Synopsis
Current ROP screening guidelines represent a simple risk model, consisting of two dichotomized factors, birth weight (BW) and gestational age at birth (GA). The pioneering work of Smith, Hellstrom, Lofqvist, and colleagues has shown that while many risk factors have been associated with severe ROP, low insulin-like growth factor 1 (IGF-1) may represent a common pathway through which many of these risk factors act to influence ROP risk, and that this influence may be captured by tracking postnatal weight gain, a surrogate measure for IGF-1. In countries with highly developed neonatal care systems, predictive models including weight gain have demonstrated accurate ROP risk assessment and a large reduction in the number of ROP examinations, compared to current guidelines based upon BW and GA alone. These models include WINROP, a computer-based algorithm that identifies cumulative deviations from expected weight gain; ROPScore, a spreadsheet-based equation; and CHOP ROP, a nomogram-based model that consists of a single equation with continuous terms for BW, GA, and weight gain. However, clinicians should appreciate important limitations of the models, including the need for larger studies to achieve a highly precise estimate of sensitivity and the poor generalizability of the models to countries where higher BW and GA infants require treatment, as low IGF-1 may play less of a role in the pathogenesis of ROP in these larger and older babies.
Keywords: Infant, nomogram, prediction model, retinopathy of prematurity, risk model
Introduction
Prognostic statistical models allow clinicians to predict a patient’s risk of developing a specific medical outcome.1 Although not typically described in such terms, our current retinopathy of prematurity (ROP) screening criteria are nothing more than a simple prediction model with two dichotomized (yes or no) predictors, birth weight (BW) and gestational age at birth (GA). A need for exams is determined by the degree of prematurity at birth by using two cut-off levels, and other risk factors are not considered in a systematic fashion. For example, in the United States, babies with either BW<1501 grams or GA≤30 weeks receive exams.2 A priority is placed upon avoiding blindness in even a single child, and this screening “model” has high sensitivity (it catches almost all cases of severe ROP), but it is not specific (most examined infants do not develop severe ROP). In fact, less than five per cent of infants examined require laser surgery, based upon multiple large US3–6, Canadian7, and UK8, 9 studies.
Growth, IGF-1, and ROP
In an effort to improve this specificity, postnatal growth-based ROP risk models have been developed. The models are based upon ground-breaking work by Smith, Hellstrom, Lofqvist, and colleagues, who have provided our current understanding of the pathophysiology underlying ROP.
ROP develops in two phases, a hypoxic preclinical phase, during which slow postnatal growth can be used to predict risk, and a subsequent proliferative clinical phase. These phases result from alterations in serum insulin-like growth factor 1 (IGF-1), a somatic growth factor, and retinal vascular endothelial growth factor (VEGF), a hypoxia-induced vasoproliferative factor necessary for normal retinal vascular development.10 Serum IGF-1 falls with premature birth from loss of maternal sources and poor endogenous production.11–14 Importantly, IGF-1 plays a permissive role in VEGF-induced retinal vascular growth.15, 16 Therefore, low serum IGF-1 hinders retinal vessel development, with localized hypoxia and VEGF accumulation, as metabolic demands increase within the developing retina. With increasing age and size, endogenous production of IGF-1 rises, permitting VEGF activity, and proliferative retinopathy develops.
Much laboratory work supports this model.10, 15–21 Clinically, multiple investigators have demonstrated that both prolonged early IGF-1 deficits and slow postnatal weight gain are associated with a higher risk of subsequent severe ROP.11, 22–30 Serum IGF1 levels correlate with fetal and postnatal growth, so postnatal growth is a good surrogate measure for serum IGF-1.12, 14, 31–33 In addition, weight measurements are simple, quick, cheap, and routinely collected, while IGF-1 assays are costly and require blood, a laboratory, and processing time.
