Abstract
Management of hepatocellular carcinoma (HCC) has been continuously evolving during recent years. HCC is a worldwide clinical and social issue and typically a complicates cirrhosis. The incidence of HCC is increasing, not only in the general population of patients with cirrhosis, but particularly in some subgroups of patients, like those with human immunodeficiency virus infection or thalassemia. Since a 3% annual HCC incidence has been estimated in cirrhosis, a bi-annual screening is generally suggested. The diagnostic criteria of HCC has recently had a dramatic evolution during recent years. HCC diagnosis is now made only on radiological criteria in the majority of the cases. In the context of cirrhosis, the universally accepted criteria for HCC diagnosis is contrast enhancement in arterial phase and washout in venous/late phase at imaging, the so called “typical pattern”. However, recently updated guidelines slightly differ in diagnostic criteria. Apart from liver transplantation, the only cure of both HCC and underlying liver cirrhosis, all the other treatments have to match with higher rate of HCC recurrence. The latter can be classified into curative (resection and percutaneous ablation) and palliative treatments. The aim of this paper was to review the current knowledge on management of HCC and to enlighten the areas of uncertainty.
Keywords: Hepatocellular carcinoma, Management, Trans-arterial chemoembolization, Orthotopic liver transplantation, Surgery, Management
Core tip: Management of hepatocellular carcinoma (HCC) has been continuously evolving during recent years. The aim of this paper was to review the current knowledge on management of HCC and to enlighten the areas of uncertainty.
INTRODUCTION
Management of hepatocellular carcinoma (HCC) has been continuously evolving during recent years. However, albeit overall similar, the main Societies of Hepatology differently defined their own guidelines. In fact, despite an evident agreement in the general lines of HCC management, some gray zones still persist[1-4]. The aim of this paper was to review the current knowledge on management of HCC and to enlighten the areas of uncertainty.
Epidemiology
HCC is a worldwide clinical and social issue. Actually, it is the sixth most common cancer and the third cause of cancer-related death. Incidence increases with advancing age, with a median age at onset of about 70 year-old in developed countries and there is a male preponderance, with a male to female ratio of about 2.4[5,6].
HCC is typically a complication of cirrhosis, although it can rarely develop in the absence of cirrhosis[1-4]. Known underlying diseases at risk for HCC development are chronic viral hepatitis C and B, alcoholic hepatitis, non- alcoholic fatty liver disease, autoimmune hepatitis, hemochromatosis, alpha-1-antitrypsin deficiency, Wilson’s disease, Budd-Chiari syndrome (BCS). Diagnostic algorithm follows the same radiological criteria for HCC (see above) despite the different aetiologies of underlying liver disease. However, an exception is HCC developing in BCS. In fact, the radiological pattern of regenerative nodules in BCS is similar to that of HCC[7]. Moreover, as a consequence of the hindered hepatic venous outflow, radiological criteria for HCC can be altered[8,9]. Finally, although the risk of procedure-related bleeding is probably increased[10], generally diagnosis of HCC in BCS still needs histological confirmation[8,9].
Overall, the incidence of HCC is increasing, not only in the general population of patients with cirrhosis[11,12], but particularly in some subgroups of patients, like those with human immunodeficiency virus (HIV) infection or thalassemia. In fact, in both HIV and thalassemia, a recent significant outcome improvement due to, respectively, iron chelating drugs in the latter and highly active antiretroviral therapy in the former, has allowed the appearance of the complication of the underlying hepatic disease[13-18].
Screening and recall policy
Surveillance of HCC is important simply because as little is HCC size at diagnosis as high is the probability of curative treatment. Since a 3% annual HCC incidence has been estimated in cirrhosis[19], a bi-annual screening is generally suggested[1-4]. Although less stringent, there is a risk of HCC development also in non-cirrhotic chronic hepatitis and treated viral hepatitis, for which an annual screening is generally performed[1-4]. Ultrasound (US) is the test of choice for surveillance. In fact, US is a cheap and safe test, due to the absence of contrast medium and radioactivity[20-23]. Moreover, differently from computed tomography (CT) and magnetic resonance (MR), US does not expose to the risk of false positive results. However, CT or MR should be preferred for surveillance of patients for which US is not adequate because of technical reasons or in liver transplantation (LT) waiting list[1-4].
Once hepatic nodules are discovered at US screening, the further work-up depends on the size[1-4]. Nodules < 1 cm should prompt a strict US surveillance every 3 or 4 mo for the first year and, in the absence of size increase, every six months after one year, like patients with cirrhosis without liver nodules. In fact, hepatic nodules < 1 cm rarely are HCC. Moreover, for such little size nodules, diagnostic value of either CT and MR is inadequate.
Nodules between 1 and 2 cm in size should be studied with CT and MR and, in case of non diagnostic imaging, undergo nodule biopsy. Moreover, a second biopsy should be contemplated in case of inconclusive findings at histology, nodule growth or change in enhancement pattern.
