Abstract
Objective: Persistent Type 2 endoleaks (PT2) after endovascular aortic aneurysm repair (EVAR) for abdominal aortic aneurysm (AAA) are associated with increased adverse outcomes, including aneurysmal sac enlargement and rupture. The aim of this study was to report early clinical outcomes of coil embolization (CE) to aortic branched vessels prior to EVAR and assess the effectiveness of this strategy in terms of prevention of sac growth due to PT2.
Materials and Methods: Between May 2007 and April 2012, EVAR was performed for 215 cases, divided into two groups (150 cases in Group A, before introduction of CE; 21 in Group B, receiving CE before EVAR). Early clinical outcomes were compared between groups.
Results: Fifty percent of cases in Group B had a marked reduction of aneurysmal sac diameter based on multi-detector row computed tomographic angiography (MDCTA) findings at the 6-month follow-up after EVAR, whereas, only 25% of cases in Group A had shrinkage of the aneurysmal sac during the same time period after EVAR.
Conclusion: This strategy has the possibility of improving late outcomes of EVAR by reducing endoleak volumes beforehand.
Keywords: EVAR, endoleaks, coil embolization
Introduction
Endovascular aortic aneurysm repair (EVAR) has become a standard treatment choice for abdominal aortic aneurysm (AAA). Randomized controlled trials have shown that EVAR significantly reduces the rate of perioperative complications as well as operative mortality and time of hospitalization, as compared to conventional open surgery (OS).1,2) However, EVAR requires continued surveillance because up to 11% of patients need reintervention for graft-related complications, in particular, for an endoleak.3) While the presence of Type 1 and Type 3 endleaks is a definite indication of reintervention, the clinical significance of a Type 2 endleak is not well established. Persistent Type 2 endoleaks (PT2) have been associated with increased adverse outcomes such as aneurysmal sac enlargement, the need for conversion to open repair, and rupture.4) Between May 2007 and May 2011 at our institute, 4 EVAR cases among 150 had required conversion to open repair for sac enlargement related to PT2 regardless of repeated catheter intervention following EVAR.5,6) On the basis of experiencing these adverse outcomes, coil embolization (CE) to aortic branch vessels, including inferior mesenteric artery (IMA), lumbar arteries (LAs), and medial sacral artery (MSA) prior to EVAR was initiated after the beginning of June, 2011. The purpose of this study was to report early clinical outcomes of preoperative CE and assess the effectiveness of this strategy in terms of the prevention of sac growth related to PT2.
Materials and Methods
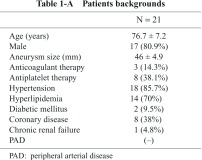
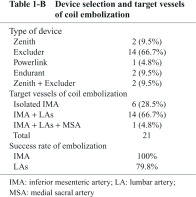
From June 2011 to April 2012, there were 21 cases of infrarenal AAA that had received CE to aortic branch vessels, including IMA, LAs, and MSA, whose patency was recognized on multi-detector row computed tomographic angiography (MDCTA) before EVAR. Selection and determination of target vessels were well discussed on the basis of imaging findings of MDCTA during the weekly vascular conference in the department of cardiology, and interventional cardiologists were mainly in charge of performing this procedure. Patient backgrounds are shown in Table 1-A. Breakdown of commercially available grafts and details of the CE procedure are demonstrated in Table 1-B; CE to both IMA and LAs was performed in 14 cases (66.7%), and CE to isolated IMA (LAs were considered to be inappropriate for CE due to small orifice diameter <2 mm on MDCTA measurement) in 6 cases (28.5%). Furthermore, CE to IMA, LAs and MSA was performed in 1 case (4.8%), and success rate of CE in this case was 100%. Overall, CE to IMA was successful in all cases (100%), and success rate of CE to LAs (total number of successful CE Las/scheduled number of target LAs) was 79.8%. 6 cases out of 21 succeeded in CE to all the scheduled target vessels.
Results
➀ MDCTA findings before discharge after EVAR revealed no Type 2 endoleaks identified in 16 cases (76%), and 2 cases showed significant shrinkage in the aneurysmal sac diameter.
