Abstract
Objective: Compared with conventional open surgery (COS), endovascular aneurysm repair (EVAR) has been reported to decrease the 30-day mortality rate in patients with ruptured abdominal aortic aneurysms (rAAAs). We developed an EVAR-first strategy for rAAAs that incorporates the Shonan ruptured abdominal aortic aneurysm protocol (SRAP). We describe short-term results with this protocol at our institution and compare them with outcomes in patients who underwent COS.
Methods: The records of all 57 patients in whom a rAAA was repaired during a 7-year period were reviewed retrospectively. Patients in the COS group (n = 30) were treated between January 2005 and December 2009; those in the SRAP group (n = 27) were treated between January 2010 and March 2012. The two groups were compared with respect to patient characteristics at admission, including severity of condition; operative and in-hospital variables; and 30-day mortality.
Results: The baseline patient characteristics in the COS and SRAP groups were similar except that the SRAP group had a significantly higher rate of cerebrovascular disease. The 30-day mortality rate was significantly higher in the COS group (43% vs. 19%), as were the intraoperative mortality rate (27% vs. 5%) and the in-hospital mortality rate (57% vs. 26%; P < 0.05 for all comparisons). The technical success rate for EVAR was 96%; no conversions to open surgery were required.
Conclusions: Use of the SRAP is a promising strategy for improving initial outcomes in patients with rAAAs.
Keywords: abdominal aortic aneurysm, rupture, EVAR
Introduction
Endoluminal therapy for abdominal aortic aneu rysms (AAAs) was first reported by Parodi, et al.1) in 1991. Subsequently, large trials found that early endovascular aneurysm repair (EVAR) provided better outcomes than laparotomy,2,3) and EVAR became the standard treatment for AAAs. Outcomes with ruptured AAAs (rAAAs), however, remain unsatisfactory because patients are usually treated on an emergency basis and their overall condition is poor, with many being in shock and having hemodynamic instability and disturbed consciousness. A 2002 meta-analysis found that the short-term mortality rate among patients with rAAAs was 48% and that the survival rate had not improved in 20 years.4)
The use of EVAR for rAAA repair was first reported by Marin, et al.5) in 1995. In 2000, Ohki, et al.6) described a series of 20 patients with rAAAs who underwent EVAR and had a 30-day mortality rate of 10%. A 2008 systematic review and meta-analysis by Mastracci, et al.7) found that EVAR for rAAAs was associated with a 21% mortality rate. Their investigation also indicated the importance of a protocol-based approach to improving rAAA treatment outcomes.
About 15 emergency operations for an rAAA are performed at our institution each year. Approximately half the patients treated have been transferred from another hospital. Until December 2009, all rAAA repairs involved laparotomy, performed according to a standard conventional open surgery (COS) protocol. Thus, when emergency room (ER) physicians suspected an rAAA after an echographic or computed tomographic (CT) evaluation, they called a surgeon. After all the surgical instruments and anesthetic agents and equipment had been prepared, the patient was transferred to the operating room (OR), where aortic clamping below the renal artery or above the celiac artery was done, and a straight graft (I-graft) was replaced. If the ER personnel determined that a patient was in shock, a thoracotomy in the left side of the chest was accomplished immediately, the descending aorta was clamped, and the patient was then transferred to the OR for rAAA repair.
