Abstract
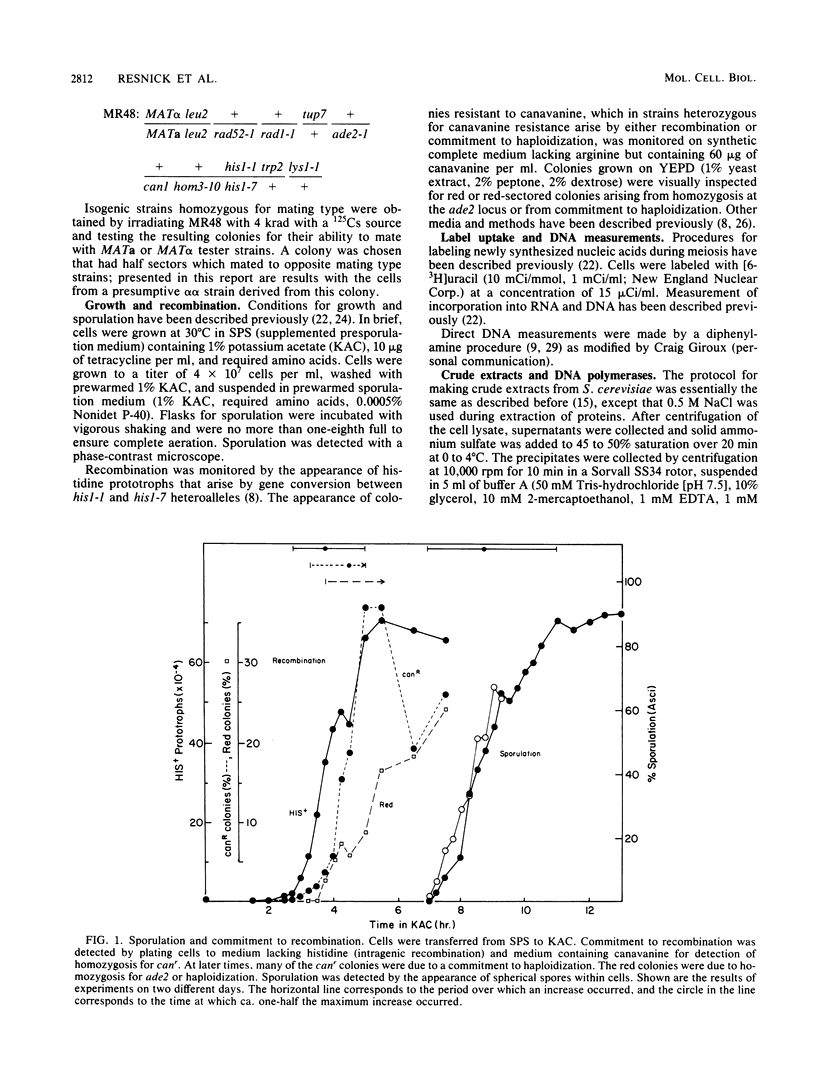
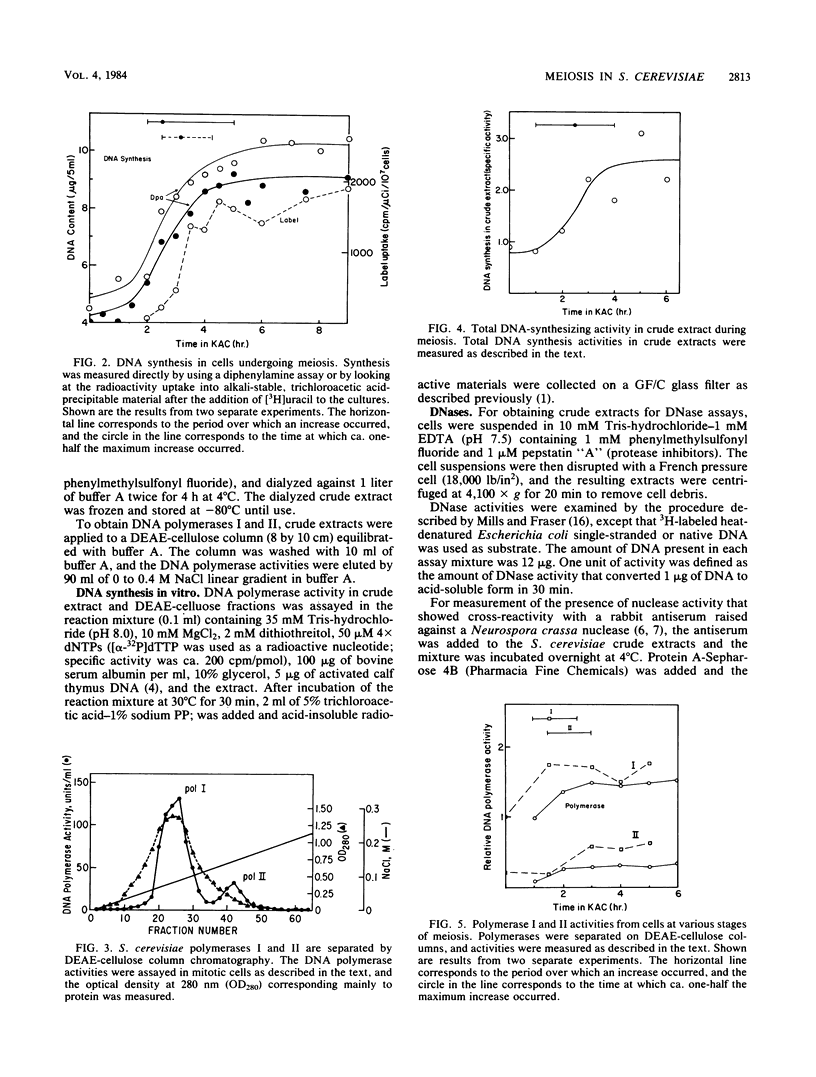
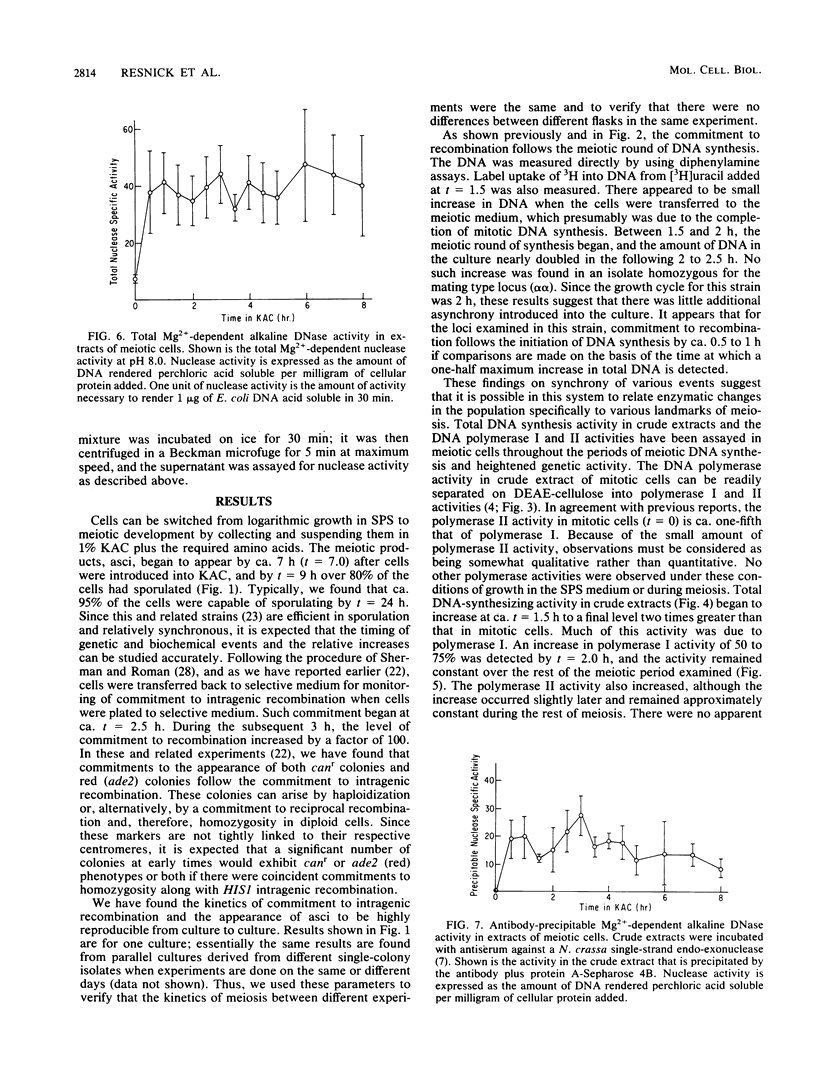
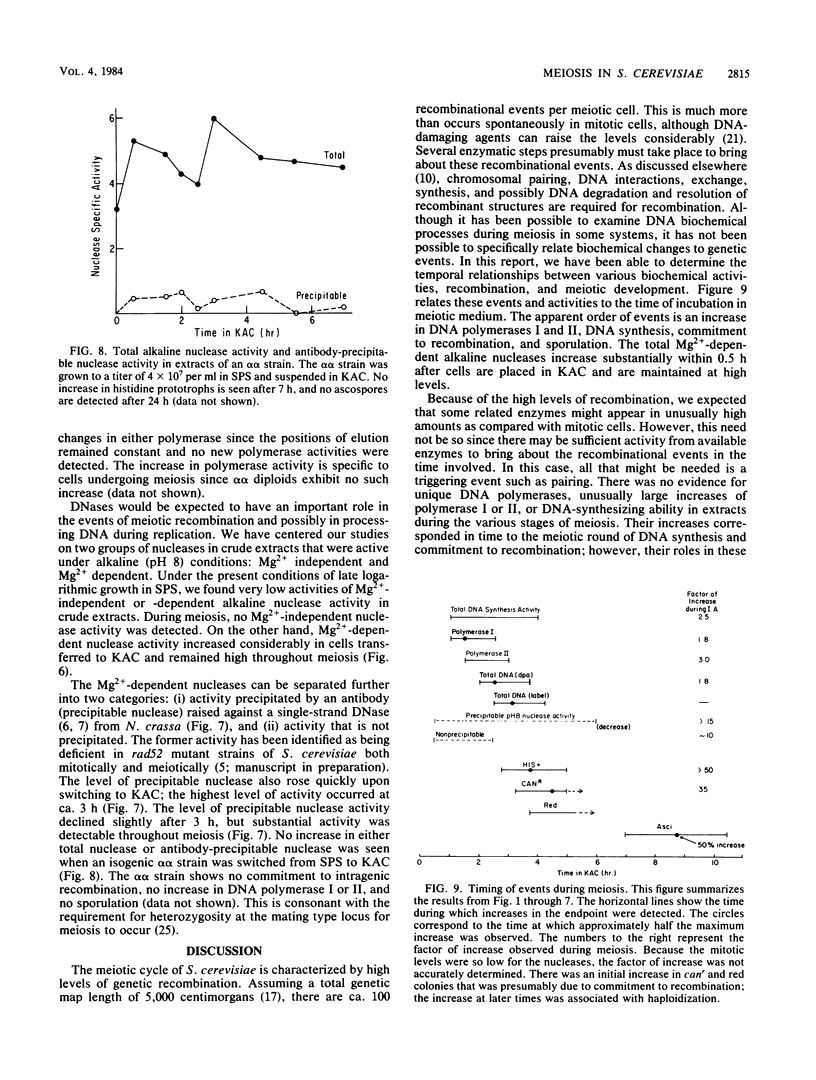
We utilized strains of Saccharomyces cerevisiae that exhibit high efficiency of synchrony of meiosis to examine several aspects of meiosis including sporulation, recombination, DNA synthesis, DNA polymerase I and II, and Mg2+-dependent alkaline DNases. The kinetics of commitment to intragenic recombination and sporulation are similar. The synthesis of DNA, as measured directly with diphenylamine, appears to precede the commitment to recombination. Both DNA polymerase I and II activities and total DNA-synthesizing activity in crude extracts increase two- to threefold before the beginning of meiotic DNA synthesis. Increases of 10- to 20-fold over mitotic levels are found for Mg2+-dependent alkaline DNase activity in crude extracts before and during the commitment to meiotic intragenic recombination. Of particular interest is the comparable increase in a nuclease under the control of the RAD52 gene; this enzyme has been identified by the use of antibody raised against a similar enzyme from Neurospora crassa. Since the RAD52 gene is essential for meiotic recombination, the nuclease is implicated in the high levels of recombination observed during meiosis. The effects observed in this report are meiosis specific since they are not observed in an alpha alpha strain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arendes J., Kim K. C., Sugino A. Yeast 2-microns plasmid DNA replication in vitro: purification of the CDC8 gene product by complementation assay. Proc Natl Acad Sci U S A. 1983 Feb;80(3):673–677. doi: 10.1073/pnas.80.