding groups (q = 3.63, p = 0.037 and q = 3.64, p = 0.036, respectively; Fig. 2A). The ANOVA and Tukey tests revealed that the SI groups spent more time immobile than the control and crowding groups (q = 5.11, p = 0.002, and q = 3.72, p = 0.031, respectively; F2,42 = 6.99, p = 0.002; Fig. 2B).
Fig. 2.
(A) Latency (s) to the first immobility episode and (B) duration of immobility (s) in the tail suspension test presented by mice that were socially isolated (SI), crowded or kept in their home cages (control) for 12 hours. Data are reported as means and standard errors. *p < 0.05 compared with the other 2 groups (1-way analysis of variance and Tukey test).
Experiment 3: Effects of SI or crowding on the ST
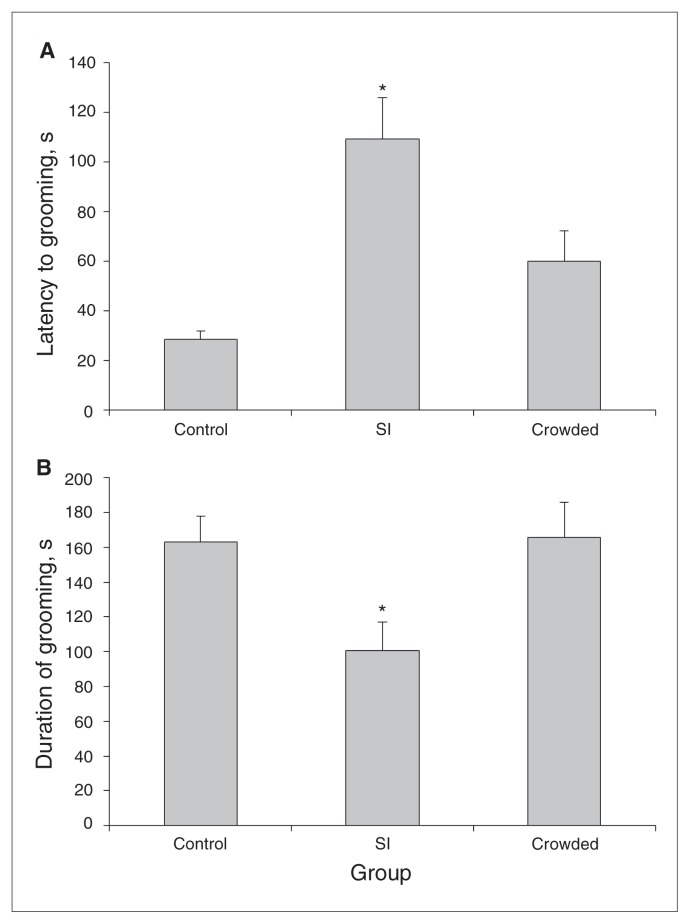
The ANOVA and Tukey tests showed that the SI group showed increased latency to the first grooming episode compared with the control and crowding groups (q = 6.59, p = 0.001 and q = 4.03, p = 0.018, respectively; F2,42 = 11.05, p < 0.001; Fig. 3A). The ANOVA and Tukey tests revealed that the SI groups displayed decreased grooming duration compared with the control and crowding groups (q = 3.61, p = 0.038 and q = 3.74, p = 0.030, respectively; F2,42 = 4.51, p = 0.017; Fig. 3B).
Fig. 3.
(A) Latency (s) to the first grooming episode and (B) duration of grooming (s) in the splash test presented by mice that were socially isolated (SI), crowded or kept in their home cages (control) for 12 hours. Data are reported as means and standard errors. *p < 0.05 compared with the other 2 groups (1-way analysis of variance and Tukey test).
Experiment 4: Effects of SI or crowding on the SPT
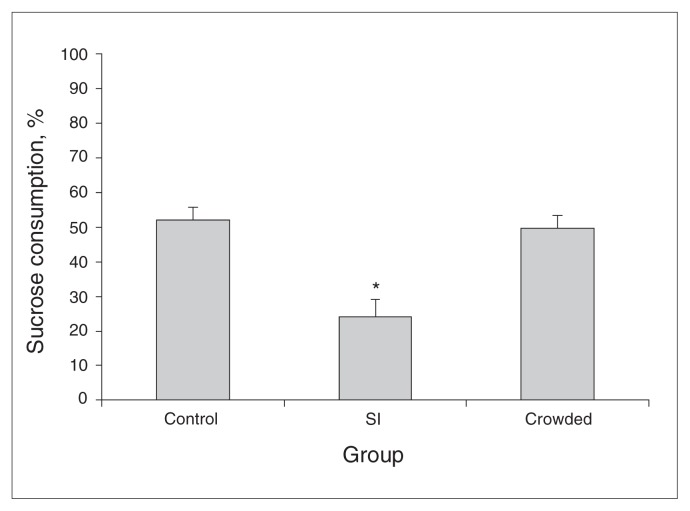
The ANOVA and Tukey tests (F2,42 = 13.11, p < 0.001) revealed that mice in the SI group consumed significantly less sucrose than the crowding or control groups (q = 5.99, p = 0.001 and q = 6.52, p = 0.001, respectively; Fig. 4).
