Abstract
AIM
To compare transforming growth factor-β1 (TGF-β1) levels in tears and the degree of corneal haze formation following epithelial laser in situ keratomileusis (Epi-LASIK) with and without the use of mitomycin C (MMC) and to investigate the effect of MMC on corneal wound healing.
METHODS
Thirty-two patients (64 eyes) with high myopia underwent Epi-LASIK surgery, and MMC was randomly used in one eye in each patient. The epithelialization process was observed, and the TGF-β1 level in tears was measured at 1 day, 3, and 7 days postoperatively for comparison with baseline. Corneal haze was graded at 1 month, 3, and 6 months after surgery.
RESULTS
Mean preoperative spherical equivalent refraction was -8.24±2.18D (range -6.00 to -10.50D) in the MMC group and -7.82±1.55D (range -6.00 to -9.75D) in the non-MMC group. There was no significant difference between the two groups (P=0.38). Mean epithelialization time was (5.02±0.68) days in the MMC group and (4.86±0.57) days in the non-MMC group (P=0.31). Tear fluid TGF-β1 levels were similar before surgery (P=0.34), but were significantly higher in the non-MMC group at 1 day, 3, and 7 days postoperatively (P=0.004, 0.008, and 0.012, respectively). Corneal haze scores 1 month after surgery were significantly higher in the non-MMC group (P=0.03), and similar at 3 and 6 months after surgery (P=0.28 and 0.62, respectively).
CONCLUSION
MMC did not delay epithelialization. In early postoperative period, lower TGF-β1 levels in tears and a lower grade of corneal haze were observed in the MMC group. Our findings suggest that the ability of MMC to inhibit Epi-LASIK-induced haze might be mediated through TGF-β1 suppression.
Keywords: Epi-LASIK, MMC, TGF-β1, haze
INTRODUCTION
Surface ablation is commonly performed in patients with moderate to high refractive error and thinner corneas, or in those where conservation of tissue is a factor[1]. Epithelial laser in situ keratomileusis (Epi-LASIK) is an alternative surface ablation procedure first developed by Pallikaris[2] in 2003. Theoretically, the preserved epithelial sheet may represent a barrier that protects the photoablated corneal stroma from inflammatory mediators to result in less postoperative pain, faster visual recovery, and less corneal haze[3].
Haze formation is a complication that occurs after surface corneal refractive surgery and cannot be completely prevented after Epi-LASIK, especially in patients with high myopia. Transforming growth factor-β1 (TGF-β1) has emerged as a key regulator of haze and scarring in the cornea and other tissues[4]. In recent years, mitomycin C (MMC) has been prophylactically and therapeutically used to ameliorate haze after surface ablation[5]. The purpose of this study was to compare tear fluid TGF-β1 levels and corneal haze scores in eyes with high myopia after Epi-LASIK with and without the use of MMC and to investigate the effect of MMC on corneal wound healing.
SUBJECTS AND METHODS
Subjects
The present study was approved by the Ethics Committee of the General Hospital of Guangzhou Military Command and performed according to the Helsinki Declaration. All patients received a full explanation of the procedure and provided written informed consent before surgery. Thirty-two patients (64 eyes), including 13 men and 19 women, who underwent Epi-LASIK between July 2008 and July 2011 in the Department of Ophthalmology of the General Hospital of Guangzhou Military Command were included in this prospective study. Mean patient age was 25.84±5.66 years (range 20 to 36 years). MMC was randomly applied to one eye of each patient, and both the doctor and the laboratory inspector knew which eye received MMC. Mean preoperative spherical equivalent refraction was -8.24±2.18D (range -6.00 to -10.50D) in the MMC group and -7.82±1.55D (range -6.00 to -9.75D) in the non-MMC group, which was not significantly different between groups (P=0.38). The patients were carefully examined before surgery, and showed no signs of ocular inflammation, allergy, or other ocular or systemic diseases. The patients were advised not to wear contact lenses at least 2 weeks prior to operation.