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. S., Carpenter A. T., Esposito M. S., Esposito R. E., Sandler L. The genetic control of meiosis. Annu Rev Genet. 1976;10:53–134. doi: 10.1146/annurev.ge.10.120176.000413. [DOI] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Electron microscopic observations on the meiotic karyotype of diploid and tetraploid Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5056–5060. doi: 10.1073/pnas.72.12.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. M. DNA polymerases from bakers' yeast. J Biol Chem. 1977 Mar 25;252(6):1873–1880. [PubMed] [Google Scholar]
- Chow T. Y., Fraser M. J. Purification and properties of single strand DNA-binding endo-exonuclease of Neurospora crassa. J Biol Chem. 1983 Oct 10;258(19):12010–12018. [PubMed] [Google Scholar]
- Chow T. Y., Fraser M. J. The major intracellular alkaline deoxyribonuclease activities expressed in wild-type and Rec-like mutants of Neurospora crassa. Can J Biochem. 1979 Jun;57(6):889–901. doi: 10.1139/o79-109. [DOI] [PubMed] [Google Scholar]
- Game J. C., Zamb T. J., Braun R. J., Resnick M., Roth R. M. The Role of Radiation (rad) Genes in Meiotic Recombination in Yeast. Genetics. 1980 Jan;94(1):51–68. doi: 10.1093/genetics/94.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Halvorson H. O. Methods in sporulation and germination of yeasts. Methods Cell Biol. 1975;11:45–69. doi: 10.1016/s0091-679x(08)60316-7. [DOI] [PubMed] [Google Scholar]
- Holliday R. Recombination and meiosis. Philos Trans R Soc Lond B Biol Sci. 1977 Mar 21;277(955):359–370. doi: 10.1098/rstb.1977.0024. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Stern H. DNA unwinding protein from meiotic cells of Lilium. Biochemistry. 1978 May 16;17(10):1872–1880. doi: 10.1021/bi00603a011. [DOI] [PubMed] [Google Scholar]