Postnatal Growth ROP Models
Specific postnatal growth based ROP predictive models are discussed below. In evaluating these models, it is most useful to consider their performance with regards to sensitivity for detecting severe ROP and the reduction in children requiring eye exams that would have resulted from their use, which is a more clinically intuitive measure than specificity. Severe ROP has been variably defined as Early Treatment of ROP Study type 1 ROP, treated ROP, and or stage 3 ROP in the studies.
All three models use weight gain as a measure of postnatal growth and consider BW and GA in the determination of risk of ROP along with growth. BW and GA are still the most significant risk factors for ROP in countries with highly developed Neonatal Intensive Care Unit (NICU) systems.3–7, 9, 34–37 The smaller and younger an infant was at birth, the greater the risk of ROP and of severe ROP. Likely pathophysiologic correlates include the degree of retinal vascular immaturity and low postnatal endogenous production of serum IGF-1. However, this predictive information is lost by using only BW and GA cut-off levels. Treating BW and GA as continuous or ordinal variables addresses this issue, but then another factor, such as slow postnatal growth, must be introduced to identify higher BW and GA infants at risk for severe ROP.
With regards to additional risk factors, current screening guidelines do include an “unstable clinical course” as judged by the neonatologist as a criteria by which to examine larger infants. However, in practice, postnatal factors are not considered in a systematic fashion, even though multiple other risk factors for ROP have been described, such as excessive supplemental oxygen, necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), anemia, apnea, sepsis, and blood transfusions.6, 35, 38–44 Based upon the studies below, it is hypothesized that most such factors may act via a common pathway, by lowering serum IGF-1 levels, and may be “captured” in a predictive model simply by considering postnatal weight gain. Supplemental oxygen is an important exception, particularly in countries with developing neonatal care systems, where higher BW and GA infants may develop severe ROP via a pathophysiologic mechanism unrelated to low serum IGF-1. The limited potential for weight gain ROP models to be used in such infants is a critical issue discussed below.
WINROP
Based upon their work on IGF-1 and ROP, Lofqvist, Hellstrom, Smith, et al. developed a computer-based ROP risk algorithm named WINROP to detect slowdowns in postnatal weight gain, predict severe ROP, and greatly reduce the number of infants requiring exams.22, 45–47 WINROP uses a cumulative-deviations statistical approach in multiple steps: each week the infant’s actual weight is compared to an expected growth curve of infants who developed no or mild ROP; the differences or deviations between the expected weight and the actual weight are accumulated from week to week; and when these cumulative deviations surpass a threshold alarm level, the risk of severe ROP is categorized using dichotomized cut off levels for BW, GA, and alarm timing to determine a need for eye exams.
WINROP demonstrated very high sensitivity for detecting severe ROP in retrospective studies: 100% in a Swedish cohort of 353 infants, reducing infants who would have received exams by 76%25; 100% in a Boston cohort of 318 infants, reducing infants who need exams by 75%29; and most recently fell slightly to 98.6% in a larger, multi-center US and Canadian cohort of 1,706 infants48. When WINROP was studied in countries with developing NICU systems, however, the sensitivity fell further: 91% in a Brazilian cohort of 366 infants49, and 55% (85% if GA<32 weeks, 5% if GA≥32 weeks) in a Mexican cohort of 352 infants50.