Nodules > 2 cm should undergo CT and MR and, in case of atypical imaging findings, nodule biopsy[1-4].
HCC diagnosis update
The diagnostic criteria of HCC has recently had a dramatic evolution during recent years[1-4,24]. In fact, till the first half of the past decade, the Golden Standard for HCC diagnosis was histology. Then, since radiological diagnostic criteria have been shaped and established[1], HCC diagnosis is now made only on radiological criteria in the majority of the cases[1-4]. In the context of cirrhosis, the universally accepted criteria for HCC diagnosis is contrast enhancement in arterial phase and washout in venous/late phase at imaging, the so called “typical pattern” (Figures 1-3). However, recently updated guidelines slightly differ in diagnostic criteria[2-4].
Figure 1.
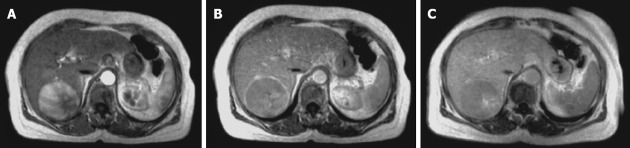
Typical hepatocellular carcinoma at magnetic resonance imaging: contrast enhancement in arterial phase (A) and washout in venous (B)/late (C) phase.
Figure 3.
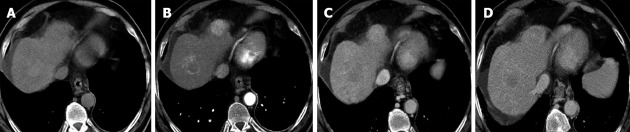
A new typical hepatocellular carcinoma found at computed tomography during follow up another hepatocellular carcinoma treated with radiofrequency ablation (central lesion). Note that also in the treated lesion there is a marginal area of vital hepatocellular carcinoma.
Figure 2.
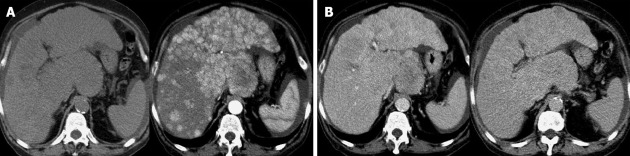
Multi-nodular hepatocellular carcinoma. A: Basal and arterial computed tomography (CT) phase; B: Venous and late CT phase.
Hepatic lesions > 1 cm with typical pattern at one imaging (CT or MR) have diagnosis of HCC in both the American Association for the Study of Liver Disease (AASLD) 2011 and European Association for the Study of the Liver (EASL) 2012 guidelines[2,4]. However, EASL 2012 guidelines specify that for lesions between 1 and 2 cm the concordance of typical pattern at two imaging (CT and MR) should be advices in suboptimal settings (technology, local skills)[4]. Both AASLD 2011 and EASL 2012 guidelines suggest lesion biopsy in the absence of typical pattern. Finally, both AASLD 2011 and EASL 2012 guidelines exclude from diagnostic criteria contrast-enhanced US (CEUS) and alpha-fetoprotein value[2,4].
Japanese Society of Hepatology (JSH) 2012 guidelines agree with the diagnostic value of typical pattern at one imaging (CT, MR, CEUS) regardless of the size, and maintains the diagnostic value of CEUS and biochemistry (AFP > 200 ng/dL, PIVKA-II > 40, AFP L3 > 15%)[3]. However, the main difference between JSH 2012 and both AASLD 2011 and EASL 2012 guidelines is that the former consider a diagnostic criteria the lesion hypo-intensity in hepatic image of Gd-EOB-MR[2-4].
HCC treatment
Apart from LT, the only cure of both HCC and underlying liver cirrhosis, all the other treatments have to match with higher rate of HCC recurrence. The latter can be classified into curative (resection and percutaneous ablation) and palliative treatments[2-4].
Resection is considered the First-Line treatment for patients with solitary tumours and preserved liver function (normal bilirubin and, either HVPG ≤ 10 mmHg, PLT > 100000 or no varices at endoscopy). Resection can also be performed for multi-focal HCC inside Milan criteria or in case of mild portal hypertension when patients are not suitable for orthotopic LT (OLT), although it is debated if such patients could benefit from other locoregional therapies, avoiding the risk of surgery and of liver de-compensation after surgery. In fact, perioperative mortality in cirrhotics after HCC resection is about 2%-3%. Moreover, there is a risk of tumor recurrence after surgery of about 70% at 5 years, enclosing both true recurrence and the novo tumours[25-39].