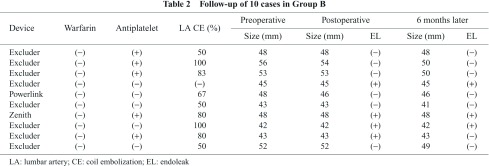
➁ Details of 10 cases, in which patients reached the 6-month follow-up MDCTA after EVAR, are revealed in Table 2. Five (50%) showed a marked reduction in the aneurysmal sac diameter. None showed sac enlargement, though 3 of 10 showed PT2 on MDCTA findings.
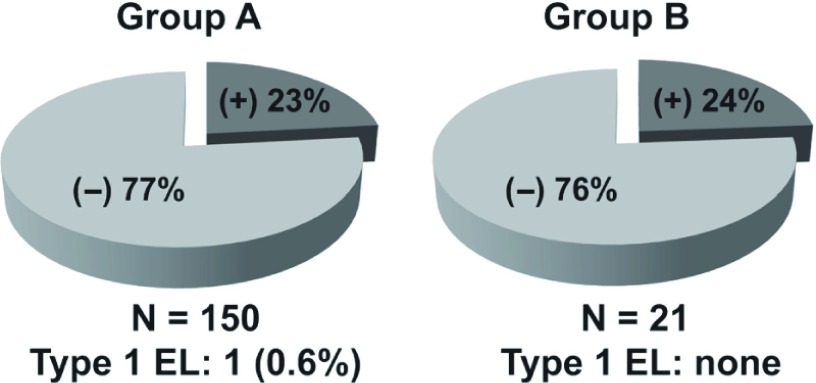
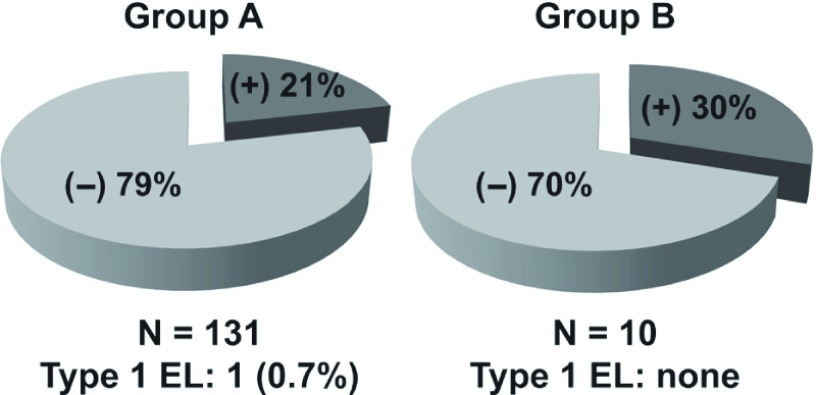
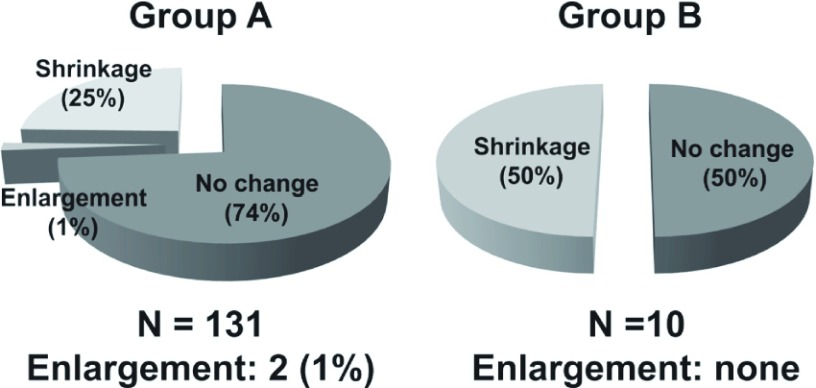
➂ Between May 2007 and April 2012 in our department, EVAR for AAA was performed for 215 cases, divided into two groups (Group A, 150 consecutive EVAR cases between May 2007 and May 2011, before the introduction of preoperative CE, and Group B, 21 EVAR cases between June 2011 and April 2012, after the introduction of preoperative CE). Early outcomes based on MDCTA findings were compared between groups. Fig. 1 reveals no significant difference between groups in the percentage of patients with Type 2 endleak before disch arge: 23% in Group A versus 24% in Group B. Although the tendency of Group B having PT2 was higher than that of Group A (30% versus 21%) at the 6-month follow-up (Fig. 2), Fig. 3 demonstrates that 50% of Group B cases had significant shrinkage of the aneurysmal sac diameter, whereas only 25% of Group A cases had shrinkage.