The WINROP algorithm involves complex calculations, which have limited transparency for the user. However, the developers have created a web-based application (www.winrop.com) that permits the user to enter BW, GA, and weight measurements, allows tracking of multiple infants, and provides a simple indication of low or high risk status for developing site-threatening ROP. The authors suggest that WINROP be used as “an adjunct to and not a replacement for standard ophthalmological screening.”48 They describe abbreviated exam schedules for low-risk infants, as opposed to no examinations at all.48
ROPScore
Eckert, Filho, et al. recently developed a less complex model named ROPScore.51 The model consists of a logistic regression equation, which is used to calculate risk only once per child, using an Excel spreadsheet. The model includes continuous rather than dichotomized terms for BW and GA, weight gain at a single time point (6 weeks postnatal age) as a proportion of BW, and dichotomous terms for blood transfusion and use of oxygen in mechanical ventilation during the first six weeks of life. Assuming a specific cut off level for low or high risk, ROPScore had a sensitivity of 98% and specificity of 56% for treatment-requiring ROP in a development cohort of 474 Brazilian infants.51 Additional studies are underway. As with WINROP, the authors suggest that ROPScore be used not to determine overall screening criteria but rather to reduce the frequency of exams in low risk infants.51
PINT ROP and CHOP ROP
Binenbaum et al. developed a simpler logistic regression based model named PINT ROP.30 Prospectively collected data from 367 infants with BW<1000 g in the Premature Infants in Need of Transfusion (PINT) randomized controlled trial were used to develop a model containing terms only for BW, GA, and daily weight gain rate, which was calculated from the current and prior week’s weight measurements. The equation is calculated on a weekly basis, and if the predicted risk of ROP is greater than a cut point level, exams are indicated and the equation does not need to be re-calculated again. In this manner, PINT ROP had 100% sensitivity for treated ROP, while reducing the number of infants requiring exams by 30% in the high risk cohort. 30
Of note, numerous additional candidate predictors were evaluated, including ethnicity, perinatal and postnatal comorbidities, and medical and surgical interventions. All these factors fell out in multivariate analyses30, supporting the hypothesis that many previously described risk factors for ROP act through a common pathway, by affecting IGF1 levels, and are therefore “captured” through weight measurements.
The PINT ROP cohort was at high risk for ROP. Therefore, the investigators applied the same modeling approach to a low risk cohort more representative of current US ROP screening criteria (BW<1501 g), to develop an updated model called CHOP ROP.52 Among 524 infants, the model had 100% sensitivity for type 1 ROP, while reducing the number of infants requiring exams by 49%. If the risk cut off was raised to miss 1 infant requiring laser, the reduction in exams was 79%, suggesting a trade off that might be explored further. There was a small (3%) advantage to using daily versus weekly weight measurements. Paper nomograms to simplify use of the models were created based upon the PINT ROP model and updated with the CHOP ROP model (Figure 1), but the authors stressed that further studies are needed prior to clinical use.30, 52
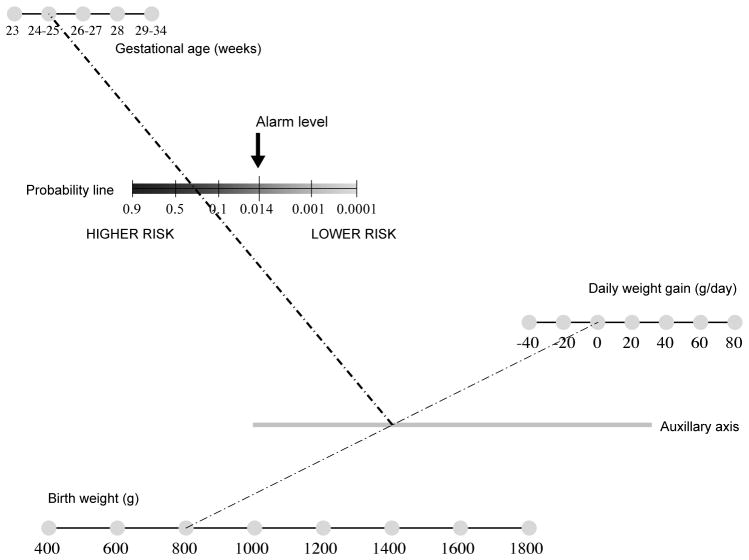
Figure 1.
Sample nomogram to predict the risk of severe retinopathy of prematurity based upon the CHOP ROP model. A straight line is drawn between the values for birth weight and daily weight gain rate. The intersection of this line with the gray auxiliary axis is then connected to the value for GA. The intersection of this second line with the probability line provides the predicted probability of severe ROP. If the risk is greater than 0.014, eye exams are indicated. NOTE: This nomogram requires further validation and is not intended for clinical use at this time. From Binenbaum G, Ying GS, Quinn GE, et al: The CHOP postnatal weight gain, birth weight, and gestaional age retinopathy of prematurity risk model. Arch Ophthalmol 2012;130(12):1560–1565; with permission.