Percutaneous local ablation, namely radiofrequency ablation (RFA) and ethanol injection (EI) are the standard of care for BCLC O-A not suitable for surgery. Although RFA is recommended in most instances as the main ablative therapy in tumors less than 5 cm, the probability of complete necrosis is very high for little tumors (< 2 cm) and progressively decreases with the increase of tumor size. In tumours ≤ 2 cm both RF and EI achieve complete responses in more than 90%, making percutaneous local ablation competitive with resection. EI is better indicated when RFA in not technically feasible. This is the case of tumors overflowing the liver margin or near organs, just like gallbladder or bowel, because of the risk of thermal injury and perforation, or when tumor is adjacent to a main vessel, because of the concern of thermal dispersion and inefficacy of RFA[40-64].
Trans-arterial chemoembolization (TACE) is the recommended treatment for BCLC stage B multinodular asymptomatic tumors without vascular invasion or extrahepatic spread. Drug-eluting beads have similar efficacy to gealfoam-lipiodol with probably less adverse events. Both should be discouraged in decompensated liver disease and in case of macroscopic vascular invasion or extrahepatic spread[65-80]. An alternative to TACE is radioembolization. However, radioembolization , although promising and with advantage of being indicated also in the case of portal vein neoplastic thrombosis, is expensive and further data are needed. In fact, to our knowledge there are not well designed studies comparing radioembolization with neither TACE nor sorafenib[81-84].
LT is actually a consolidated therapeutic option for HCC because it cures both tumor and underlying cirrhosis[1-4]. However, the indication of LT for HCC treatment has evolved over recent years[85-109]. Moreover, initial experiences tended to offer LT as the last therapeutic chance when resection was not feasible. In fact, till the first half of nineties, the discouraging results of some experiences had questioned the possibility of LT efficacy as HCC treatment. In 1996, the publication of a pivotal prospective study on less than 50 patients, transplanted for HCC under predefined criteria (single HCC ≤ 5 cm or 3 HCC ≤ 3 cm each), the so called “Milan criteria”, showed a 4-year survival of 75%[85]. Subsequent experiences of LT for HCC inside the Milan criteria, confirmed a survival rate exceeding 70% at 5 years, with a recurrence in less than 15%. Due to these data, LT is now the first-line treatment for one HCC ≤ 5 cm or 3 HCC ≤ 3 cm each[1-4].
Although all published guidelines go on considering the Milan criteria as the only fence inside which LT should be considered as treatment of HCC[2-4], the possibility of an extension of Milan criteria as indication for LT is already a debated issue. In fact, while it is universally recognized that LT for HCC inside Milan criteria guaranties an acceptable outcome, numerous heterogeneous experiences explore the possibility of extending the Milan criteria.
A line of experiences studies the applicability of the University of California San Francisco (UCSF) criteria, that is single nodule ≤ 6.5 cm or 2-3 nodules ≤ 4.5 cm and total diameter ≤ 8 cm. In fact, UCSF criteria on explant identified retrospectively a cohort of patients whose survival was not significantly different from those of patient transplanted for HCC inside the Milan criteria[93]. The same results had other retrospective experiences by other groups using UCSF criteria. Moreover, a recent prospective study showed a 5-year survival not significantly different in patients transplanted for HCC inside Milan and UCSF criteria[103].
A recent multicenter restrospective study on over 1700 found that HCC inside the “up-to-seven” criteria at explant (those HCCs having the number 7 as the sum of the size of the larger tumor and the number of tumors) and without microvascular invasion had a 5-year survival not significantly different from those inside the Milan criteria, while survival was significantly worst in case of HCC inside the “up-to-seven” criteria and with microvascular invasion[104].
Another line of studies suggest that down-staging for HCC exceeding conventional criteria could be effective for extending LT without worsening survival. These studies are heterogeneous in design, inclusion criteria and philosophy. In fact, while some suggests to offer LT to those patients who achieve an effective downstaging, so selecting patients with a less aggressive HCC and likely reducing the probability of HCC recurrence after LT, others indicate LT for those HCC without an effective downstaging, as a rescue treatment[107-109].
Despite all the above studies, guidelines still give indication to LT only to HCC inside Milan criteria[2-4]. However, as published experiences show, many center actually perform LT outside the Milan criteria, using criteria different from centre to centre[85-109].
Whatever the criteria adopted, a significant problem of HCC candidates for LT is the drop out, that is patients who do not reach the goal of LT because of progression of HCC or of causes unrelated to HCC. Many studies have investigated the risk of drop out that remains difficult to define, although some factors, like tumor multinodularity, neoadjuvant treatment failures, elevated AFP or model for end-stage liver disease score, have been correlated with a higher probability of drop out. From an opposite point of view, given the organ shortage, some patients with single HCC < 2 cm may benefit from alternative treatments and avoid LT at least until recurrence occours, highlighting the possibility of salvage transplantation in low risk population. Moreover, it is still uncertain which is the role of LT after surgery and high risk of recurrence at pathology[2-4].