Fig. 1.
Differences in the rate of type 2 endoleaks between Group A and Group B before discharge.
Fig. 2.
Differences in the rate of persistent type 2 endoleaks (PT2) between Group A and Group B at 6-month follow-up.
Fig. 3.
Changes of aneurysmal sac diameters and comparisons between Group A and Group B at 6-month follow-up.
Discussion
EVAR has become a standard treatment of option for AAA, and it has been associated with significant reduction of operative mortality as well as a faster recovery time because of its being less invasive, compared to OS.1,2) However, continued surveillance is necessary after EVAR because up to 11% of cases require reintervention for graft-related complications, in particular, an endoleak.3) Although there are no doubts about a definite indication of reintervention for the presence of Type 1 and Type 3 endleaks, the clinical significance of a Type 2 endoleak has not been clearly established yet. When compared with Type 1 and Type 3 endoleaks, a Type 2 endoleak is considered to be usually benign because as many as 80% of Type 2 endleak resolves spontaneously within 6 months after EVAR.7,8) However, PT2 (present >6 months) is associated with adverse late outcomes, including aneurysmal sac enlargement, conversion to open repair, and rupture.4)
As transarterial CE to aortic branch vessels and translumbar embolization to aneurysmal sac using prothrombotic material are considered to be efficient interventional techniques for prevention of PT2 following EVAR with a reported success rate of 10% to 100%,3,9,10) we had performed transarterial retrograde CE to vessels arising from the aneurysmal bodies for applicable cases with PT2 between May, 2007 and May, 2011. Among 150 consecutive EVAR cases during this period, four cases finally received conversions to open repair for aneurysmal sac growth, including 1 emergency ruptured case, regardless of repeated catheter intervention for PT2.5,6)
Taking advantage of these unfavorable results, CE to aortic branched vessels whose patency were identified on MDCTA prior to EVAR was initiated after June, 2011 so as to reduce the risk of PT2. Although we could not show statistically significant benefits of this interventional strategy before EVAR because of marked differences in sample sizes between Group A and Group B in this study, it is remarkable to note that 50% of cases in Group B showed significant shrinkage in the aneurysmal sac diameter whereas only 25% of cases in Group A showed it. In patients with PT2 after EVAR, maximum diameter of the endoleak cavity or nidus is an important predictor of aneurysm growth, and might indicate the need for more aggressive surv eillance as well as earlier additional treatment.11) Also, Abularrage, et al. revealed that the combination of a patent IMA and more than six patent Las on MDCTA before EVAR increased the odds of PT2 approximately 9 fold, which were associated with adverse events, including aneurysmal growth.12)
So, our present basic strategy for successful EVAR is reducing the endoleak cavity as less as possible not after EVAR but before EVAR as well as completely eradicating Type 1 endoleak intraoperatively by conforming to instruction for use (IFU). Also, in cases with internal iliac artery occlusions due to CE before EVAR or atherosclerotic changes, it is technically impossible to perform retrograde CE to LAs through iliolumbar arteries. Therefore, performing CE to aortic branched vessels before EVAR is expected to have merits to improve the late outcomes of EVAR. Furthermore, prosthetic endograft infection may at times be concerned after aggressive and repeated catheter interventions.13,14) Therefore, we consider it is more desirable to perform additional catheter intervention after EVAR as less as possible to reduce the risk of this devastating complication. However, in terms of the demerits of CE before EVAR, putting the catheter into the orifices of target vessels inside the aneurysmal sac is considered to be technically dema nding. Also, it may be at times difficult to detect minor or tiny PT2 after EVAR on the MDCTA findings because of the artifacts derived from the coiling materials.