Model Development and Complexity
The creation of a usable clinical prediction model is a stepwise process.1 First, a development study is undertaken, containing as large a sample size as possible in order to avoid over fitting of the model.1, 53 Over fitting may occur when the complexity of the model is high relative to the number of outcome events (cases of severe ROP) in the cohort, and the model effectively describes random error rather than a true association. Such a model will not perform well in a new groups of patients. Second, the model must be validated in new patients and if necessary updated, preferably using both the original and new datasets.1, 54, 55 Model updating may involve adjusting the model coefficients or even adding new variables if necessary.55 In the case of the CHOP ROP model, the study cohort had a significantly different risk profile than the PINT ROP cohort, so the model coefficients and alarm cut point needed to be adjusted.
The final step is to evaluate the impact of the model in clinical use.1,56 Successful implementation of a clinical predictive model requires physician acceptance, which depends upon transparency, ease of use, and in this case confidence that no cases of severe ROP will be missed.56 Transparency and ease of use relate to model complexity, while “confidence” relates most directly to the precision of the point estimate of sensitivity of the model for predicting severe ROP. A cumulative-deviations model is relatively more complex and to some degree lacks transparency with regards to the calculations being done to determine risk. The process becomes more user-friendly with the use of a web-based application.22, 23, 25 A logistic regression equation based model provides a simpler calculation of risk, and it can be represented as a paper nomogram, which does not require any calculation to be done (Figure 1).
Sample Size: An important limitation
Despite studies in cohorts ranging from 300 to 1700 infants, sample size remains a limiting factor. While a point estimate of sensitivity of 100% has been reported, the confidence interval (CI) around that point estimate is likely too wide for clinicians to have confidence that infants with severe ROP would not be missed. The reason is that the width of the CI is driven by the number of cases of severe ROP, not the overall number of infants. For example, the CHOP ROP study involved 524 infants, but only 20 infants had severe ROP, so while the sensitivity was 100%, the 95% CI was 84% to 100%. Even in the most recent, multi-center WINROP study, which involved 1706 infants and reported a sensitivity of 98.6%, the CI was only 96.7% to 100%.
To have sufficient confidence in a model to justify its use for primary “all or none” screening decisions, the lower boundary of the CI arguably should be much higher, perhaps above 99%, which would require a dataset with hundreds of cases of ROP. Therefore, it may be best to conservatively consider these studies as still in the “development” stage, and only once that high degree of precision of sensitivity (very narrow CI) is obtained would subsequent validation and updating studies be undertaken. Larger studies might also identify confounding medical factors, the presence of which would default an infant to receive eye examinations. However, alternative perspectives and approaches may be considered. Rather than replacing current screening guidelines, a model may be used alongside current guidelines, changing exam frequency or timing based upon predicted risk. WINROP has been used in Sweden in this fashion, based upon repeatedly high sensitivity demonstrated across multiple WINROP studies.48 Alternatively, a sensitivity of less than 100% might be acceptable under some circumstances, such as when ophthalmologic resources are limited and must be rationed. Finally, a model’s alarm level could be lowered to more confidently ensure 100% sensitivity, while accepting a decrease in specificity. This approach could work well in a multi-tiered screening approach together with telemedicine, once both modalities have been sufficiently validated: the risk model determines which infants require fundus photographs, and photographic grading determines which infants require eye examination by an ophthalmologist.