Fig. 4.
Percentage of sucrose consumption in the sucrose preference test presented by mice that were socially isolated (SI), crowded or kept in their home cages (control) for 12 hours. Data are reported as means and standard errors. *p < 0.05 compared with the other 2 groups (1-way analysis of variance and Tukey test).
Experiment 5: Effects of SI or crowding on serum corticosterone concentrations
The ANOVA and Tukey tests (F2,33 = 17.79, p < 0.001) revealed a significant increase in corticosterone concentration in the SI and crowding groups compared with the control group (q = 8.31, p < 0.001 and q = 5.43, p = 0.002, respectively; Table 1).
Table 1.
Effects of social isolation or crowding for 12 hours on serum corticosterone concentrations
| Group | Corticosterone, mean (SE) ng/mL |
|---|---|
| Control | 76.7 (7.24) |
| Socially isolated | 167.5 (15.34)* |
| Crowded | 136.0 (8.43)* |
SE = standard error.
p < 0.05 compared with the control group (1-way analysis of variance and Tukey tests).
Experiment 6: Determination of the temporal forgetting curve of the PM-DAT
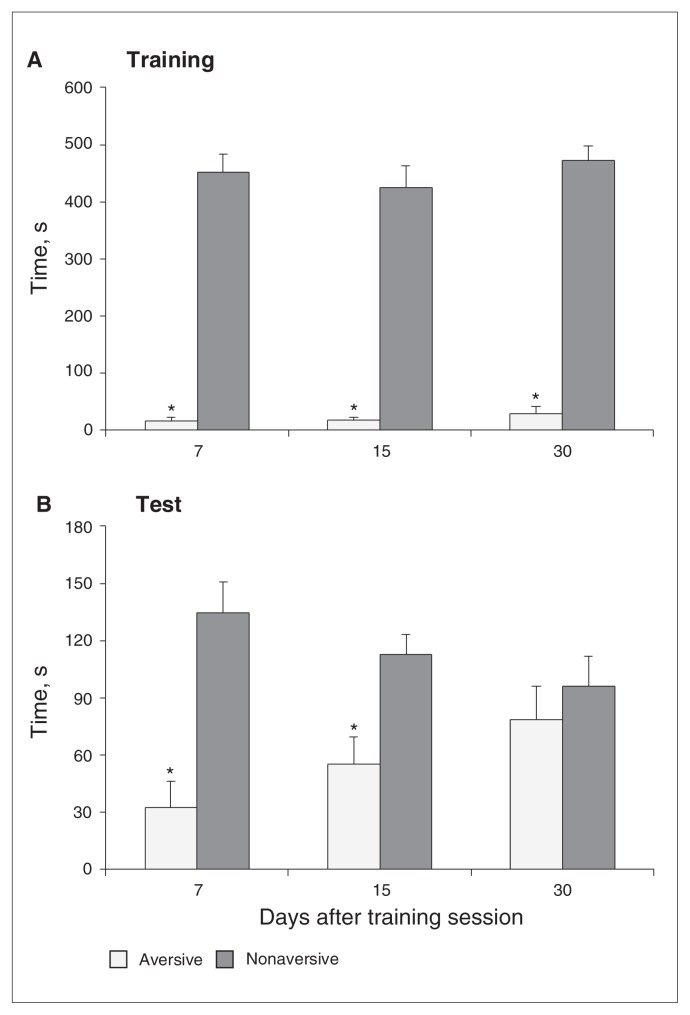
Two-way ANOVA revealed significant arm type (aversive v. nonaversive) effects (F1,27 = 525, p < 0.001) in the training. Still, there were no significant group (7 d, 15 d and 30 d; F2,27 = 0.84, all p > 0.05) or arm type × group interaction (F2,27 = 0.33, all p > 0.05) effects. The Tukey test revealed that all groups spent significantly less time in the aversive enclosed arm than in the nonaversive one (Fig. 5A). No significant differences were found among groups in the percentage of time spent in the open arms or in the total number of entries (all p > 0.05, data not shown).
Fig. 5.
Time spent in the aversive and nonaversive enclosed arms of the plus-maze discriminative avoidance task apparatus in the (A) training and (B) test sessions by mice subjected to the test session 7, 15 or 30 days after the training session. Data are reported as means and standard errors. *p < 0.05 compared with the time spent in the nonaversive enclosed arm (2-way analysis of variance and Tukey test).
In the test session, 2-way ANOVA revealed significant arm type (F1,27 = 24.33, p < 0.001) and arm type × group interaction effects (F2,27 = 3.62, p = 0.003), but there were no significant group effects (F2,27 = 0.04, all p > 0.05; Fig. 5B). The 7- and 15-day groups presented a significant decrease in the time spent in the aversive enclosed arm than in the nonaversive arm. Animals tested 30 days after training did not prefer the non-aversive enclosed arm. In addition, ANOVA showed no significant differences among groups in the percentage of time spent in the open arms or the total number of entries during the testing (all p > 0.05, data not shown).