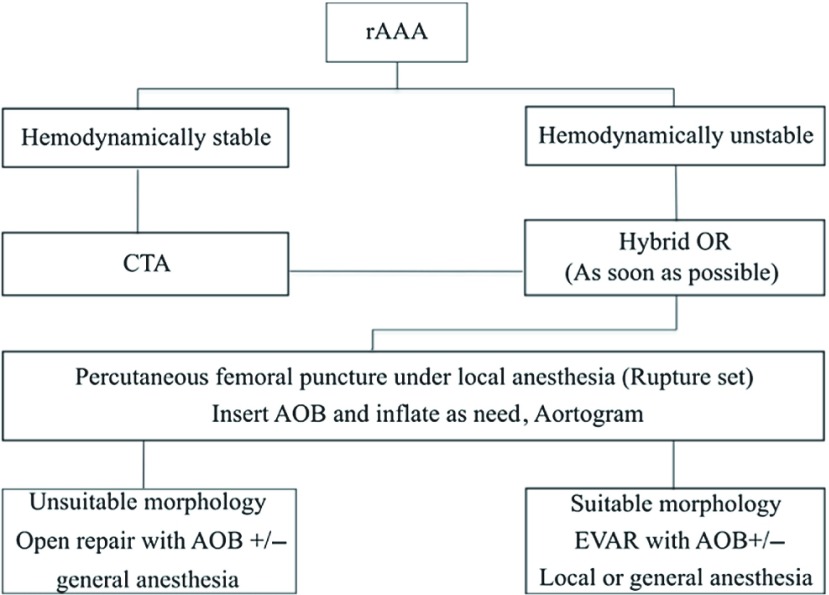
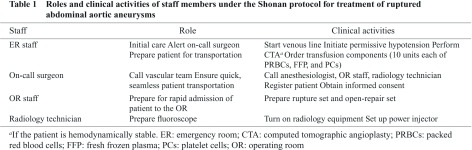
In 2010, we developed a new protocol for treating patients with rAAAs: the Shonan ruptured abdominal aortic aneurysm protocol (SRAP; Fig. 1). The protocol, which is based on an EVAR-first approach and an algorithm described by Mehta, et al.,8) clearly specifies the roles and responsibilities of the ER staff, on-call surgeon, anesthesiologists, OR staff, and radiology staff (Table 1). It also provides detailed instructions for all procedures, including patient transfers. Because the primary goal of development of the SRAP was to use EVAR as the initial treatment for rAAAs in as many cases as possible, our morphologic indications for EVAR were less strict than those in the manufacturer’s instructions for use (IFU) for the endoprosthesis employed. A secondary aim of creation of the SRAP was to prioritize patients’ transfer to the OR and thereby expedite the progression from diagnosis to insertion of an aortic occlusion balloon (AOB).
Fig. 1.
Shonan ruptured abdominal aortic aneurysm (rAAA) protocol (SRAP). The rupture set consists of 12F sheaths (Ultimum Introducer; St Jude Medical, St Paul, Minnesota, USA); a 0.035-inch, 200-cm guide wire (Radifocus; Terumo Europe, Leuven, Belgium); a 0.035-inch, 180-cm Super stiff guide wire (Boston Scientific, Natick, Massachusetts, USA); a 60-cm KMP catheter (COOK MEDICAL, Bloomington, Indiana, USA); a 110-cm pigtail catheter (COOK); and an aortic occlusion balloon (AOB) (Reliant; Medtronic Vascular, Santa Rosa, California, USA). CTA: computed tomographic angiography; OR: operation room; EVAR: endovascular aneurysm repair
We describe a retrospective comparison between short-term patient outcomes achieved with the SRAP and those obtained with COS.
Methods
All patients or their representatives provided informed consent to use of the SRAP, which was approved by both an in-house and an external institutional review board. Under the SRAP, when ER physicians suspected a rAAA after an initial echographic evaluation, hemodynamically stable patients undergo CT angiography (CTA) in the ER, whereas hemodynamically unstable patients are taken to the OR immediately. In all patients, percutaneous insertion of a 12F sheath (Ultimum Introducer; St Jude Medical, St Paul, Minnesota, USA) into the femoral artery is performed, followed by insertion of an AOB (Reliant; Medtronic Vascular, Santa Rosa, California, USA). If the patient is hemodynamically unstable, the balloon is inflated, and the aorta is occluded. If CTA confirms that the morphologic features of the aneurysm are suitable for EVAR, the patient is considered eligible for this procedure. Under the SRAP, suitable morphologic features are an aneurysm neck diameter of between 18 and 30 mm, a neck angulation of less than 90 degrees, and a neck longer than 5 mm. An Excluder AAA Endo-prosthesis (WL Gore & Associates, Flagstaff, Arizona, USA) is used in all endovascular rAAA repairs. In hemodynamically unstable patients in whom contralateral cannulation would be difficult to achieve, an aortic extender is used to occlude the contralateral leg (aorto-uni-iliac [AUI] technique and femorofemoral [FF] bypass).
If the morphologic features of the aneurysm are unsuitable for EVAR, the patient undergoes COS under general anesthesia. During the operation, the aorta is replaced while the bleeding is controlled with an AOB. All patients who have an rAAA repair, regardless of whether an endovascular or open approach is used, are transferred to the intensive care unit after their procedure.
In this study, patients who underwent EVAR under the SRAP and those who had COS under the older protocol were compared with respect to baseline characteristics, operating time for rAAA repair, units of blood transfused during the repair, intraoperative mortality rate, in-hospital mortality rate, duration of hospital stay, 30-day mortality rate (primary endpoint), and cause of postoperative death. The severity of the preoperative condition of all patients was assessed by using the Glasgow aneurysm score,9) the Fitzgerald classification,10) and the Hardman index.11) A t-test, chi-square test, or Fisher exact test was used to compare variables in the COS and SRAP groups. A P value of less than .05 was considered to represent a significant difference. All statistical analyses used Microsoft Excel 2008 for Mac, Version 12.3.3 (Microsoft, Redmond, Washington, USA).