Generalizability: A second important limitation
There are several reasons why a predictive model may behave poorly in new patients, including the methods used to design the model and differences in health care systems and patient characteristics.54 There is good evidence that the generalizability of models such as WINROP and CHOP ROP to countries where higher BW and GA infants develop severe ROP will be limited, and that separate model development studies need to be carried out in such populations. When WINROP was applied to a Brazilian cohort, it demonstrated less than 90% sensitivity49, and when it was applied to a Mexican cohort, sensitivity was only 55%50. ROPScore was developed in Brazil, and model performance was higher. 51 However, additional terms for blood transfusion and oxygen supplementation are included in the model51, and performance across multiple settings still must be assessed, as there is high variability in ROP risk profile across neonatal centers in the country, even within the same city57.
The poor performance of postnatal weight gain ROP models in countries with developing neonatal care system may be related to differences in ROP pathophysiology, particularly in older GA infants. At older post menstrual ages, endogenous production of IGF-1 has already increased, so that low IGF-1 may play less of a role in the pathogenesis of severe ROP. Rather, ROP in such infants might be driven primarily by high oxygen exposure, which causes inhibition of VEGF and retinal blood vessel destruction, as it does in oxygen-induced animal models of ROP. This hypothesis is supported by the dramatic difference in the performance of WINROP among Mexican infants with GA<32 weeks (85% sensitivity) and GA≥32 weeks (5% sensitivity).
Benefits and Future Studies
Improved ROP risk assessment could have broad-reaching benefits in neonatology, ophthalmology, and public health. Revised ROP screening guidelines might reduce both the number of children requiring stressful diagnostic eye examinations and the frequency of exams for lower-risk infants. Professional and infrastructure resources may be better allocated to high risk infants, particularly in areas and countries with limited resources, where better targeted resources could lower the burden of blindness due to ROP. Tiered approaches, combining a growth-based risk model with telemedicine assessments, might further improve the efficiency of screening. In general, the cost effectiveness of ROP diagnostic examinations would be increased.
Postnatal weight gain based ROP prediction models have great potential to influence the clinical management of ROP. Further development, validation, and clinical impact studies across multiple international settings can and should be pursued, but there are no set epidemiological rules by which to determine when sufficient validation has been achieved to safely use a clinical prediction model; criteria depend upon the clinical context.55 ROP screening involves high stakes decisions, and perhaps model validation and updating will best be viewed as an ongoing process. The studies discussed above have established “proof of concept.” And if even larger development and validation studies continue to demonstrate very high sensitivity for predicting severe ROP, then the research objective will shift towards simply maximizing the predictive value of the data upon which ROP screening guidelines are based. Retrospective pooling of datasets by investigators and an ongoing prospective data registry would permit a model to be updated in an ongoing fashion and maximize the use of available data. This type of collaborative approach would best ensure that all infants at risk for site threatening ROP undergo diagnostic eye examinations, while the potential benefits of postnatal growth based ROP models are realized.
Key points.
Slow postnatal growth is a surrogate measure for low serum insulin-like growth factor 1, which is an important risk factor for severe ROP.
Risk models that consider postnatal weight gain, along with birth weight and gestational age, include WINROP, ROPScore, and CHOP ROP.
These models predict severe ROP with much greater specificity than current ROP screening guidelines.
Two important limitations are study sample size and poor generalizability in countries with developing neonatal care systems.