Experiment 7: Effects of pretest SI or crowding on the retrieval of the PM-DAT
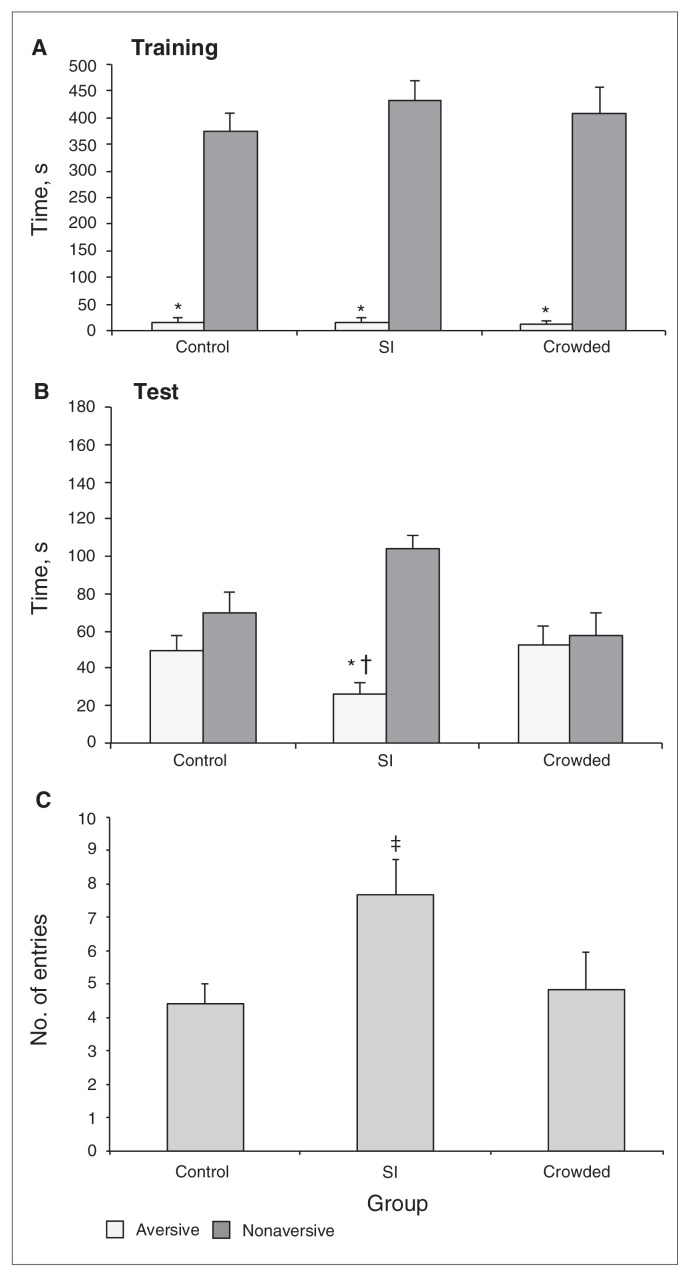
Two-way ANOVA revealed significant arm type effects (F1,42 = 525.96, p < 0.001) in the training. There were no significant housing condition (control, SI and crowding; F2,42 = 0.56, all p > 0.05) or arm type × housing condition interaction effects (F2,42 = 0.57, all p > 0.05). All groups spent significantly less time in the aversive enclosed arm than in the nonaversive arm before the animals were subjected to SI or crowding (Fig. 6A). No significant differences were found in the percentage of time spent in the open arms or in the total number of entries (all p > 0.05, data not shown).
Fig. 6.
Time spent in the aversive and nonaversive enclosed arms of the plus-maze discriminative avoidance task apparatus for all of the groups — control, socially isolated (SI) and crowded in the test session, respectively — in the (A) training and (B) test sessions, as well as (C) the total number of entries into all arms of the apparatus during the test session. Data are reported as means and standard errors. *p < 0.05 compared with the time spent in the nonaversive enclosed arm; †p < 0.05 compared with the time spent in the aversive enclosed arm of the other groups and ‡p < 0.05 compared with the other 2 groups (panel C, 1-way analysis of variance and Tukey test).
In the test session, 2-way ANOVA revealed significant effects for both arm type (F1,42 = 17.7, p < 0.001) and arm type × housing condition interaction (F2,42 = 8.17, p < 0.001). There were no significant effects of housing condition (F2,42 = 0.73, all p > 0.05). Only the SI group spent significantly less time in the aversive enclosed arm than in the nonaversive one (Fig. 6B). Additional analysis showed that the SI group spent significantly less time in the aversive arm than the other 2 groups.
The ANOVA and Tukey tests revealed a significant effect of SI on the total number of entries (F2,42 = 3.55, p = 0.002). Thus, the SI group exhibited a higher number of entries than the control or crowding groups (q = 3.72, p = 0.031, and q = 3.49, p = 0.045, respectively; Fig. 6C). No significant differences were found among the groups in the percentage of time spent in the open arms during testing (all p > 0.05, data not shown).