It is no exaggeration to say that surgical and hospital outcome of EVAR is superior to that of open graft replacement of abdominal aorta because EVAR is a much less invasive procedure. However, making efforts to reduce the risk of aneurysmal sac enlargement due to PT2 is crucial to attain satisfactory late outcome of EVAR, which is equivalent to that of open surgical repair. In this regard, we consider preoperative CE to aortic branched vessels can be one of the efficient endovascular treatment options.
In conclusion, although statistically significant benefits of CE to aortic branched vessels before EVAR cannot be shown in this study due to marked differences in sample size between both groups, considering dramatic shrinkage of aneurismal sac in Group B at 6 months after EVAR, this strategy will have the possib ility of contributing to improving late outcome of EVAR.
Disclosure Statement
The authors of this article do not have any conflicts of interest in this study.
References
- Lee WA, Carter JW, Upchurch G, et al. Perioperative outcomes after open and endovascular repair of intact abdominal aortic aneurysms in the United States during 2001. J Vasc Surg 2004; 39: 491-6. [DOI] [PubMed] [Google Scholar]
- Greenhalgh RM, Brown LC, Powell JT, et al. Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med 2010; 362: 1872-80. [DOI] [PubMed] [Google Scholar]
- Conrad MF, Adams AB, Guest JM, et al. Secondary intervention after endovascular aortic aneurysm repair. Ann Surg 2009; 250: 383-9. [DOI] [PubMed] [Google Scholar]
- Jones JE, Atkins MD, Brewster DC, et al. Persistent type 2 endoleak after endovascular repair of abdominal aortic aneurysm is associated with adverse late outcomes. J Vasc Surg 2007; 46: 1-8 [DOI] [PubMed] [Google Scholar]
- Hiraoka A, Yoshitaka H, Chikazawa G, et al. A modified technique of open surgical treatment for aneurysmal sac enlargement after endovascular repair. Eur J Vasc Endovasc Surg Extra 2012; 23: e45-7. [Google Scholar]
- Tanaka K, Yoshitaka H, Kuinose M, et al. A case of late conversion for type II endoleak after EVAR. Jpn J Vasc Surg 2012; 21: 599-602. [in Japanese] [Google Scholar]
- Gelfand DV, White GH, Wilson SE. Clinical significance of type II endoleak after endovascular repair of abdominal aortic aneurysm. Ann Vasc Surg 2006; 20: 69-74. [DOI] [PubMed] [Google Scholar]
- Rayt HS, Sanford RM, Salem M, et al. Conservative management of type 2 endoleaks is not associated with increased risk of aneurysm rupture. Eur J Vasc Endovasc Surg 2009; 38: 718-23. [DOI] [PubMed] [Google Scholar]
- Rial R, Serrano Fj Fj, Vega M, et al. Treatment of type II endoleaks after endovascular repair of abdominal aortic aneurysms: Translumbar puncture and injection of thrombin into the aneurysm sac. Eur J Vasc Endovasc Surg 2004; 27: 333-5. [DOI] [PubMed] [Google Scholar]
- Gorlitzer M, Mertikian G, Trnka H, et al. Translumbar treatment of type II enodleaks after endovascular repair of abdominal aortic aneurysm. Interact Cardiovasc Thorac Surg 2008; 7: 781-4. [DOI] [PubMed] [Google Scholar]
- Timaran CH, Ohki T, Rhee SJ, et al. Predicting aneurysm enlargement in patients with persistent type II endoleaks. J Vasc Surg 2004; 39: 1157-62. [DOI] [PubMed] [Google Scholar]
- Abularrage CJ, Crawford RS, Conrad MF, et al. Preoperative variables predict persistent type 2 endoleak after endovascular aneurysm repair. J Vasc Surg 2010; 52: 19-24. [DOI] [PubMed] [Google Scholar]
- Chaar CI, Eid R, Park T, et al. Delayed open conversions after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2012; 55: 1562-9. [DOI] [PubMed] [Google Scholar]
- Moulakakis KG, Dalainas I, Mylonas S, et al. Conversion to open repair after endografting for abdominal aortic aneurysm: a review of causes, incidence, results, and surgical techniques of reconstruction. J Endovasc Ther 2010; 17: 694-702. [DOI] [PubMed] [Google Scholar]