Postnatal growth based models have great potential to reduce the number of diagnostic ROP examinations being performed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moons KG, Royston P, Vergouwe Y, et al. Prognosis and prognostic research: what, why, and how? BMJ. 2009;338:1317–1320. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 2.Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013 Jan;131(1):189–195. doi: 10.1542/peds.2012-2996. [DOI] [PubMed] [Google Scholar]
- 3.The natural ocular outcome of premature birth and retinopathy. Status at 1 year. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol. 1994 Jul;112(7):903–912. doi: 10.1001/archopht.1994.01090190051021. [DOI] [PubMed] [Google Scholar]
- 4.Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1991 Nov;98(11):1628–1640. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 5.Early Treatment For Retinopathy Of Prematurity Cooperative G. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003 Dec;121(12):1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 6.Chiang MF, Arons RR, Flynn JT, et al. Incidence of retinopathy of prematurity from 1996 to 2000: analysis of a comprehensive New York state patient database. Ophthalmology. 2004 Jul;111(7):1317–1325. doi: 10.1016/j.ophtha.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Lee SK, Normand C, McMillan D, et al. Evidence for changing guidelines for routine screening for retinopathy of prematurity. Arch Pediatr Adolesc Med. 2001 Mar;155(3):387–395. doi: 10.1001/archpedi.155.3.387. [DOI] [PubMed] [Google Scholar]
- 8.Haines L, Fielder AR, Scrivener R, et al. Retinopathy of prematurity in the UK I: the organisation of services for screening and treatment. Eye (Lond) 2002 Jan;16(1):33–38. doi: 10.1038/sj.eye.6700030. [DOI] [PubMed] [Google Scholar]
- 9.Royal College of Paediatrics and Child Health RCoO, British Association of Perinatal Medicine. UK Retinopathy of Prematurity Guideline May 2008. [PDF] [Accessed Sep 17, 2012];UK Retinopathy of Prematurity Guideline. 2008 Available at: http://www.rcophth.ac.uk/core/core_picker/download.asp?id=180&filetitle=UK+Retinopathy+of+Prematurity+Guideline+May+2008.
- 10.Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996 Oct;114(10):1219–1228. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- 11.Hellstrom A, Engstrom E, Hard AL, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003 Nov;112(5):1016–1020. doi: 10.1542/peds.112.5.1016. [DOI] [PubMed] [Google Scholar]
- 12.Langford K, Nicolaides K, Miell JP. Maternal and fetal insulin-like growth factors and their binding proteins in the second and third trimesters of human pregnancy. Hum Reprod. 1998 May;13(5):1389–1393. doi: 10.1093/humrep/13.5.1389. [DOI] [PubMed] [Google Scholar]
- 13.Lassarre C, Hardouin S, Daffos F, et al. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res. 1991 Mar;29(3):219–225. doi: 10.1203/00006450-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Lineham JD, Smith RM, Dahlenburg GW, et al. Circulating insulin-like growth factor I levels in newborn premature and full-term infants followed longitudinally. Early Hum Dev. 1986 Feb;13(1):37–46. doi: 10.1016/0378-3782(86)90096-4. [DOI] [PubMed] [Google Scholar]
- 15.Smith LE, Shen W, Perruzzi C, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999 Dec;5(12):1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 16.Hellstrom A, Perruzzi C, Ju M, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci U S A. 2001 May 8;98(10):5804–5808. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995 Oct;1(10):1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 19.Pierce EA, Avery RL, Foley ED, et al. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson GS, Pierce EA, Rook SL, et al. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih SC, Ju M, Liu N, et al. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. J Clin Invest. 2003 Jul;112(1):50–57. doi: 10.1172/JCI17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lofqvist C, Andersson E, Sigurdsson J, et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006 Dec;124(12):1711–1718. doi: 10.1001/archopht.124.12.1711. [DOI] [PubMed] [Google Scholar]