- Howell S. H., Stern H. The appearance of DNA breakage and repair activities in the synchronous meiotic cycle of Lilium. J Mol Biol. 1971 Feb 14;55(3):357–378. doi: 10.1016/0022-2836(71)90323-8. [DOI] [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo H., Greenberg B. D., Sugino A. Yeast 2-micrometer plasmid DNA replication in vitro: origin and direction. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7261–7265. doi: 10.1073/pnas.78.12.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C., Fraser M. J. Different chromatographic forms of Neurospora crassa nucleases specific for single-stranded nucleic acids. Can J Biochem. 1973 Jun;51(6):888–895. doi: 10.1139/o73-110. [DOI] [PubMed] [Google Scholar]
- Mortimer R. K., Schild D. Genetic map of Saccharomyces cerevisiae. Microbiol Rev. 1980 Dec;44(4):519–571. doi: 10.1128/mr.44.4.519-571.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Prakash L., Burke W., Montelone B. A. Effects of the RAD52 Gene on Recombination in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Jan;94(1):31–50. doi: 10.1093/genetics/94.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukkila P. J. Biochemical analysis of genetic recombination in eukaryotes. Heredity (Edinb) 1977 Oct;39(2):193–217. doi: 10.1038/hdy.1977.61. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Game J. C., Stasiewicz S. Genetic effects of UV irradiation on excision-proficient and -deficient yeast during meiosis. Genetics. 1983 Aug;104(4):603–618. doi: 10.1093/genetics/104.