Abstract
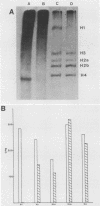
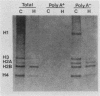
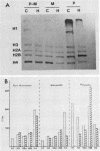
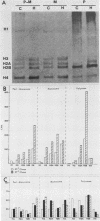
Transferring Drosophila tissue culture cells from 25 to 37 degrees C (heat shock) causes histone protein synthesis to become noncoordinate. To determine the level at which this is controlled, the synthesis, degradation, and translation of individual histone mRNAs was studied under both heat shock and control conditions. The increased synthesis of histone H2b protein during heat shock appears to be controlled primarily at the level of translation. During heat shock, H2b mRNA is transcribed at about the same level as in the control. However, H2b mRNA is more stable under heat shock than under control conditions and is predominantly found in polysomes. The reduction in synthesis of H2a, H3, and H4 protein during heat shock appears to be controlled at both the transcriptional and translational levels. Although transcription of H2a, H3, and H4 mRNAs is reduced during heat shock, like H2b mRNA, they are more stable. However, unlike H2b mRNA, these mRNAs are not predominantly associated with polysomes during heat shock. Regulation of H1 synthesis during heat shock is completely different from that of the other histones. During heat shock, H1 mRNA is not transcribed, and unlike all of the other Drosophila mRNAs studied to date, its mRNA is not stable in heat-shocked cells. Results from in vitro translation studies support the conclusion that noncoordinate synthesis of the core histone proteins during heat shock is controlled at the level of translation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Ballinger D. G., Pardue M. L. The control of protein synthesis during heat shock in Drosophila cells involves altered polypeptide elongation rates. Cell. 1983 May;33(1):103–113. doi: 10.1016/0092-8674(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Baumbach L. L., Marashi F., Plumb M., Stein G., Stein J. Inhibition of DNA replication coordinately reduces cellular levels of core and H1 histone mRNAs: requirement for protein synthesis. Biochemistry. 1984 Apr 10;23(8):1618–1625. doi: 10.1021/bi00303a006. [DOI] [PubMed] [Google Scholar]
- Biessmann H., Levy B., McCarthy B. J. In vitro transcription of heat-shock-specific RNA from chromatin of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1978 Feb;75(2):759–763. doi: 10.1073/pnas.75.2.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breindl M., Gallwitz D. Effects of cordycepin, hydroxyurea and cycloheximide on histone mRNA synthesis in synchronized HeLa cells. Mol Biol Rep. 1974 Feb;1(5):263–268. doi: 10.1007/BF00417581. [DOI] [PubMed] [Google Scholar]
- Burckhardt J., Birnstiel M. L. Analysis of histone messenger RNA of Drosophila melanogaster by two-dimensional gel electrophoresis. J Mol Biol. 1978 Jan 5;118(1):61–79. doi: 10.1016/0022-2836(78)90244-9. [DOI] [PubMed] [Google Scholar]
- Butler W. B., Mueller G. C. Control of histone synthesis in HeLa cells. Biochim Biophys Acta. 1973 Feb 4;294(1):481–496. doi: 10.1016/0005-2787(73)90104-4. [DOI] [PubMed] [Google Scholar]
- Childs G., Maxson R., Cohn R. H., Kedes L. Orphons: dispersed genetic elements derived from tandem repetitive genes of eucaryotes. Cell. 1981 Mar;23(3):651–663. doi: 10.1016/0092-8674(81)90428-1. [DOI] [PubMed] [Google Scholar]
- Echalier G., Ohanessian A. In vitro culture of Drosophila melanogaster embryonic cells. In Vitro. 1970 Nov-Dec;6(3):162–172. doi: 10.1007/BF02617759. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Saumweber H., Biessmann H. Two Drosophila melanogaster proteins related to intermediate filament proteins of vertebrate cells. J Cell Biol. 1981 Oct;91(1):175–183. doi: 10.1083/jcb.91.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D. Kinetics of inactivation of histone mRNA in the cytoplasm after inhibition of DNA replication in synchronised HeLa cells. Nature. 1975 Sep 18;257(5523):247–248. doi: 10.1038/257247a0. [DOI] [PubMed] [Google Scholar]
- Gillies S., Stollar V. Translation of vesicular stomatitis and Sindbis virus mRNAs in cell-free extracts of Aedes albopictus cells. J Biol Chem. 1981 Dec 25;256(24):13188–13192. [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L., Lifton R. P., Stark G. R., Williams J. G. Isolation of specific RNA's using DNA covalently linked to diazobenzyloxymethyl cellulose or paper. Methods Enzymol. 1979;68:206–220. doi: 10.1016/0076-6879(79)68016-3. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Krüger C., Benecke B. J. In vitro translation of Drosophila heat-shock and non--heat-shock mRNAs in heterologous and homologous cell-free systems. Cell. 1981 Feb;23(2):595–603. doi: 10.1016/0092-8674(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lengyel J. A., Penman S. Differential stability of cytoplasmic RNA in a Drosophila cell line. Dev Biol. 1977 Jun;57(2):243–253. doi: 10.1016/0012-1606(77)90212-3. [DOI] [PubMed] [Google Scholar]