- 23.Lofqvist C, Hansen-Pupp I, Andersson E, et al. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulinlike growth factor I. Arch Ophthalmol. 2009 May;127(5):622–627. doi: 10.1001/archophthalmol.2009.69. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Munuzuri A, Fernandez-Lorenzo J, Couce-Pico M, et al. Serum levels of IGF1 are a useful predictor of retinopathy of prematurity. Acta Paediatr. 2010 Jan 18; doi: 10.1111/j.1651-2227.2009.01677.x. [DOI] [PubMed] [Google Scholar]
- 25.Hellstrom A, Hard AL, Engstrom E, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009 Apr;123(4):e638–645. doi: 10.1542/peds.2008-2697. [DOI] [PubMed] [Google Scholar]
- 26.Wallace DK, Kylstra JA, Phillips SJ, et al. Poor postnatal weight gain: a risk factor for severe retinopathy of prematurity. J AAPOS. 2000 Dec;4(6):343–347. doi: 10.1067/mpa.2000.110342. [DOI] [PubMed] [Google Scholar]
- 27.Fortes Filho JB, Bonomo PP, Maia M, et al. Weight gain measured at 6 weeks after birth as a predictor for severe retinopathy of prematurity: study with 317 very low birth weight preterm babies. Graefes Arch Clin Exp Ophthalmol. 2009 Jun;247(6):831–836. doi: 10.1007/s00417-008-1012-3. [DOI] [PubMed] [Google Scholar]
- 28.Villegas-Becerril EG-FR, Perula-Torres L, Gllarado-Galera JM. IGF-1, VEGF, and bFGF as Predictive Factors for the Onset of Retinopathy of Prematurity (ROP) Arch Soc Esp Oftalmol. 2006;81:641–646. doi: 10.4321/s0365-66912006001100005. [DOI] [PubMed] [Google Scholar]
- 29.Wu C, Vanderveen DK, Hellstrom A, et al. Longitudinal postnatal weight measurements for the prediction of retinopathy of prematurity. Arch Ophthalmol. 2010 Apr;128(4):443–447. doi: 10.1001/archophthalmol.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binenbaum G, Ying GS, Quinn GE, et al. A clinical prediction model to stratify retinopathy of prematurity risk using postnatal weight gain. Pediatrics. 2011 Mar;127(3):e607–614. doi: 10.1542/peds.2010-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad I, Zaldivar F, Iwanaga K, et al. Inflammatory and growth mediators in growing preterm infants. J Pediatr Endocrinol Metab. 2007 Mar;20(3):387–396. doi: 10.1515/jpem.2007.20.3.387. [DOI] [PubMed] [Google Scholar]
- 32.Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 2000 Apr;183(1):1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hikino S, Ihara K, Yamamoto J, et al. Physical growth and retinopathy in preterm infants: involvement of IGF-I and GH. Pediatr Res. 2001 Dec;50(6):732–736. doi: 10.1203/00006450-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 34.Duhaime AC, Alario AJ, Lewander WJ, et al. Head injury in very young children: mechanisms, injury types, and ophthalmologic findings in 100 hospitalized patients younger than 2 years of age. Pediatrics. 1992 Aug;90(2 Pt 1):179–185. [PubMed] [Google Scholar]
- 35.Lucey JF, Dangman B. A reexamination of the role of oxygen in retrolental fibroplasia. Pediatrics. 1984 Jan;73(1):82–96. [PubMed] [Google Scholar]
- 36.Ng YK, Fielder AR, Shaw DE, et al. Epidemiology of retinopathy of prematurity. Lancet. 1988 Nov 26;2(8622):1235–1238. doi: 10.1016/s0140-6736(88)90820-3. [DOI] [PubMed] [Google Scholar]
- 37.Schaffer DB, Palmer EA, Plotsky DF, et al. Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1993 Feb;100(2):230–237. doi: 10.1016/s0161-6420(93)31665-9. [DOI] [PubMed] [Google Scholar]
- 38.Hutchinson AK, O’Neil JW, Morgan EN, et al. Retinopathy of prematurity in infants with birth weights greater than 1250 grams. J AAPOS. 2003 Jun;7(3):190–194. doi: 10.1016/s1091-8531(02)42018-6. [DOI] [PubMed] [Google Scholar]
