Abstract
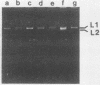
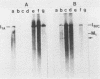
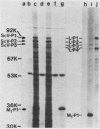
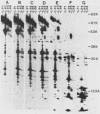
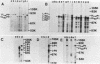
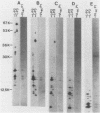
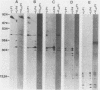
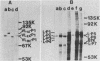
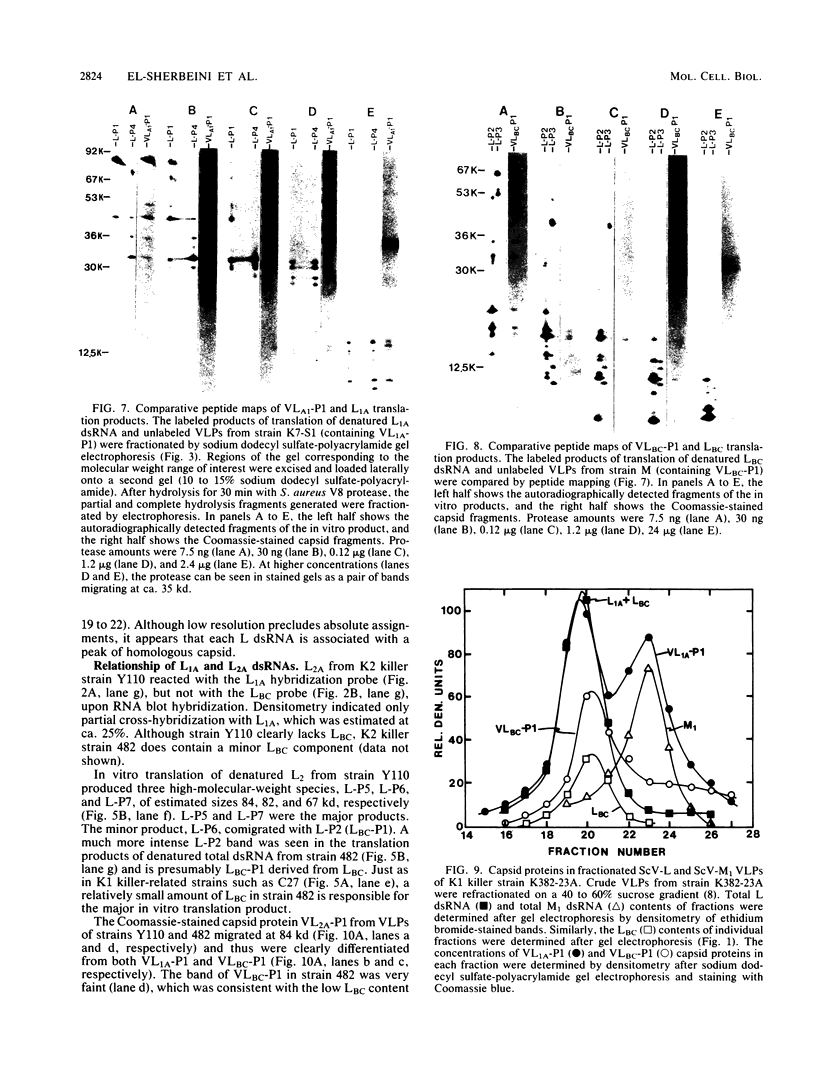
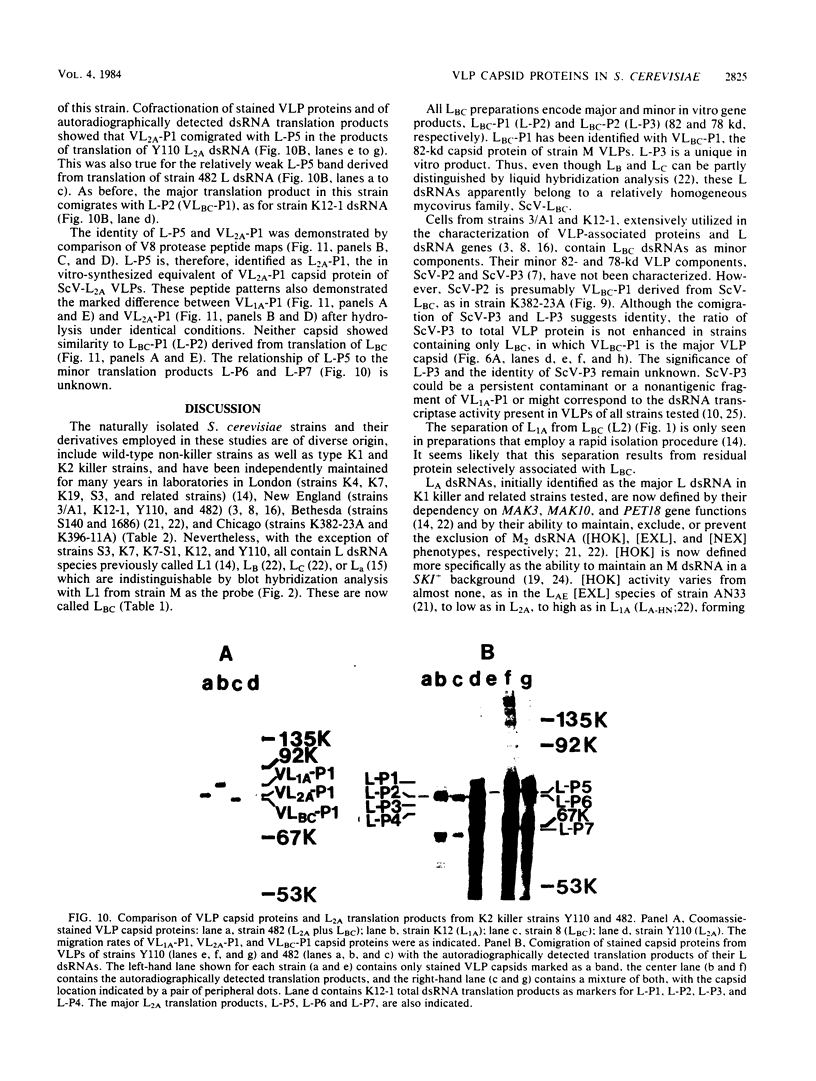
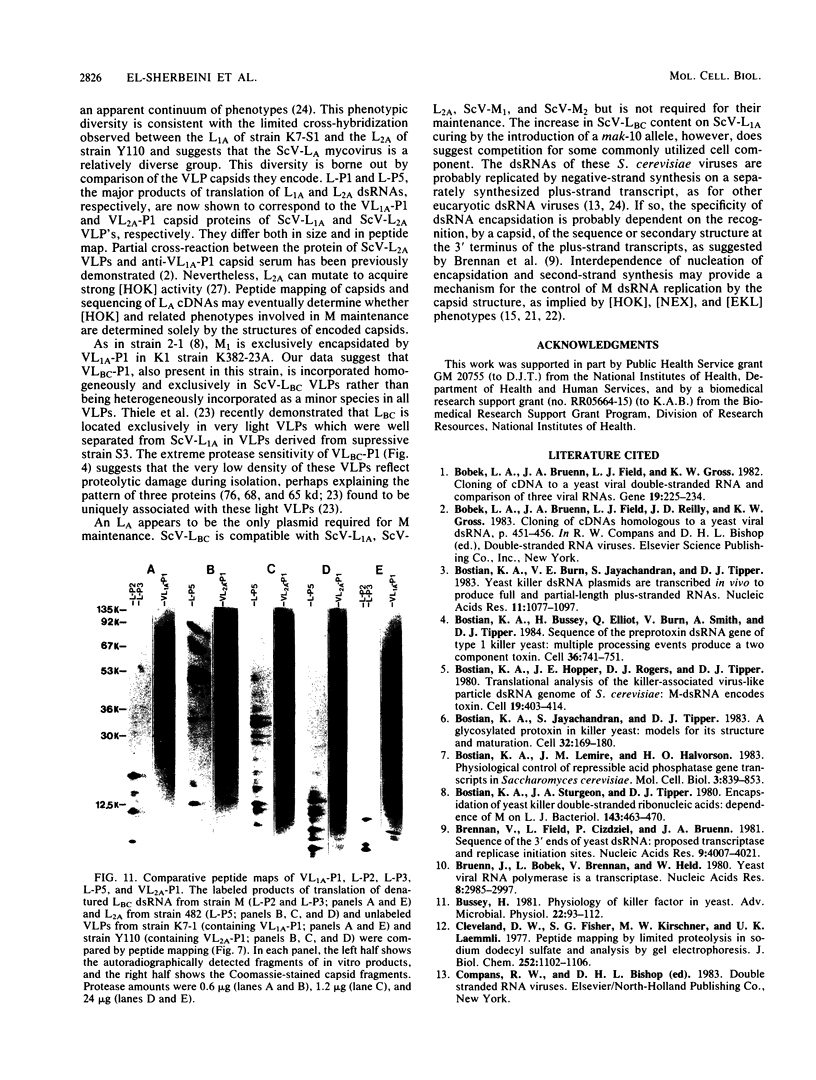
The plasmid determinants of killer phenotypes in type K1 and K2 killer yeast cells are the 1.9-kilobase (kb) M1 and 1.7-kb M2 double-stranded RNAs (dsRNAs), respectively. These are dependent for their maintenance and encapsidation, in Saccharomyces cerevisiae virus ScV-M1 or ScV-M2 virus-like particles, on the capsid provided by one of a group of moderately related 4.7-kb dsRNAs called LA. The L1A and L2A dsRNAs found in naturally isolated K1 and K2 killers encode 88-kilodalton VL1A-P1 and 86-kilodalton VL2A-P1 capsids, respectively. These are competent for encapsidating homologous LA dsRNAs as well as M dsRNAs. Most strains of S. cerevisiae, including killers, contain one of a second group of closely related 4.7-kb dsRNAs called LBC. These encode their own 82-kilodalton capsid protein, VLBC-P1, which, at least in strains containing only LBC, encapsidates homologous dsRNA in ScV-LBC virus-like particles. In a K1 killer strain containing both L1A and LBC, ScV-M1 particles contain only VL1A-P1. In such strains it is probable that each virus-like particle contains a single capsid type and that each L dsRNA is encapsidated by a homologous capsid.