Abstract
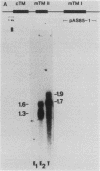
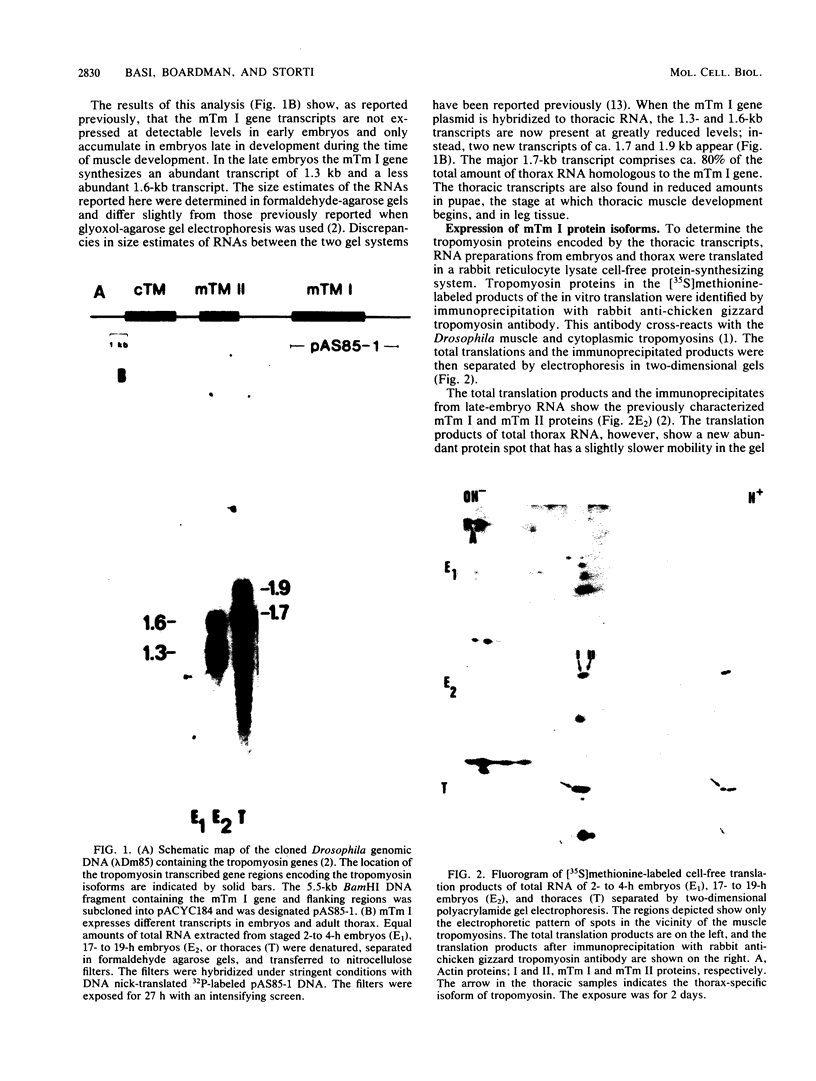
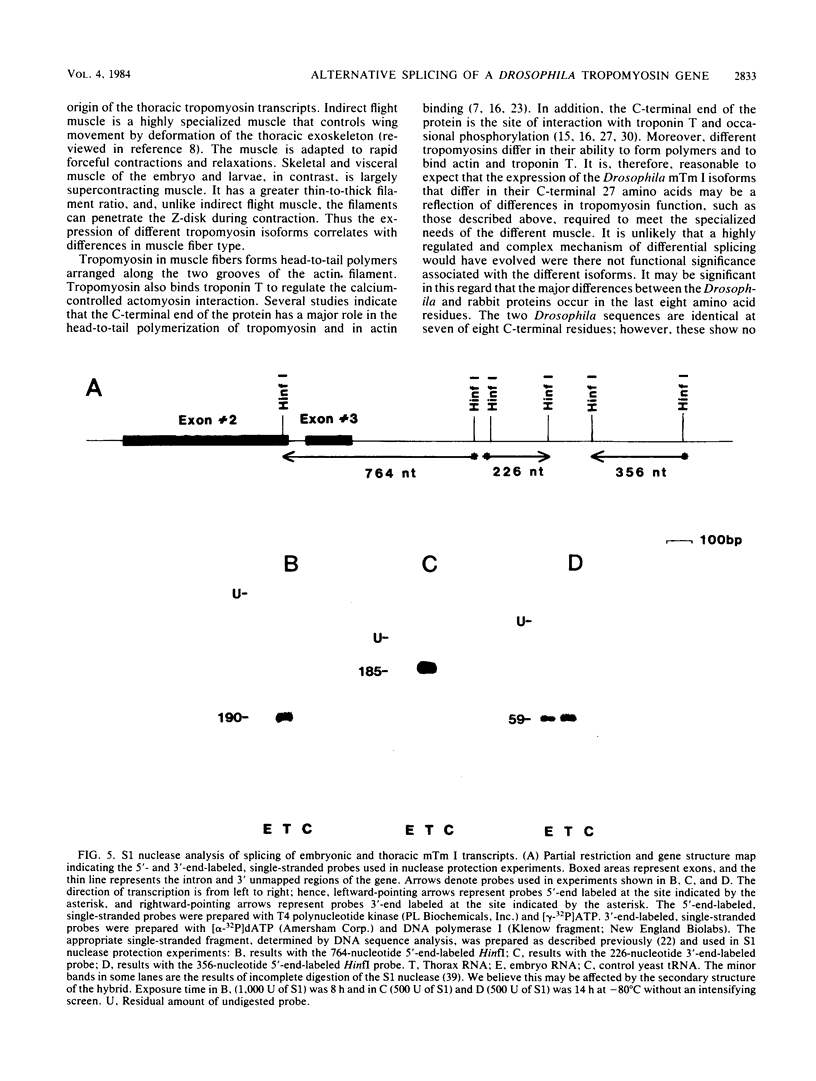
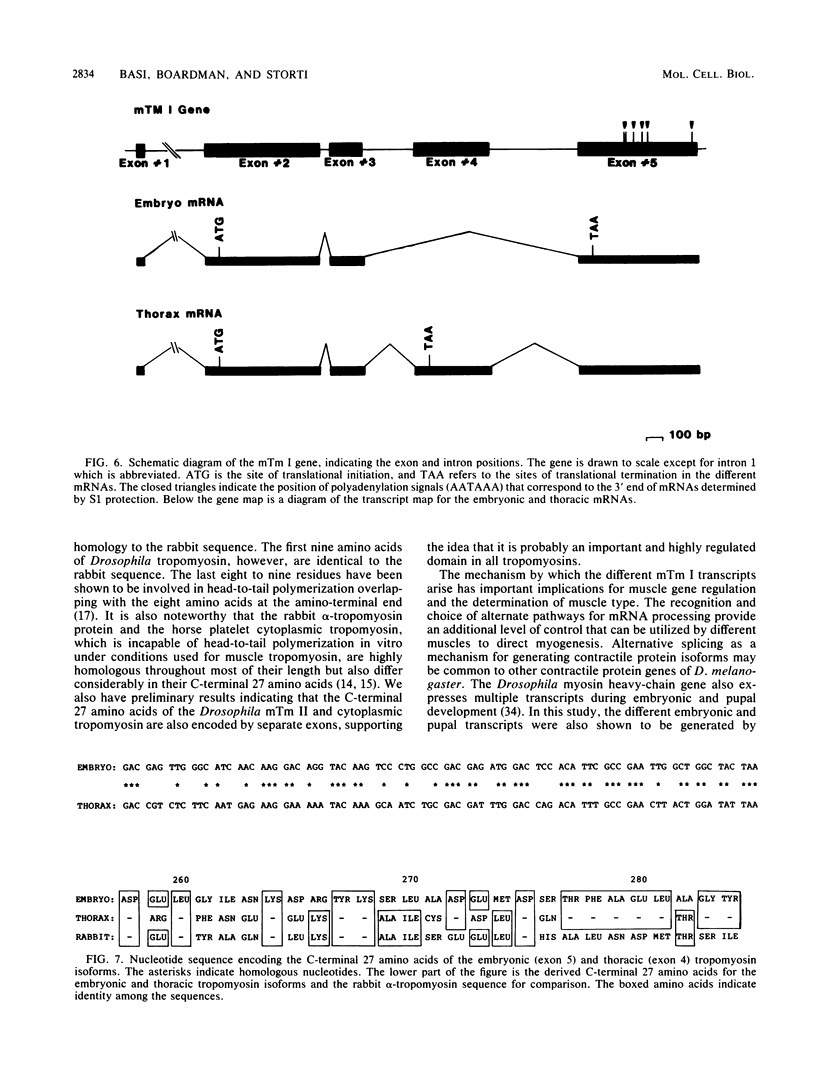
The muscle tropomyosin I (mTm I) gene from Drosophila melanogaster has been analyzed and shown to express a complex transcription unit consisting of two sets of tissue-specific mRNAs. A 1.3- and 1.6-kilobase set of mRNAs is expressed during myogenesis in embryos, and in myogenic cell cultures. The mRNAs encode a 34,000-dalton muscle tropomyosin isoform. The same mTm I gene expresses a different set of 1.7- and 1.9-kilobase mRNAs in thoracic flight muscle tissue of the adult. The thorax RNAs encode a new tropomyosin isoform resolved on two-dimensional gels. The structure of the gene has been determined, and we show that the embryonic and thoracic mRNAs are generated by alternative splicing. The alternate exon splicing patterns determine a different 27 amino acids at the carboxy-terminal end of the two tropomyosin isoforms. These results show that the carboxy-terminal domain of tropomyosin is highly regulated in determining tropomyosin function. The results also show that contractile protein isoforms can be generated by single as well as multiple genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bautch V. L., Storti R. V. Identification of a cytoplasmic tropomyosin gene linked to two muscle tropomyosin genes in Drosophila. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7123–7127. doi: 10.1073/pnas.80.23.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautch V. L., Storti R. V., Mischke D., Pardue M. L. Organization and expression of Drosophila tropomyosin genes. J Mol Biol. 1982 Dec 5;162(2):231–250. doi: 10.1016/0022-2836(82)90524-1. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Bernstein S. I., Mogami K., Donady J. J., Emerson C. P., Jr Drosophila muscle myosin heavy chain encoded by a single gene in a cluster of muscle mutations. 1983 Mar 31-Apr 6Nature. 