Living donor LT (LDLT) is an alternative option if waiting list is long and offers the possibility of a LT after a short time. However, there is a donor risk of death of about 0.3% and of life threatening complications of about 2%. In fact, LDLT should be restricted to centers of excellence[110-120].
The mainstay of palliative therapy for advanced HCC is Sorafenib, which is indicated in advanced HCC (BCLC C) or HCC progressing upon loco-regional therapies in patients with well preserved liver function and good performance status. Registrative studies have shown a 3-mo increase in median overall survival of sorafenib compared to placebo. Moreover, adverse events are frequent a can be severe (Diarrea, Hand-foot skin-reaction)[121-125].
The role of Sorafenib in some subgroup of patients is debated. In fact, while some experiences report encouraging results of sorafenib therapy after HCC recurrence post OLT, others suggests that adverse events could affect sorafenib efficacy[126-135]. Moreover, our preliminary experience on HCC in HIV positive patient report a high incidence of hepatotoxicity. In both the subgroup the possibility of drug interections should be investigated.
ACKNOWLEDGMENTS
I thank Prof Angelo Vanzulli for imaging supply.
Footnotes
P- Reviewers Kumar V, Wang B S- Editor Wen LL L- Editor A E- Editor Li JY
References
- 1.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–364. doi: 10.1159/000327577. [DOI] [PubMed] [Google Scholar]
- 4.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 7.Flor N, Zuin M, Brovelli F, Maggioni M, Tentori A, Sardanelli F, Cornalba GP. Regenerative nodules in patients with chronic Budd-Chiari syndrome: a longitudinal study using multiphase contrast-enhanced multidetector CT. Eur J Radiol. 2010;73:588–593. doi: 10.1016/j.ejrad.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Moucari R, Rautou PE, Cazals-Hatem D, Geara A, Bureau C, Consigny Y, Francoz C, Denninger MH, Vilgrain V, Belghiti J, Durand F, Valla D, Plessier A. Hepatocellular carcinoma in Budd-Chiari syndrome: characteristics and risk factors. Gut. 2008;57:828–835. doi: 10.1136/gut.2007.139477. [DOI] [PubMed] [Google Scholar]
- 9.Gwon D, Ko GY, Yoon HK, Sung KB, Kim JH, Lee SS, Lee JM, Ohm JY, Shin JH, Song HY. Hepatocellular carcinoma associated with membranous obstruction of the inferior vena cava: incidence, characteristics, and risk factors and clinical efficacy of TACE. Radiology. 2010;254:617–626. doi: 10.1148/radiol.09090738. [DOI] [PubMed] [Google Scholar]
- 10.Rautou PE, Douarin L, Denninger MH, Escolano S, Lebrec D, Moreau R, Vidaud M, Itzykson R, Moucari R, Bezeaud A, et al. Bleeding in patients with Budd-Chiari syndrome. J Hepatol. 2011;54:56–63. doi: 10.1016/j.jhep.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 11.Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology. 2008;48:137–145. doi: 10.1002/hep.22312. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 13.Ioannou GN, Bryson CL, Weiss NS, Miller R, Scott JD, Boyko EJ. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57:249–257. doi: 10.1002/hep.25800. [DOI] [PubMed] [Google Scholar]
- 14.Sahasrabuddhe VV, Shiels MS, McGlynn KA, Engels EA. The risk of hepatocellular carcinoma among individuals with acquired immunodeficiency syndrome in the United States. Cancer. 2012;118:6226–6233. doi: 10.1002/cncr.27694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merchante N, Merino E, López-Aldeguer J, Jover F, Delgado-Fernández M, Galindo MJ, Ortega E, Rivero A, Mínguez C, Romero-Palacios A, et al. Increasing incidence of hepatocellular carcinoma in HIV-infected patients in Spain. Clin Infect Dis. 2013;56:143–150. doi: 10.1093/cid/cis777. [DOI] [PubMed] [Google Scholar]
- 16.Mancuso A, Rigano P, Renda D, Di Salvo V, Pignatti CB, Guddo F, Buccellato A, Nicoli N, Maggio A. Hepatocellular carcinoma on cirrhosis-free liver in a HCV-infected thalassemic. Am J Hematol. 2005;78:158–159. doi: 10.1002/ajh.20289. [DOI] [PubMed] [Google Scholar]
- 17.Mancuso A, Sciarrino E, Renda MC, Maggio A. A prospective study of hepatocellular carcinoma incidence in thalassemia. Hemoglobin. 2006;30:119–124. doi: 10.1080/03630260500455565. [DOI] [PubMed] [Google Scholar]
- 18.Mancuso A. Hepatocellular carcinoma in thalassemia: A critical review. World J Hepatol. 2010;2:171–174. doi: 10.4254/wjh.v2.i5.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, Piva A, Di Carlo V, Dioguardi N. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675–680. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 20.Bolondi L. Screening for hepatocellular carcinoma in cirrhosis. J Hepatol. 2003;39:1076–1084. doi: 10.1016/s0168-8278(03)00349-0. [DOI] [PubMed] [Google Scholar]
- 21.Kim CK, Lim JH, Lee WJ. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver: accuracy of ultrasonography in transplant patients. J Ultrasound Med. 2001;20:99–104. doi: 10.7863/jum.2001.20.2.99. [DOI] [PubMed] [Google Scholar]