Experiment 8: Effects of metyrapone pretreatment on the reinstatement of retrieval induced by 12 hours of SI
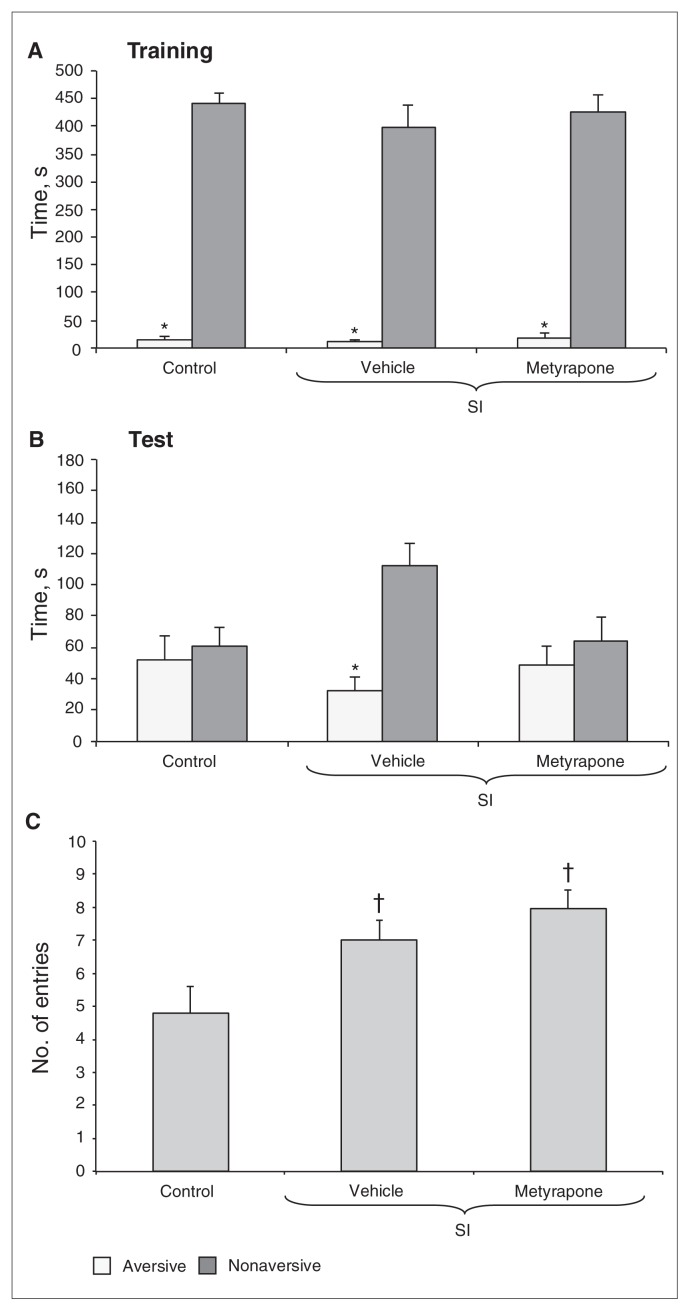
Two-way ANOVA revealed a significant effect of arm type (F1,42 = 10.13, p < 0.001) during training. There were no significant effects of group (F2,42 = 0.63, all p > 0.05) or arm type × group interaction (F2,42 = 0.54, all p > 0.05). All groups spent significantly less time in the aversive enclosed arm than in the nonaversive one (Fig. 7A). No significant differences were found in the percentage of time spent in the open arms or in the total number of entries (all p > 0.05, data not shown).
Fig. 7.
Time spent in the aversive and nonaversive enclosed arms of the of the plus-maze discriminative avoidance task apparatus by all of the groups — control, socially isolated (SI) animals treated with vehicle and SI animals treated with metyrapone in the test session —in the (A) training and (B) test sessions, as well as (C) the total number of entries into all of the arms of the apparatus in the test session. Data are reported as means and standard errors. *p < 0.05 compared with the time spent in the nonaversive enclosed arm (panels A and B, 2-way analysis of variance and Tukey test). †p < 0.05 compared with the control group (panel C, 1-way analysis of variance and Tukey test).
In the test session, 2-way ANOVA revealed significant arm type (F1,42 = 11.03, p = 0.001) and arm type × group interaction effects (F2,42 = 4.48, p = 0.008). There was no significant group effect (F2,42 = 0.98, all p > 0.05). Only the VEH-SI group spent significantly less time in the aversive enclosed arm than in the nonaversive one (Fig. 7B). The total number of entries of the VEH-SI or MET-SI groups was significantly higher than that in the VEH-CTRL group (q = 3.48, p = 0.046 and q = 4.55, p = 0.007, respectively) in the testing (F2,42 = 5.78, p = 0.006; Fig. 7C). No significant differences were found in the percentage of time spent in the open arms during testing (all p > 0.05, data not show).
Experiment 9: Effects of metyrapone pretreatment on the increase of corticosterone induced by 12 hours of SI
The ANOVA and Tukey tests (F2,35 = 10.87, p < 0.001; Table 2) revealed that corticosterone concentration in the VEH-SI group was significantly higher than that in the VEH-CTRL or MET-SI groups (q = 6.21, p = 0.001, and q = 5.11, p = 0.003, respectively).
Table 2.