- Lifton R. P., Goldberg M. L., Karp R. W., Hogness D. S. The organization of the histone genes in Drosophila melanogaster: functional and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1047–1051. doi: 10.1101/sqb.1978.042.01.105. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Varying patterns of protein synthesis in Drosophila during heat shock: implications for regulation. Dev Biol. 1980 Jun 15;77(2):463–479. doi: 10.1016/0012-1606(80)90488-1. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Translational control of protein synthesis after infection by vesicular stomatitis virus. J Virol. 1980 Dec;36(3):719–733. doi: 10.1128/jvi.36.3.719-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- McKenzie S. L., Henikoff S., Meselson M. Localization of RNA from heat-induced polysomes at puff sites in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1117–1121. doi: 10.1073/pnas.72.3.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie S. L., Meselson M. Translation in vitro of Drosophila heat-shock messages. J Mol Biol. 1977 Nov 25;117(1):279–283. doi: 10.1016/0022-2836(77)90035-3. [DOI] [PubMed] [Google Scholar]
- Mirault M. E., Goldschmidt-Clermont M., Moran L., Arrigo A. P., Tissières A. The effect of heat shock on gene expression in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):819–827. doi: 10.1101/sqb.1978.042.01.082. [DOI] [PubMed] [Google Scholar]
- O'Connor D., Lis J. T. Two closely linked transcription units within the 63B heat shock puff locus of D. melanogaster display strikingly different regulation. Nucleic Acids Res. 1981 Oct 10;9(19):5075–5092. doi: 10.1093/nar/9.19.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal E. T., Brandhorst B. P., Ruderman J. V. Translationally mediated changes in patterns of protein synthesis during maturation of starfish oocytes. Dev Biol. 1982 May;91(1):215–220. doi: 10.1016/0012-1606(82)90026-4. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Sanders M. M., Groppi V. E., Jr, Browning E. T. Resolution of basic cellular proteins including histone variants by two-dimensional gel electrophoresis: evaluation of lysine to arginine ratios and phosphorylation. Anal Biochem. 1980 Mar 15;103(1):157–165. doi: 10.1016/0003-2697(80)90250-x. [DOI] [PubMed] [Google Scholar]
- Sanders M. M. Identification of histone H2b as a heat-shock protein in Drosophila. J Cell Biol. 1981 Nov;91(2 Pt 1):579–583. doi: 10.1083/jcb.91.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Storti R. V., Pardue M. L., Rich A. Cell-free protein synthesis in lysates of Drosophila melanogaster cells. Biochemistry. 1979 Apr 17;18(8):1588–1594. doi: 10.1021/bi00575a032. [DOI] [PubMed] [Google Scholar]
- Spradling A., Hui H., Penman S. Two very different components of messenger RNA in an insect cell line. Cell. 1975 Feb;4(2):131–137. doi: 10.1016/0092-8674(75)90119-1. [DOI] [PubMed] [Google Scholar]
- Spradling A., Pardue M. L., Penman S. Messenger RNA in heat-shocked Drosophila cells. J Mol Biol. 1977 Feb 5;109(4):559–587. doi: 10.1016/s0022-2836(77)80091-0. [DOI] [PubMed] [Google Scholar]
- Spradling A., Penman S., Pardue M. L. Analysis of drosophila mRNA by in situ hybridization: sequences transcribed in normal and heat shocked cultured cells. Cell. 1975 Apr;4(4):395–404. doi: 10.1016/0092-8674(75)90160-9. [DOI] [PubMed] [Google Scholar]
- Stahl H., Gallwitz D. Fate of histone messenger RNA in synchronized HeLa cells in the absence of initiation of protein synthesis. Eur J Biochem. 1977 Jan;72(2):385–392. doi: 10.1111/j.1432-1033.1977.tb11263.x. [DOI] [PubMed] [Google Scholar]
- Stimac E., Groppi V. E., Coffino P. Increased histone mRNA levels during inhibition of protein synthesis. Biochem Biophys Res Commun. 1983 Jul 18;114(1):131–137. doi: 10.1016/0006-291x(83)91604-2. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Tanguay R. M., Camato R., Lettre F., Vincent M. Expression of histone genes during heat shock and in arsenite-treated Drosophila Kc cells. Can J Biochem Cell Biol. 1983 Jun;61(6):414–420. doi: 10.1139/o83-056. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]