- 22.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato T, Tateishi R, Yoshida H, Ohki T, Masuzaki R, Imamura J, Goto T, Kanai F, Obi S, Kato N, et al. Ultrasound surveillance for early detection of hepatocellular carcinoma among patients with chronic hepatitis C. Hepatol Int. 2009;3:544–550. doi: 10.1007/s12072-009-9145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fung KT, Li FT, Raimondo ML, Maudgil D, Mancuso A, Tibballs JM, Watkinson AA, Patch D, Burroughs AK. Systematic review of radiological imaging for hepatocellular carcinoma in cirrhotic patients. Br J Radiol. 2004;77:633–640. doi: 10.1259/bjr/31556748. [DOI] [PubMed] [Google Scholar]
- 25.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17 Suppl 2:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 26.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 27.Lang H, Sotiropoulos GC, Dömland M, Frühauf NR, Paul A, Hüsing J, Malagó M, Broelsch CE. Liver resection for hepatocellular carcinoma in non-cirrhotic liver without underlying viral hepatitis. Br J Surg. 2005;92:198–202. doi: 10.1002/bjs.4763. [DOI] [PubMed] [Google Scholar]
- 28.Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 29.Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli G, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 30.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602–611. doi: 10.1097/00000658-200211000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makuuchi M, Sano K. The surgical approach to HCC: our progress and results in Japan. Liver Transpl. 2004;10:S46–S52. doi: 10.1002/lt.20044. [DOI] [PubMed] [Google Scholar]
- 32.Arii S, Tanaka S, Mitsunori Y, Nakamura N, Kudo A, Noguchi N, Irie T. Surgical strategies for hepatocellular carcinoma with special reference to anatomical hepatic resection and intraoperative contrast-enhanced ultrasonography. Oncology. 2010;78 Suppl 1:125–130. doi: 10.1159/000315240. [DOI] [PubMed] [Google Scholar]
- 33.Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298–304. doi: 10.1002/ssu.2980090404. [DOI] [PubMed] [Google Scholar]
- 34.Shi M, Guo RP, Lin XJ, Zhang YQ, Chen MS, Zhang CQ, Lau WY, Li JQ. Partial hepatectomy with wide versus narrow resection margin for solitary hepatocellular carcinoma: a prospective randomized trial. Ann Surg. 2007;245:36–43. doi: 10.1097/01.sla.0000231758.07868.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 36.Simpson KJ, Finlayson ND. Clinical evaluation of liver disease. Baillieres Clin Gastroenterol. 1995;9:639–659. doi: 10.1016/0950-3528(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 37.Cucchetti A, Piscaglia F, Caturelli E, Benvegnù L, Vivarelli M, Ercolani G, Cescon M, Ravaioli M, Grazi GL, Bolondi L, et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. 2009;16:413–422. doi: 10.1245/s10434-008-0232-4. [DOI] [PubMed] [Google Scholar]
- 38.Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 40.Lencioni R, Bartolozzi C, Caramella D, Paolicchi A, Carrai M, Maltinti G, Capria A, Tafi A, Conte PF, Bevilacqua G. Treatment of small hepatocellular carcinoma with percutaneous ethanol injection. Analysis of prognostic factors in 105 Western patients. Cancer. 1995;76:1737–1746. doi: 10.1002/1097-0142(19951115)76:10<1737::aid-cncr2820761010>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 41.Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, Pompili M, Brunello F, Lazzaroni S, Torzilli G. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101–108. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- 42.Livraghi T, Bolondi L, Lazzaroni S, Marin G, Morabito A, Rapaccini GL, Salmi A, Torzilli G. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer. 1992;69:925–929. doi: 10.1002/1097-0142(19920215)69:4<925::aid-cncr2820690415>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 43.Kuang M, Lu MD, Xie XY, Xu HX, Xu ZF, Liu GJ, Yin XY, Huang JF, Lencioni R. Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology. 2009;253:552–561. doi: 10.1148/radiol.2532082021. [DOI] [PubMed] [Google Scholar]
- 44.Khan KN, Yatsuhashi H, Yamasaki K, Yamasaki M, Inoue O, Koga M, Yano M. Prospective analysis of risk factors for early intrahepatic recurrence of hepatocellular carcinoma following ethanol injection. J Hepatol. 2000;32:269–278. doi: 10.1016/s0168-8278(00)80072-0. [DOI] [PubMed] [Google Scholar]
- 45.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma & lt; or =4 cm. Gastroenterology. 2004;127:1714–1723. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 47.Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, Sacchetto P, Gandini G, Rizzetto M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727–735. doi: 10.1080/00365520701885481. [DOI] [PubMed] [Google Scholar]
- 48.Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–459. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 49.Huo TI, Huang YH, Wu JC, Lee PC, Chang FY, Lee SD. Comparison of percutaneous acetic acid injection and percutaneous ethanol injection for hepatocellular carcinoma in cirrhotic patients: a prospective study. Scand J Gastroenterol. 2003;38:770–778. doi: 10.1080/00365520310003048. [DOI] [PubMed] [Google Scholar]