Effects of metyrapone pretreatment on serum corticoster one concentrations induced by 12 hours of social isolation
| Group/treatment | Corticosterone, mean (SE) ng/mL |
|---|---|
| Control/vehicle | 86.3 (7.67) |
| Socially isolated/vehicle | 135.83 (9.54)*† |
| Socially isolated/metyrapone | 95.07 (6.47) |
SE = standard error.
p < 0.05 compared with the control group (1-way analysis of variance and Tukey tests).
p < 0.05 compared with the social isolation/metyrapone group (1-way analysis of variance and Tukey tests).
Discussion
Here, we report that short-term SI induced depressive-like behaviour in mice and reinstated retrieval of a forgotten discriminative memory. Since the PM-DAT is aversive and, consequently, negatively valenced, these results suggest the occurrence of MCM in mice. This notion is strengthened by the fact that another social stressor (crowding) increased, by the same magnitude, serum corticosterone concentration, but promoted neither depressive-like behaviour nor memory reinstatement. Importantly, stress-induced corticosterone secretion, though insufficient, was a necessary condition for the retrieval reinstatement because metyrapone abolished both the corticosterone increase and the facilitative effect on retrieval produced by SI.
In humans, it has been demonstrated that depressive mood is associated with enhanced recall of negative information. However, this MCM has been primarily reported in patients who were clinically depressed.8–10,26,27 As memory processing takes place during the same depressive state, the MCM could be related to the state-dependent phenomenon.28 In state-dependency, the endogenous context — a physiologic state, is incorporated into the conglomerate of memory attributes, exerting control over retrieval.29 Consequently, the retrieval of a memory may require that the organism be in a similar state in which the memory was acquired.30–33 The individual influences of MCM and state-dependency phenomena on memory retrieval under a depressive mood can be dissociated by the experimental induction of a depressive mood before recollection. A few studies have demonstrated MCM by experimentally inducing a depressive mood in humans.6,34
In the present study, depressive-like behaviour was induced before the PM-DAT retrieval through 12 hours of SI. We applied SI to induce depressive-like behaviour for 2 reasons. First, social stressors offer high face validity and are of particular interest in humans.35 Second, depression is often described as a psychogenic stress-related disorder,36 and SI in particular has been reported to increase resistance to anti-depressant treatment in depressed patients.37 Importantly, unpublished data from our laboratory reveal that longer periods of SI did not increase immobility time in the FST, suggesting a compensatory mechanism.
Animal models of depression have been developed to address the complex environmental, physiologic and genetic interactions that occur in this disorder.38,39 Thus, measures of depression in mice may depend on genotype,40,41 and performance in behavioural tests may be strain-specific. The duration of immobility in the FST appeared shorter than that described in other studies.16,42 These discrepancies could be explained by the differences in strain, sex and housing conditions.
The PM-DAT was chosen in this study because, in addition to being an aversive task, it can simultaneously evaluate anxiety-like behaviour and motor activity. These behaviours can modify retrieval performance, and therefore must be carefully controlled.17,19,43–46 In addition, compared with other aversive memory tests, a difference in motor activity would be a less critical methodological concern in the PM-DAT interpretation because retention is evaluated by the time spent in the aversive versus nonaversive enclosed arms during testing. This was particularly important in the present study because SI increased locomotion in the PM-DAT despite increasing immobility in the FST and in the TST. This increase in locomotor activity induced by SI is in line with the results of previous studies47–50 that have linked it to both a decrease in the basal turnover of serotonin in the nucleus accumbens48 and an increase in the activity of dopamine neurons in the mesoaccumbens system.50
Avoidance of the aversive enclosed arm in the PM-DAT on testing has been validated as a measurement of retention because amnestic manipulations decrease it.17,19,43,45,46,51 We examined whether the SI-induced depressive-like behaviour would facilitate the retrieval, thereby characterizing an MCM in mice. Thus, the effects of SI were evaluated in an experimental condition in which facilitation of retrieval would not be masked by a ceiling effect produced by an optimal retrieval response in the control group. Hence, a forgetting curve for the task was determined (experiment 6). The results show that naive mice recalled the discriminative avoidance task 7 or 15 days after training but did not recall it 30 days later.
Pretest SI (but not pretest crowding) reinstated avoidance in mice tested 30 days after training. The facilitative effect of pretest SI on PM-DAT retrieval was abolished by metyrapone pretreatment at a dose that also abolished the observed increase in serum corticosterone concentration. Conversely, crowding for 12 hours produced the same magnitude of corticosterone increase as SI, but did not reinstate memory retrieval. Together, these data suggest that stress-induced corticosterone release is necessary, albeit insufficient, for MCM-facilitated retrieval. In addition, with the result that crowding was not effective at inducing depressive-like behaviour, these data suggest that SI produced an MCM improvement in the retrieval of an aversive task and that corticosterone secretion had a permissive role in this phenomenon.