Results
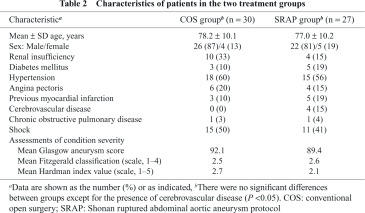
The study included the 57 patients with rAAAs treated consecutively at our institution between January 2005 and March 2012. Thirty of these patients underwent COS between January 2005 and December 2006. The other 27 had a procedure (24 EVAR and 3 open repairs) under the SRAP, between January 2010 and March 2012. The three open repairs in the SRAP group were performed because the morphologic features of the aneurysm neck did not meet the criteria for EVAR specified in the protocol. Table 2 shows baseline characteristics of the patients in the study. The only significant difference between the COS and EVAR groups was the higher rate of cerebrovascular disease in the SRAP group (P <0.05). The results of Glasgow aneurysm scoring indicated that all 57 patients were at high risk of death after open surgery for AAA.
Table 3 shows operative and perioperative data for the two treatment groups. The 30-day mortality rate was 43.3% in the COS group (13 of 30 patients) and 18.5% (5 of 27) in the SRAP group, with the difference being significant (P <0.05). Moreover, compared with patients in the COS group, those in the SRAP group had significantly lower rates of both intraoperative and in-hospital mortality. There were no significant differences between groups in operating time, units of blood transfused, duration of hospital stay, or deaths due to multiple-organ failure (MOF).
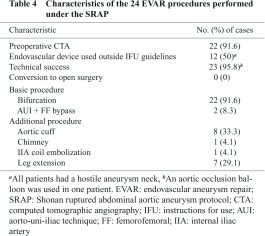
Characteristics of the 24 EVAR procedures done under the SRAP are shown in Table 4. Technical success was achieved in all but one patient. The patient in whom the endoprosthesis could not be placed was a 98-year-old man who was in shock at arrival at our hospital. After aortic cross-clamping with an AOB, he had a cardiac arrest and died, despite cardiopulmonary resuscitation attempts. In two patients, the AUI technique and FF bypass were required because of hemodynamic instability. All 12 patients in whom the endoprosthesis was used outside the IFU guidelines had a hostile aneurysm neck, but the neck did meet the morphologic criteria stipulated in the SRAP. In two patients, preoperative CT scanning was not feasible because the patients were hemodynamically unstable, but images were obtained intraoperatively for both. Seventeen of 24 patients who had undergone EVAR had an additional procedure.
Discussion
Our findings suggest that the introduction of the SRAP resulted in an improvement in early outcomes of a procedure for treating rAAAs that is less invasive than COS. Factors that probably contributed to the improvement were careful patient selection according to morphologic criteria and a multidisciplinary approach that expedited the treatment process. Our study was not designed to assess whether results achieved with EVAR of rAAAs are superior to those with COS. A formal comparison between EVAR and COS would require a randomized study, but we believe that such a study would not be ethically acceptable. However, a nonrandomized prospective study might provide useful comparative data.
With the use of the SRAP at our institution, EVAR could be accomplished in all but one case meeting the basic criteria for an endovascular repair. The aneurysm neck in an rAAA is often hostile because of the large diameter of the aneurysm sac and the steep neck angle; therefore, implantation of the endoprosthesis must be tailored to the specific morphologic features of the neck. We prefer to withdraw the floppy portion of the stiff wire that reaches the tip of the delivery system and deploy the device while pressing on the delivery system. We think this method is the easiest to perform and the most appropriate for a hectic emergency situation. Others have suggested bending the stiff wire.12–14) Furthermore, to facilitate a good fit between the rigid body of the endoprosthesis and a torturous neck, we implant the device with the body in a lower position and add an aortic extender. If large endoleak and hemodynamic instability develop, however, conversion to open surgery becomes unavoidable. Conversion in this situation is challenging and requires extensive surgical experience.
The reduction in intraoperative mortality we observed under the SRAP was probably due to minimization of blood loss achieved by prompt aortic-cross cramping with an AOB, which allowed patients to recover from shock. We anticipated that maintenance of blood perfusion to the organs and prevention of coagulopathy would reduce postoperative deaths from MOF, but the COS and SRAP groups had a similar MOF-related mortality rate. Our results, therefore, suggest that the introduction of new techniques to mitigate the risk of MOF in patients who have undergone rAAA repair will be a key factor in increasing survival rate.