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan D. R., Archer D., Donati F., Ferguson A., Higgs B. D. Antagonism of pancuronium in renal failure: no recurarization. Br J Anaesth. 1982 Jan;54(1):63–68. doi: 10.1093/bja/54.1.63. [DOI] [PubMed] [Google Scholar]
- Bobek L. A., Bruenn J. A., Field L. J., Gross K. W. Cloning of cDNA to a yeast viral double-stranded RNA and comparison of three viral RNAs. Gene. 1982 Sep;19(2):225–230. doi: 10.1016/0378-1119(82)90010-5. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Burn V. E., Jayachandran S., Tipper D. J. Yeast killer dsRNA plasmids are transcribed in vivo to produce full and partial-length plus-stranded RNAs. Nucleic Acids Res. 1983 Feb 25;11(4):1077–1097. doi: 10.1093/nar/11.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Elliott Q., Bussey H., Burn V., Smith A., Tipper D. J. Sequence of the preprotoxin dsRNA gene of type I killer yeast: multiple processing events produce a two-component toxin. Cell. 1984 Mar;36(3):741–751. doi: 10.1016/0092-8674(84)90354-4. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Jayachandran S., Tipper D. J. A glycosylated protoxin in killer yeast: models for its structure and maturation. Cell. 1983 Jan;32(1):169–180. doi: 10.1016/0092-8674(83)90507-x. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Lemire J. M., Halvorson H. O. Physiological control of repressible acid phosphatase gene transcripts in Saccharomyces cerevisiae. Mol Cell Biol. 1983 May;3(5):839–853. doi: 10.1128/mcb.3.5.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Sturgeon J. A., Tipper D. J. Encapsidation of yeast killer double-stranded ribonucleic acids: dependence of M on L. J Bacteriol. 