The effects of SI and corticosterone in promoting MCM seem to contrast with the findings of a considerable number of studies showing that stress, and glucocorticoids in particular, impairs memory retrieval. This has been documented for spatial and contextual memory in rats and declarative (most episodic) memory in humans.2,23,24 However, while the previously mentioned studies demonstrated that stress and glucocorticoids can impair the retrieval of a nonforgotten task, earlier studies reported that weak foot shock facilitated retrieval after experimental amnesia.25 In addition, amphetamine, a drug that has stressful effects,52 facilitates retrieval of a spontaneously forgotten maze task53 and a forgotten conditioned emotional response.54,55 It is noteworthy that each of the experimental manipulations reported to directly facilitate retrieval after forgetting share the common action of increasing arousal or vigilance.56 Accordingly, the locus coeruleus is a key brainstem region involved in arousal,57 and electrical stimulation of its neurons or a pharmacological increase in the availability of forebrain noradrenaline can facilitate retrieval of remote memory in rats. However, these effects only occurred when the stimulus is applied in the context in which training took place.29 Given that increased corticosterone concentration enhances arousal58 and that stress exposure increases noradrenergic activity in the locus coeruleus,59 the facilitative effect of SI on memory retrieval in the present study could be explained by a combination of increased arousal (induced by increased corticosterone or stress-induced locus coeruleus activation) and memory activation by context cues and mood congruency.
Limitations
Although our study demonstrates the critical influence of mood congruency in animals, we exclusively investigated a negatively valenced task. The reproduction of these results with a positively valenced task would be of considerable interest.
Conclusion
The depressive-like state, as measured by depressive-like behaviour, induced by 12 hours of SI was critical for facilitating the reinstatement of an aversive memory. Twelve hours of crowding neither induced depressive-like behaviour nor enhanced retrieval. Importantly, both the SI and crowding conditions increased corticosterone concentration by the same magnitude. Because the corticosterone synthesis inhibitor metyrapone abolished SI-induced retrieval reinstatement, SI-induced corticosterone increase seems to be a necessary but insufficient condition for MCM.
Acknowledgments
This research was supported by FAPESP/CEPID (Fundação de Amparo à Pesquisa do Estado de São Paulo/Centro de Pesquisa, Inovação e Difusão) grant #1998/14303-3, CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Co-ordenação de Aperfeiçoamento de Pessoal de Nível Superior) and AFIP (Associação Fundo de Incentivo à Pesquisa). The English language has been proofread and edited by EK Sawyer, but we are entirely responsible for the scientific content. The authors thank Ms. Teotila Amaral, Ms. Marilde Costa, Ms. Claudenice Santos, Mr. Cleomar Ferreira and Mr. Antonio Santos for their capable technical assistance. R. Frussa-Filho, M.L. Andersen and S. Tufik are recipients of the CNPq fellowship.
Footnotes
Competing interests: The authors declare having received grant support as above and as follows: A.L. Takatsu-Coleman, S. Tufik, M.L. Andersen and R. Frussa-Filho from CNPq; C.L. Patti from AFIP and FAPESP; K.A. Zanin, A. Zager, S. Tufik and L.F. Berro from FAPESP; and R.C. Carvalho, A.R. Borçoi and L.M.B. Ceccon from CAPES.
Contributors: A.L. Takatsu-Coleman, S. Tufik, M.L. Andersen and R. Frussa-Filho designed the study. A.L. Takatsu-Coleman, A. Zager, R.C. Carvalho, A.R. Boirçoi, L.M.B. Ceccon and L.F. Berro acquired the data; C.L. Patti, K.A. Zanin and R. Frussa-Filho analyzed it. A.L. Takatsu-Coleman, C.L. Patti, K.A. Zanin, A.R. Boirçoi, L.M.B. Ceccon, L.F. Berro and R. Frussa-Filho wrote the article; C.L. Patti, A. Zager, R.C. Carvalho, S. Tufik, M.L. Andersen and R. Frussa-Filho reviewed it. All authors approved publication.
References
- 1.McGaugh JL. Dissociating learning and performance: drug and hormone enhancement of memory storage. Brain Res Bull. 1989;23:339–45. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- 2.Wolf OT. Stress and memory in humans: Twelve years of progress? Brain Res. 2009;1293:142–54. doi: 10.1016/j.brainres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 3.James W. The principles of psychology. New York (NY): Holt; 1890. [Google Scholar]
- 4.Sara SJ. Reactivation, retrieval, replay and reconsolidation in and out of sleep: connecting the dots. Front Behav Neurosci. 2010;4:185. doi: 10.3389/fnbeh.2010.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaney PH. Affect and memory: a review. Psychol Bull. 1986;99:229–46. [PubMed] [Google Scholar]
- 6.Lewis PA, Critchley HD, Smith AP, et al. Brain mechanisms for mood congruent memory facilitation. Neuroimage. 2005;25:1214–23. doi: 10.1016/j.neuroimage.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 7.Colombel F. Memory bias and depression: a critical commentary. Encephale. 2007;33:242–8. doi: 10.1016/s0013-7006(07)92035-7. [DOI] [PubMed] [Google Scholar]