Abdominal compartment syndrome (ACS), a life-threatening condition, has been found to be associated with endovascular repair of rAAAs. Mehta, et al.15) reported that the development of ACS is related to the hemodynamics of the specific aneurysm and that patients with ACS are more likely to have an increased activated partial thromboplastin time and require an AOB and massive transfusion. They also observed that the mortality rate among patients in whom ACS occurs after AAA repair is significantly higher than that in patients who do not have an onset of ACS (67% vs. 10%; P = 0.01). Mehta, et al. recommended that intravesicular pressure be monitored closely in patients who have undergone endovascular rAAA repair and that decompressive laparotomy be performed promptly when the pressure increases or worsening of ACS is observed. The SRAP does not currently specify postoperative management practices, but adding such specifications to future versions of the protocol may enhance its effectiveness in improving rAAA treatment outcomes.
Conclusions
With the introduction of the SRAP, an EVAR-first strategy for repairing rAAAs, intraoperative, in-hospital, and 30-day mortality rates decreased in comparison with rates associated with COS. Thus, the SRAP appears to be useful when performed by EVAR experienced physician in improving early outcomes of rAAA treatment. Midterm and long-term results with the use of the SRAP remain to be determined.
Acknowledgement
The authors thank Renée J. Robillard, MA, ELS, for editorial assistance.
Disclosure Statement
Hidemitsu Ogino and coauthors have no conflicts of interest.
References
- Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg 1991; 5: 491-9. [DOI] [PubMed] [Google Scholar]
- EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005; 365: 2179-86. [DOI] [PubMed] [Google Scholar]
- Prinssen M, Verhoeven EL, Buth J, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med 2004; 351: 1607-18. [DOI] [PubMed] [Google Scholar]
- Bown MJ, Sutton AJ, Bell PR, et al. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg 2002; 89: 714-30. [DOI] [PubMed] [Google Scholar]
- Marin ML, Veith FJ, Cynamon J, et al. Initial experience with transluminally placed endovascular grafts for the treatment of complex vascular lesions. Ann Surg 1995; 222: 449-65; discussion 465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki T, Veith FJ. Endovascular grafts and other image-guided catheter-based adjuncts to improve the treatment of ruptured aortoiliac aneurysms. Ann Surg 2000; 232: 466-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastracci TM, Garrido-Olivares L, Cinà CS, et al. Endovascular repair of ruptured abdominal aortic aneurysms: a systematic review and meta-analysis. J Vasc Surg 2008; 47: 214-21. [DOI] [PubMed] [Google Scholar]
- Mehta M, Taggert J, Darling RC, 3rd, et al. Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis. J Vasc Surg 2006; 44: 1-8; discussion 8. [DOI] [PubMed] [Google Scholar]
- Samy AK, Murray G, MacBain G. Glasgow aneurysm score. Cardiovasc Surg 1994; 2: 41-4. [PubMed] [Google Scholar]
- Fitzgerald JF, Stillman RM, Powers JC. A suggested classification and reappraisal of mortality statistics for ruptured atherosclerotic infrarenal aortic aneurysms. Surg Gynecol Obstet 1978; 146: 344-6. [PubMed] [Google Scholar]
- Hardman DT, Fisher CM, Patel MI, et al. Ruptured abdominal aortic aneurysms: who should be offered surgery? J Vasc Surg 1996; 23: 123-9. [DOI] [PubMed] [Google Scholar]
- Minion DJ, Yancey A, Patterson DE, et al. The endowedge and kilt techniques to achieve additional juxtarenal seal during deployment of the Gore Excluder endoprosthesis. Ann Vasc Surg 2006; 20: 472-7. [DOI] [PubMed] [Google Scholar]
- Ghouri MA, Dougherty KG, Krajcer Z. Technical tips for endovascular treatment of abdominal aortic aneurysms with challenging infrarenal neck anatomy using the Excluder endoprosthesis. J Endovasc Ther 2010; 17: 705-11. [DOI] [PubMed] [Google Scholar]
- Quinones-Baldrich WJ, Chandra A. Angled guidewire delivery of aortic endovascular prostheses for angulated landing zones. Ann Vasc Surg 2009; 23: 425-7. [DOI] [PubMed] [Google Scholar]
- Mehta M, Darling RC, Roddy SP, et al. Factors associated with abdominal compartment syndrome complicating endovascular repair of ruptured abdominal aortic aneurysms. J Vasc Surg 2005; 42: 1047-51. [DOI] [PubMed] [Google Scholar]