- 50.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 52.Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgrò G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol. 2010;52:380–388. doi: 10.1016/j.jhep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Bouza C, López-Cuadrado T, Alcázar R, Saz-Parkinson Z, Amate JM. Meta-analysis of percutaneous radiofrequency ablation versus ethanol injection in hepatocellular carcinoma. BMC Gastroenterol. 2009;9:31. doi: 10.1186/1471-230X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Omata M, Tateishi R, Yoshida H, Shiina S. Treatment of hepatocellular carcinoma by percutaneous tumor ablation methods: Ethanol injection therapy and radiofrequency ablation. Gastroenterology. 2004;127:S159–S166. doi: 10.1053/j.gastro.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 55.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 56.Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, Tong MJ, Amado RG, Busuttil RW. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954–960. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- 57.Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K, Shigenobu S, Ishibashi K, Arima T. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253–1262. doi: 10.1002/cncr.11168. [DOI] [PubMed] [Google Scholar]
- 58.Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 59.Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101–1108. doi: 10.1002/hep.21164. [DOI] [PubMed] [Google Scholar]
- 60.Lencioni R, Llovet JM. Percutaneous ethanol injection for hepatocellular carcinoma: alive or dead? J Hepatol. 2005;43:377–380. doi: 10.1016/j.jhep.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Imamura J, Tateishi R, Shiina S, Goto E, Sato T, Ohki T, Masuzaki R, Goto T, Yoshida H, Kanai F, et al. Neoplastic seeding after radiofrequency ablation for hepatocellular carcinoma. Am J Gastroenterol. 2008;103:3057–3062. doi: 10.1111/j.1572-0241.2008.02153.x. [DOI] [PubMed] [Google Scholar]
- 62.Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 63.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 65.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 66.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 67.Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796–802. doi: 10.1002/cncr.20426. [DOI] [PubMed] [Google Scholar]
- 68.Lin DY, Liaw YF, Lee TY, Lai CM. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma--a randomized controlled trial. Gastroenterology. 1988;94:453–456. doi: 10.1016/0016-5085(88)90436-2. [DOI] [PubMed] [Google Scholar]
- 69.Pelletier G, Roche A, Ink O, Anciaux ML, Derhy S, Rougier P, Lenoir C, Attali P, Etienne JP. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol. 1990;11:181–184. doi: 10.1016/0168-8278(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 70.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 71.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 72.Bruix J, Llovet JM, Castells A, Montañá X, Brú C, Ayuso MC, Vilana R, Rodés J. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578–1583. doi: 10.1002/hep.510270617. [DOI] [PubMed] [Google Scholar]
- 73.Pelletier G, Ducreux M, Gay F, Luboinski M, Hagège H, Dao T, Van Steenbergen W, Buffet C, Rougier P, Adler M, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. 1998;29:129–134. doi: 10.1016/s0168-8278(98)80187-6. [DOI] [PubMed] [Google Scholar]
- 74.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 75.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20:S377–S390. doi: 10.1016/j.jvir.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 76.A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med. 1995;332:1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 77.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 78.Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)-embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;(3):CD004787. doi: 10.1002/14651858.CD004787.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, Pitton M, Sergent G, Pfammatter T, Terraz S, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 81.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, Sato KT, Benson A, Nemcek AA, Gates VL, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 82.Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, Heusner T, Cicinnati VR, Paul A, Bockisch A, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–1749. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 83.Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, Marchianò A, Bongini M, Lanocita R, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–1837. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 84.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 85.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 86.Mela M, Mancuso A, Burroughs AK. Review article: hepatocellular carcinoma: indications for liver transplantation. Aliment Pharmacol Ther. 2003;17 Suppl 2:130–137. doi: 10.1046/j.1365-2036.17.s2.16.x. [DOI] [PubMed] [Google Scholar]