1980 Jul;143(1):463–470. doi: 10.1128/jb.143.1.463-470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan V. E., Field L., Cizdziel P., Bruenn J. A. Sequences at the 3' ends of yeast viral dsRNAs: proposed transcriptase and replicase initiation sites. Nucleic Acids Res. 1981 Aug 25;9(16):4007–4021. doi: 10.1093/nar/9.16.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J., Bobek L., Brennan V., Held W. Yeast viral RNA polymerase is a transcriptase. Nucleic Acids Res. 1980 Jul 11;8(13):2985–2997. doi: 10.1093/nar/8.13.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Field L. J., Bobek L. A., Brennan V. E., Reilly J. D., Bruenn J. A. There are at least two yeast viral double-stranded RNAs of the same size: an explanation for viral exclusion. Cell. 1982 Nov;31(1):193–200. doi: 10.1016/0092-8674(82)90419-6. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Palfree R. G., Bussey H. Yeast killer toxin: purification and characterisation of the protein toxin from Saccharomyces cerevisiae. Eur J Biochem. 1979 Feb 1;93(3):487–493. doi: 10.1111/j.1432-1033.1979.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ridley S. P., Sommer S. S., Wickner R. B. Superkiller mutations in Saccharomyces cerevisiae suppress exclusion of M2 double-stranded RNA by L-A-HN and confer cold sensitivity in the presence of M and L-A-HN. Mol Cell Biol. 1984 Apr;4(4):761–770. doi: 10.1128/mcb.4.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Co-curing of plasmids affecting killer double-stranded RNAs of Saccharomyces cerevisiae: [HOK], [NEX], and the abundance of L are related and further evidence that M1 requires L. J Bacteriol. 