302(5907):393–397. doi: 10.1038/302393a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Côté G. P., Smillie L. B. The interaction of equine platelet tropomyosin with skeletal muscle actin. J Biol Chem. 1981 Jul 25;256(14):7257–7261. [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Henikoff S., Sloan J. S., Kelly J. D. A Drosophila metabolic gene transcript is alternatively processed. Cell. 1983 Sep;34(2):405–414. doi: 10.1016/0092-8674(83)90374-4. [DOI] [PubMed] [Google Scholar]
- Karlik C. C., Mahaffey J. W., Coutu M. D., Fyrberg E. A. Organization of contractile protein genes within the 88F subdivision of the D. melanogaster third chromosome. Cell. 1984 Jun;37(2):469–481. doi: 10.1016/0092-8674(84)90377-5. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lewis W. G., Cote G. P., Mak A. S., Smillie L. B. Amino acid sequence of equine platelet tropomyosin. Correlation with interaction properties. FEBS Lett. 1983 Jun 13;156(2):269–273. doi: 10.1016/0014-5793(83)80511-0. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Golosinska K., Smillie L. B. Induction of nonpolymerizable tropomyosin binding to F-actin by troponin and its components. J Biol Chem. 1983 Dec 10;258(23):14330–14334. [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B. Non-polymerizable tropomyosin: preparation, some properties and F-actin binding. Biochem Biophys Res Commun. 1981 Jul 16;101(1):208–214. doi: 10.1016/s0006-291x(81)80032-0. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B., Stewart G. R. A comparison of the amino acid sequences of rabbit skeletal muscle alpha- and beta-tropomyosins. J Biol Chem. 1980 Apr 25;255(8):3647–3655. [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B. Structural interpretation of the two-site binding of troponin on the muscle thin filament. J Mol Biol. 1981 Jul 5;149(3):541–550. doi: 10.1016/0022-2836(81)90486-1. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Bandman E., Strohman R. C. Regional differences in the expression of myosin light chains and tropomyosin subunits during development of chicken breast muscle. Dev Biol. 1983 Feb;95(2):484–491. doi: 10.1016/0012-1606(83)90050-7. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Yamashiro-Matsumura S., Lin J. J. Isolation and characterization of tropomyosin-containing microfilaments from cultured cells. J Biol Chem. 1983 May 25;258(10):6636–6644. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M. Tropomyosin coiled-coil interactions: evidence for an unstaggered structure. J Mol Biol. 1975 Oct 25;98(2):293–304. doi: 10.1016/s0022-2836(75)80119-7. [DOI] [PubMed] [Google Scholar]
- Mogami K., Hotta Y. Isolation of Drosophila flightless mutants which affect myofibrillar proteins of indirect flight muscle. Mol Gen Genet. 1981;183(3):409–417. doi: 10.1007/BF00268758. [DOI] [PubMed] [Google Scholar]
- Montarras D., Fiszman M. Y., Gros F. Characterization of the tropomyosin present in various chick embryo muscle types and in muscle cells differentiated in vitro. J Biol Chem. 1981 Apr 25;256(8):4081–4086. [PubMed] [Google Scholar]
- Montgomery K., Mak A. S. In vitro phosphorylation of tropomyosin by a kinase from chicken embryo. J Biol Chem. 1984 May 10;259(9):5555–5560. [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Pearlstone J. R., Smillie L. B. Binding of troponin-T fragments to several types of tropomyosin. Sensitivity to Ca2+ in the presence of troponin-C. J Biol Chem. 1982 Sep 25;257(18):10587–10592. [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Berk A. J., Berget S. M. Transcription maps of adenovirus. Methods Enzymol. 1980;65(1):750–768. doi: 10.1016/s0076-6879(80)65071-x. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Szwast A. E. Molecular cloning and characterization of Drosophila genes and their expression during embryonic development and in primary muscle cell cultures. Dev Biol. 1982 Apr;90(2):272–283. doi: 10.1016/0012-1606(82)90376-1. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin S. L., Zulauf E., Sánchez F., Craig E. A., McCarthy B. J. Multiple actin-related sequences in the Drosophila melanogaster genome. Cell. 1980 Jan;19(1):121–131. doi: 10.1016/0092-8674(80)90393-1. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]