- 87.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, Tzakis AG, Van Thiel DH, Carr B, Selby R. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221–228; discussion 228-229. doi: 10.1097/00000658-199109000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwatsuki S, Esquivel CO, Gordon RD, Shaw BW, Starzl TE, Shade RR, Van Thiel DH. Liver transplantation for fulminant hepatic failure. Semin Liver Dis. 1985;5:325–328. doi: 10.1055/s-2008-1040628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19:311–322. doi: 10.1055/s-2007-1007120. [DOI] [PubMed] [Google Scholar]
- 91.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 93.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 94.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 95.Pomfret EA, Washburn K, Wald C, Nalesnik MA, Douglas D, Russo M, Roberts J, Reich DJ, Schwartz ME, Mieles L, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010;16:262–278. doi: 10.1002/lt.21999. [DOI] [PubMed] [Google Scholar]
- 96.Sala M, Fuster J, Llovet JM, Navasa M, Solé M, Varela M, Pons F, Rimola A, García-Valdecasas JC, Brú C, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl. 2004;10:1294–1300. doi: 10.1002/lt.20202. [DOI] [PubMed] [Google Scholar]
- 97.Mazzaferro V, Battiston C, Perrone S, Pulvirenti A, Regalia E, Romito R, Sarli D, Schiavo M, Garbagnati F, Marchianò A, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240:900–909. doi: 10.1097/01.sla.0000143301.56154.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu DS, Yu NC, Raman SS, Lassman C, Tong MJ, Britten C, Durazo F, Saab S, Han S, Finn R, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130–1137. doi: 10.1002/hep.20688. [DOI] [PubMed] [Google Scholar]
- 99.Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, Perrin H, Azoulay D. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688–701; discussion 701-703. doi: 10.1097/00000658-199712000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Decaens T, Roudot-Thoraval F, Bresson-Hadni S, Meyer C, Gugenheim J, Durand F, Bernard PH, Boillot O, Boudjema K, Calmus Y, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11:767–775. doi: 10.1002/lt.20418. [DOI] [PubMed] [Google Scholar]
- 101.Porrett PM, Peterman H, Rosen M, Sonnad S, Soulen M, Markmann JF, Shaked A, Furth E, Reddy KR, Olthoff K. Lack of benefit of pre-transplant locoregional hepatic therapy for hepatocellular cancer in the current MELD era. Liver Transpl. 2006;12:665–673. doi: 10.1002/lt.20636. [DOI] [PubMed] [Google Scholar]
- 102.Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, Rodés J, Bruix J. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123–128. doi: 10.1136/gut.50.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 104.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 105.Raj A, McCall J, Gane E. Validation of the “Metroticket” predictor in a cohort of patients transplanted for predominantly HBV-related hepatocellular carcinoma. J Hepatol. 2011;55:1063–1068. doi: 10.1016/j.jhep.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 106.Schwartz M, Dvorchik I, Roayaie S, Fiel MI, Finkelstein S, Marsh JW, Martignetti JA, Llovet JM. Liver transplantation for hepatocellular carcinoma: extension of indications based on molecular markers. J Hepatol. 2008;49:581–588. doi: 10.1016/j.jhep.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yao FY, Kerlan RK, Hirose R, Davern TJ, Bass NM, Feng S, Peters M, Terrault N, Freise CE, Ascher NL, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D’Errico Grigioni A, Panzini I, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547–2557. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]
- 109.De Carlis L, Di Sandro S, Giacomoni A, Slim A, Lauterio A, Mangoni I, Mihaylov P, Pirotta V, Aseni P, Rampoldi A. Beyond the Milan criteria: what risks for patients with hepatocellular carcinoma progression before liver transplantation? J Clin Gastroenterol. 2012;46:78–86. doi: 10.1097/MCG.0b013e31822b36f6. [DOI] [PubMed] [Google Scholar]
- 110.Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–1082. doi: 10.1056/NEJMra011629. [DOI] [PubMed] [Google Scholar]
- 111.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 112.Siegler M, Simmerling MC, Siegler JH, Cronin DC. Recipient deaths during donor surgery: a new ethical problem in living donor liver transplantation (LDLT) Liver Transpl. 2006;12:358–360. doi: 10.1002/lt.20670. [DOI] [PubMed] [Google Scholar]
- 113.Ghobrial RM, Freise CE, Trotter JF, Tong L, Ojo AO, Fair JH, Fisher RA, Emond JC, Koffron AJ, Pruett TL, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008;135:468–476. doi: 10.1053/j.gastro.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown RS. Live donors in liver transplantation. Gastroenterology. 2008;134:1802–1813. doi: 10.1053/j.gastro.2008.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cronin DC, Millis JM. Living donor liver transplantation: The ethics and the practice. Hepatology. 2008;47:11–13. doi: 10.1002/hep.22150. [DOI] [PubMed] [Google Scholar]