- 8.Watkins PC, Martin CK, Stern LD. Unconscious memory bias in depression: perceptual and conceptual processes. J Abnorm Psychol. 2000;109:282–9. [PubMed] [Google Scholar]
- 9.Watkins PC, Vache K, Verney SP, et al. Unconscious mood-congruent memory bias in depression. J Abnorm Psychol. 1996;105:34–41. doi: 10.1037//0021-843x.105.1.34. [DOI] [PubMed] [Google Scholar]
- 10.Watkins PC, Mathews A, Williamson DA, et al. Mood-congruent memory in depression: Emotional priming or elaboration? J Abnorm Psychol. 1992;101:581–6. doi: 10.1037//0021-843x.101.3.581. [DOI] [PubMed] [Google Scholar]
- 11.Koster EH, De Raedt R, Leyman L, et al. Mood-congruent attention and memory bias in dysphoria: exploring the coherence among information-processing biases. Behav Res Ther. 2010;48:219–25. doi: 10.1016/j.brat.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Teasdale JD, Russell ML. Differential effects of induced mood on the recall of positive, negative and neutral words. Br J Clin Psychol. 1983;22:163–71. doi: 10.1111/j.2044-8260.1983.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 13.Blazer DG, II, Hybels CF. Origins of depression in later life. Psychol Med. 2005;35:1241–52. doi: 10.1017/S0033291705004411. [DOI] [PubMed] [Google Scholar]
- 14.Bekhet AK, Zauszniewski JA, Nakhla WE. Loneliness: a concept analysis. Nurs Forum. 2008;43:207–13. doi: 10.1111/j.1744-6198.2008.00114.x. [DOI] [PubMed] [Google Scholar]
- 15.Heinrich LM, Gullone E. The clinical significance of loneliness: a literature review. Clin Psychol Rev. 2006;26:695–718. doi: 10.1016/j.cpr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Martin AL, Brown RE. The lonely mouse: verification of a separation-induced model of depression in female mice. Behav Brain Res. 2010;207:196–207. doi: 10.1016/j.bbr.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Silva RH, Frussa-Filho R. The plus-maze discriminative avoidance task: a new model to study memory-anxiety interactions. Effects of chlordiazepoxide and caffeine. J Neurosci Methods. 2000;102:117–25. doi: 10.1016/s0165-0270(00)00289-2. [DOI] [PubMed] [Google Scholar]
- 18.Silva RH, Felicio LF, Frussa-Filho R. Ganglioside GM1 attenuates scopolamine-induced amnesia in rats and mice. Psychopharmacology (Berl) 1999;141:111–7. doi: 10.1007/s002130050814. [DOI] [PubMed] [Google Scholar]
- 19.Gulick D, Gould TJ. Nicotine acts in the anterior cingulate, but not dorsal or ventral hippocampus, to reverse ethanol-induced learning impairments in the plus-maze discriminative avoidance task. Addict Biol. 2011;16:176–88. doi: 10.1111/j.1369-1600.2010.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss IC, Pryce CR, Jongen-Relo AL, et al. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–95. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Akirav I, Kozenicky M, Tal D, et al. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learn Mem. 2004;11:188–95. doi: 10.1101/lm.61704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandi C, Pinelo-Nava MT. Stress and memory: behavioral effects and neurobiological mechanisms. Neural Plast. 2007;2007:78970. doi: 10.1155/2007/78970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Quervain DJ, Aerni A, Schelling G, et al. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30:358–70. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Cazakoff BN, Johnson KJ, Howland JG. Converging effects of acute stress on spatial and recognition memory in rodents: a review of recent behavioural and pharmacological findings. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:733–41. doi: 10.1016/j.pnpbp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Albers HE, Yogev L, Todd RB, et al. Adrenal corticoids in hamsters: role in circadian timing. Am J Physiol. 1985;248:R434–8. doi: 10.1152/ajpregu.1985.248.4.R434. [DOI] [PubMed] [Google Scholar]
- 26.Power MJ, Cameron CM, Dalgleish T. Emotional priming in clinically depressed subjects. J Affect Disord. 1996;38:1–11. doi: 10.1016/0165-0327(95)00083-6. [DOI] [PubMed] [Google Scholar]
- 27.Ellwart T, Rinck M, Becker ES. Selective memory and memory deficits in depressed inpatients. Depress Anxiety. 2003;17:197–206. doi: 10.1002/da.10102. [DOI] [PubMed] [Google Scholar]
- 28.Haaga DA. Mood state-dependent retention using identical or nonidentical mood inductions at learning and recall. Br J Clin Psychol. 1989;28:75–83. doi: 10.1111/j.2044-8260.1989.tb00814.x. [DOI] [PubMed] [Google Scholar]
- 29.Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 30.Izquierdo I. Endogenous state dependency: memory depends on the relation between the neurohumoral and hormonal states present after training and at the time of testing. In: Lynch G, McGaugh JL, Weinberger NM, editors. Neurobiology of learning and memory. New York (NY): Guilford; 1984. pp. 333–50. [Google Scholar]
- 31.Bruins Slot LA, Colpaert FC. Opiate states of memory: receptor mechanisms. J Neurosci. 1999;19:10520–9. doi: 10.1523/JNEUROSCI.19-23-10520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patti CL, Zanin KA, Sanday L, et al. Effects of sleep deprivation on memory in mice: role of state-dependent learning. Sleep. 2010;33:1669–79. doi: 10.1093/sleep/33.12.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patti CL, Kameda SR, Carvalho RC, et al. Effects of morphine on the plus-maze discriminative avoidance task: role of state-dependent learning. Psychopharmacology (Berl) 2006;184:1–12. doi: 10.1007/s00213-005-0238-6. [DOI] [PubMed] [Google Scholar]