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Kasimos J. N., Game J. C., Braun R. J., Roth R. M. Changes in DNA during meiosis in a repair-deficient mutant (rad 52) of yeast. Science. 1981 May 1;212(4494):543–545. doi: 10.1126/science.7010606. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Martin P. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol Gen Genet. 1976 Jan 16;143(2):119–129. doi: 10.1007/BF00266917. [DOI] [PubMed] [Google Scholar]
- Resnick M. A. The repair of double-strand breaks in chromosomal DNA of yeast. Basic Life Sci. 1975;5B:549–556. doi: 10.1007/978-1-4684-2898-8_20. [DOI] [PubMed] [Google Scholar]
- Roman H., Sands S. M. Heterogeneity of Clones of Saccharomyces Derived from Haploid Ascospores. Proc Natl Acad Sci U S A. 1953 Mar;39(3):171–179. doi: 10.1073/pnas.39.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. Temperature-sensitive yeast mutants defective in meiotic recombination and replication. Genetics. 1976 Aug;83(4):675–686. doi: 10.1093/genetics/83.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERMAN F., ROMAN H. Evidence for two types of allelic recombination in yeast. Genetics. 1963 Feb;48:255–261. doi: 10.1093/genetics/48.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi K., Lu B. C. Meiosis in Coprinus: characterization and activities of two forms of DNA polymerase during meiotic stages. Mol Cell Biol. 1982 Jul;2(7):752–757. doi: 10.1128/mcb.2.7.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamb T. J., Roth R. Role of mitotic replication genes in chromosome duplication during meiosis. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3951–3955. doi: 10.1073/pnas.74.9.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]