- 116.Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: A life-expectancy and cost-effectiveness perspective. Hepatology. 2001;33:1073–1079. doi: 10.1053/jhep.2001.23311. [DOI] [PubMed] [Google Scholar]
- 117.Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg. 2007;94:78–86. doi: 10.1002/bjs.5528. [DOI] [PubMed] [Google Scholar]
- 118.Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS, Ghobrial RM, Fair JH, Olthoff KM, Kam I, et al. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007;7:1601–1608. doi: 10.1111/j.1600-6143.2007.01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kulik L, Abecassis M. Living donor liver transplantation for hepatocellular carcinoma. Gastroenterology. 2004;127:S277–S282. doi: 10.1053/j.gastro.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 120.Majno P, Mazzaferro V. Living donor liver transplantation for hepatocellular carcinoma exceeding conventional criteria: questions, answers and demands for a common language. Liver Transpl. 2006;12:896–898. doi: 10.1002/lt.20808. [DOI] [PubMed] [Google Scholar]
- 121.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 122.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 123.Kim JE, Ryoo BY, Ryu MH, Chang HM, Suh DJ, Lee HC, Lim YS, Kim KM, Kang YK. Sorafenib for hepatocellular carcinoma according to Child-Pugh class of liver function. Cancer Chemother Pharmacol. 2011;68:1285–1290. doi: 10.1007/s00280-011-1616-x. [DOI] [PubMed] [Google Scholar]
- 124.Hollebecque A, Cattan S, Romano O, Sergent G, Mourad A, Louvet A, Dharancy S, Boleslawski E, Truant S, Pruvot FR, et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: the impact of the Child-Pugh score. Aliment Pharmacol Ther. 2011;34:1193–1201. doi: 10.1111/j.1365-2036.2011.04860.x. [DOI] [PubMed] [Google Scholar]
- 125.Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055–2063. doi: 10.1002/hep.24644. [DOI] [PubMed] [Google Scholar]
- 126.Fujiki M, Aucejo F, Kim R. Adjuvant treatment of hepatocellular carcinoma after orthotopic liver transplantation: do we really need this? Clin Transplant. 2013;27:169–177. doi: 10.1111/ctr.12042. [DOI] [PubMed] [Google Scholar]
- 127.Sotiropoulos GC, Nowak KW, Fouzas I, Vernadakis S, Kykalos S, Klein CG, Paul A. Sorafenib treatment for recurrent hepatocellular carcinoma after liver transplantation. Transplant Proc. 2012;44:2754–2756. doi: 10.1016/j.transproceed.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 128.Zavaglia C, Airoldi A, Mancuso A, Vangeli M, Viganò R, Cordone G, Gentiluomo M, Belli LS. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature. Eur J Gastroenterol Hepatol. 2013;25:180–186. doi: 10.1097/MEG.0b013e328359e550. [DOI] [PubMed] [Google Scholar]
- 129.Welker MW, Bechstein WO, Zeuzem S, Trojan J. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int. 2013;26:109–118. doi: 10.1111/j.1432-2277.2012.01562.x. [DOI] [PubMed] [Google Scholar]
- 130.Vitale A, Boccagni P, Kertusha X, Zanus G, D’Amico F, Lodo E, Pastorelli D, Ramirez Morales R, Lombardi G, Senzolo M, et al. Sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation? Transplant Proc. 2012;44:1989–1991. doi: 10.1016/j.transproceed.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 131.Castroagudín JF, Molina-Pérez E, Ferreiro-Iglesias R, Abdulkader I, Otero-Antón E, Tomé S, Varo-Pérez E. Late recurrence of hepatocellular carcinoma after liver transplantation: is an active surveillance for recurrence needed? Transplant Proc. 2012;44:1565–1567. doi: 10.1016/j.transproceed.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 132.Teng CL, Hwang WL, Chen YJ, Chang KH, Cheng SB. Sorafenib for hepatocellular carcinoma patients beyond Milan criteria after orthotopic liver transplantation: a case control study. World J Surg Oncol. 2012;10:41. doi: 10.1186/1477-7819-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Truesdale AE, Caldwell SH, Shah NL, Argo CK, Al-Osaimi AM, Schmitt TM, Northup PG. Sorafenib therapy for hepatocellular carcinoma prior to liver transplant is associated with increased complications after transplant. Transpl Int. 2011;24:991–998. doi: 10.1111/j.1432-2277.2011.01299.x. [DOI] [PubMed] [Google Scholar]
- 134.Saidi RF, Shah SA, Rawson AP, Grossman S, Piperdi B, Bozorgzadeh A. Treating hepatocellular carcinoma with sorafenib in liver transplant patients: an initial experience. Transplant Proc. 2010;42:4582–4584. doi: 10.1016/j.transproceed.2010.09.147. [DOI] [PubMed] [Google Scholar]
- 135.Kim R, El-Gazzaz G, Tan A, Elson P, Byrne M, Chang YD, Aucejo F. Safety and feasibility of using sorafenib in recurrent hepatocellular carcinoma after orthotopic liver transplantation. Oncology. 2010;79:62–66. doi: 10.1159/000319548. [DOI] [PubMed] [Google Scholar]