- 39.Yanovitch TL, Siatkowski RM, McCaffree M, et al. Retinopathy of prematurity in infants with birth weight>or=1250 grams-incidence, severity, and screening guideline cost-analysis. J AAPOS. 2006 Apr;10(2):128–134. doi: 10.1016/j.jaapos.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008 Feb;84(2):77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Mohamed S, Schaa K, Cooper ME, et al. Genetic contributions to the development of retinopathy of prematurity. Pediatr Res. 2009 Feb;65(2):193–197. doi: 10.1203/PDR.0b013e31818d1dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben Sira I, Nissenkorn I, Kremer I. Retinopathy of prematurity. Surv Ophthalmol. 1988 Jul-Aug;33(1):1–16. doi: 10.1016/0039-6257(88)90068-9. [DOI] [PubMed] [Google Scholar]
- 43.Fielder AR, Shaw DE, Robinson J, et al. Natural history of retinopathy of prematurity: a prospective study. Eye (Lond) 1992;6( Pt 3):233–242. doi: 10.1038/eye.1992.46. [DOI] [PubMed] [Google Scholar]
- 44.Shohat M, Reisner SH, Krikler R, et al. Retinopathy of prematurity: incidence and risk factors. Pediatrics. 1983 Aug;72(2):159–163. [PubMed] [Google Scholar]
- 45.Frisen M. Statistical surveillance: optimality and methods. Int Stat Rev. 2003;71(2):403–434. [Google Scholar]
- 46.Roberts SW. A comparison of some control chart procedures. Technometrics. 1966;8(3):411–430. 435. [Google Scholar]
- 47.Shiryaev A. On optimum methods in quickest detection problems. Theory Probab Appl. 1963;8(1):22–46. [Google Scholar]
- 48.Wu C, Lofqvist C, Smith LE, et al. Importance of Early Postnatal Weight Gain for Normal Retinal Angiogenesis in Very Preterm Infants: A Multicenter Study Analyzing Weight Velocity Deviations for the Prediction of Retinopathy of Prematurity. Arch Ophthalmol. 2012 Apr 9; doi: 10.1001/archophthalmol.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hard AL, Lofqvist C, Fortes Filho JB, et al. Predicting proliferative retinopathy in a Brazilian population of preterm infants with the screening algorithm WINROP. Arch Ophthalmol. 2010 Nov;128(11):1432–1436. doi: 10.1001/archophthalmol.2010.255. [DOI] [PubMed] [Google Scholar]
- 50.Zepeda-Romero LC, Hard AL, Gomez-Ruiz LM, et al. Prediction of retinopathy of prematurity using the screening algorithm WINROP in a Mexican population of preterm infants. Arch Ophthalmol. 2012 Jun;130(6):720–723. doi: 10.1001/archophthalmol.2012.215. [DOI] [PubMed] [Google Scholar]
- 51.Eckert GU, Fortes Filho JB, Maia M, et al. A predictive score for retinopathy of prematurity in very low birth weight preterm infants. Eye (Lond) 2012 Mar;26(3):400–406. doi: 10.1038/eye.2011.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Binenbaum G, Ying GS, Quinn GE, et al. The CHOP postnatal weight gain, birth weight, and gestational age retinopathy of prematurity risk model. Arch Ophthalmol. 2012;130(12):1560–1565. doi: 10.1001/archophthalmol.2012.2524. [DOI] [PubMed] [Google Scholar]
- 53.Royston P, Moons KG, Altman DG, et al. Prognosis and prognostic research: Developing a prognostic model. BMJ. 2009;338:b604. doi: 10.1136/bmj.b604. [DOI] [PubMed] [Google Scholar]
- 54.Altman DG, Vergouwe Y, Royston P, et al. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. doi: 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]
- 55.Moons KG, Altman DG, Vergouwe Y, et al. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. doi: 10.1136/bmj.b606. [DOI] [PubMed] [Google Scholar]
- 56.Wyatt JCAD. Commentary: Prognostic models: clinically useful or quickly forgotten. Br Med J. 1995;311:1539–1541. [Google Scholar]
- 57.Zin AA, Moreira ME, Bunce C, et al. Retinopathy of prematurity in 7 neonatal units in Rio de Janeiro: screening criteria and workload implications. Pediatrics. 2010 Aug;126(2):e410–417. doi: 10.1542/peds.2010-0090. [DOI] [PubMed] [Google Scholar]