1982 May;150(2):545–551. doi: 10.1128/jb.150.2.545-551.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Yeast L dsRNA consists of at least three distinct RNAs; evidence that the non-Mendelian genes [HOK], [NEX] and [EXL] are on one of these dsRNAs. Cell. 1982 Dec;31(2 Pt 1):429–441. doi: 10.1016/0092-8674(82)90136-2. [DOI] [PubMed] [Google Scholar]
- Thiele D. J., Hannig E. M., Leibowitz M. J. Multiple L double-stranded RNA species of Saccharomyces cerevisiae: evidence for separate encapsidation. Mol Cell Biol. 1984 Jan;4(1):92–100. doi: 10.1128/mcb.4.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Bostian K. A. Double-stranded ribonucleic acid killer systems in yeasts. Microbiol Rev. 1984 Jun;48(2):125–156. doi: 10.1128/mr.48.2.125-156.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh D., Leibowitz M. J. Transcription of killer virion double-stranded RNA in vitro. Nucleic Acids Res. 1980 Jun 11;8(11):2365–2375. doi: 10.1093/nar/8.11.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Killer systems in Saccharomyces cerevisiae: three distinct modes of exclusion of M2 double-stranded RNA by three species of double-stranded RNA, M1, L-A-E, and L-A-HN. Mol Cell Biol. 1983 Apr;3(4):654–661. doi: 10.1128/mcb.3.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. R., Bevan E. A. Studies on the nature of the killer factor produced by Saccharomyces cerevisiae. J Gen Microbiol. 1968 Apr;51(1):115–126. doi: 10.1099/00221287-51-1-115. [DOI] [PubMed] [Google Scholar]