- 34.Klaassen T, Riedel WJ, Deutz NE, et al. Mood congruent memory bias induced by tryptophan depletion. Psychol Med. 2002;32:167–72. doi: 10.1017/s003329170100438x. [DOI] [PubMed] [Google Scholar]
- 35.Brown KJ, Grunberg NE. Effects of housing on male and female rats: crowding stresses male but calm females. Physiol Behav. 1995;58:1085–9. doi: 10.1016/0031-9384(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 36.Gourley SL, Wu FJ, Kiraly DD, et al. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008;63:353–9. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rush AJ, Bernstein IH, Trivedi MH, et al. An evaluation of the quick inventory of depressive symptomatology and the Hamilton rating scale for depression: a sequenced treatment alternatives to relieve depression trial report. Biol Psychiatry. 2006;59:493–501. doi: 10.1016/j.biopsych.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–90. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 39.Markou A, Chiamulera C, Geyer MA, et al. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2009;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cervo L, Canetta A, Calcagno E, et al. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J Neurosci. 2005;25:8165–72. doi: 10.1523/JNEUROSCI.1816-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Can A, Blackwell RA, Piantadosi SC, et al. Antidepressant-like responses to lithium in genetically diverse mouse strains. Genes Brain Behav. 2011;10:434–43. doi: 10.1111/j.1601-183X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 43.Kameda SR, Frussa-Filho R, Carvalho RC, et al. Dissociation of the effects of ethanol on memory, anxiety, and motor behavior in mice tested in the plus-maze discriminative avoidance task. Psychopharmacology (Berl) 2007;192:39–48. doi: 10.1007/s00213-006-0684-9. [DOI] [PubMed] [Google Scholar]
- 44.Carvalho RC, Patti CC, Takatsu-Coleman AL, et al. Effects of reserpine on the plus-maze discriminative avoidance task: dissociation between memory and motor impairments. Brain Res. 2006;1122:179–83. doi: 10.1016/j.brainres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Gulick D, Gould TJ. Effects of ethanol and caffeine on behavior in C57BL/6 mice in the plus-maze discriminative avoidance task. Behav Neurosci. 2009;123:1271–8. doi: 10.1037/a0017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gulick D, Gould TJ. Interactive effects of ethanol and nicotine on learning, anxiety, and locomotion in C57BL/6 mice in the plus-maze discriminative avoidance task. Neuropharmacology. 2009;57:302–10. doi: 10.1016/j.neuropharm.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siegfried B, Alleva E, Oliverio A, et al. Effects of isolation on activity, reactivity, excitability and aggressive behavior in two inbred strains of mice. Behav Brain Res. 1981;2:211–8. doi: 10.1016/0166-4328(81)90056-5. [DOI] [PubMed] [Google Scholar]
- 48.Heidbreder CA, Weiss IC, Domeney AM, et al. Behavioral, neuro-chemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–68. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 49.Guo M, Wu CF, Liu W, et al. Sex difference in psychological behavior changes induced by long-term social isolation in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:115–21. doi: 10.1016/j.pnpbp.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 50.Fabricius K, Helboe L, Fink-Jensen A, et al. Increased dopaminergic activity in socially isolated rats: an electrophysiological study. Neurosci Lett. 2010;482:117–22. doi: 10.1016/j.neulet.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 51.Silva RH, Kameda SR, Carvalho RC, et al. Effects of amphetamine on the plus-maze discriminative avoidance task in mice. Psychopharmacology (Berl) 2002;160:9–18. doi: 10.1007/s00213-001-0948-3. [DOI] [PubMed] [Google Scholar]
- 52.Fukushiro DF, Frussa-Filho R. Chronic amphetamine transforms the emotional significance of a novel but not a familiar environment: implications for addiction. Int J Neuropsychopharmacol. 2011;14:955–65. doi: 10.1017/S1461145710001379. [DOI] [PubMed] [Google Scholar]
- 53.Sara SJ, Deweer B. Memory retrieval enhanced by amphetamine after a long retention interval. Behav Neural Biol. 1982;36:146–60. doi: 10.1016/s0163-1047(82)90145-5. [DOI] [PubMed] [Google Scholar]
- 54.Sara SJ. Forgetting of a conditioned emotional response and its alleviation by pretest amphetamine. Physiol Psychol. 1984;12:17–22. [Google Scholar]
- 55.Quartermain D, Judge ME, Jung H. Amphetamine enhances retrieval following diverse sources of forgetting. Physiol Behav. 1988;43:239–41. doi: 10.1016/0031-9384(88)90245-4. [DOI] [PubMed] [Google Scholar]
- 56.Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 57.Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J Neuroendocrinol. 1999;11:361–9. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]
- 58.Fietta P. Glucocorticoids and brain functions. Riv Biol. 2007;100:403–18. [PubMed] [Google Scholar]
- 59.McDevitt RA, Szot P, Baratta MV, et al. Stress-induced activity in the locus coeruleus is not sensitive to stressor controllability. Brain Res. 2009;1285:109–18. doi: 10.1016/j.brainres.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]