Methods
Technique
All patients underwent bilateral Epi-LASIK performed by the same surgeon in the same session. Epithelial separation was performed using an instrument designed at Kangning Company (KM-5000d, Wuxi, China). An epithelial flap with a superior hinge was created, and the flap was retracted using a spatula. The laser treatment was performed using an excimer laser (VISX Star S4, Santa Clara, CA, USA). After ablation, a 6.0mm circular corneal light shield soaked in 0.02% MMC was placed on the residual bed for 15 to 20 seconds in one random eye in each patient. The stroma was gently washed, and the flap was repositioned over the bed. After allowing the edges of the flap to adhere, two drops of tobramycin and dexamethasone (S.A. Alcon Couvreur, N.V, Puur, Belgium) and ketorolac (Allergan, Irvine, CA, USA.) were instilled into the eye. A PureVision bandage contact lens (diameter 14.0mm, base curve 8.6mm, power -0.50D; Bausch & Lomb, Rochester, NY, USA) was placed onto the eye at the end of the procedure.
Tear fluid TGF-β1 measurements
Tears were collected preoperatively and 1 day, 3, and 7 days postoperatively from the lower conjunctival sac without topical anesthesia using a scaled 20µL microcapillary tube. Special attention was paid not to irritate the cornea or conjunctiva and collect the tear fluid samples without trauma, and the time required to collect 20µL was recorded. All of the tear samples were collected by the same individual at the same time of day.
TGF-β1 levels were determined by the quantitative sandwich enzyme immunoassay technique (Quantikine human TGF-β1, R and D Systems Inc., Minneapolis, MN, USA). Microtiter plates were coated with recombinant human TGF-β1 receptor, and 0.1mL 1mol/L HCl was added to activate latent TGF-β1 and incubated for 10 minutes. The acidified samples were neutralized by adding 0.1mL 1.2mol/L NaOH/0.5mol/L HEPES. Subsequently, 200mL activated samples were added to the precoated wells and incubated for 3 hours. The unbound material was removed with washing buffer, and 200mL of antibody human TGF-β1 conjugate (diluted 1:1 000) was added and incubated at room temperature for 1.5 hours. After three washes, 200mL of substrate solution was added to each well and incubated at room temperature for 20 minutes. We added 50mL of stop solution to each well and measured the optical density with an EIA reader (LP 400, Sanofi, Diagnostics Pasteur, France) at 450nm within 30 minutes.
Postoperative treatment and follow-up
Postoperative medication included Pranoprofen eye drops (Senju Pharmaceutical Co., Ltd., Osaka, Japan) and eye drops containing tobramycin and dexamethasone (S.A. Alcon Couvreur) both administered four times daily until the therapeutic lens was removed. After lens removal, all treated eyes received 0.1% fluorometholone eye drops (Santen Pharmaceutical Co., Ltd., Osaka Japan) four times daily in a tapered dose for 5 weeks. Artificial tears (Allergan) were prescribed for use according to the patient's discretion.
Follow-up visits were scheduled at 1 day, 3, and 5 to 7 days postoperatively and then at 1 month, 3, and 6 months postoperatively. The contact lens was removed when epithelialization was complete (usually between postoperative days 5 to 7). The postoperative corneal haze score at 1 month, 3, and 6 months was considered for statistical analysis.
Haze levels were reported based on Fantes' classification[6] (0=normal, 0.5=opacity under indirect light, 1= opacity under direct light, 2=possible to observe iris in detail, 3=difficult to observe iris in detail, 4=not possible to observe iris in detail).
Statistical Analyses
Results are presented as mean±standard deviation (SD). All data were analyzed using SPSS version 13.0 software for Windows (SPSS, Chicago, IL, USA). Group differences for continuous variables were tested using paired Student's t tests. Haze scores were evaluated with Pearson's Chi-squared test with contingency tables. P values less than 0.05 were regarded as statistically significant.
RESULTS
Epithelial healing time
Mean epithelialization time was 5.02±0.68 days in the MMC group and 4.86±0.57 days in the non-MMC group. This difference was not statistically significant (P=0.31), suggesting that MMC did not delay epithelialization.
Tear fluid TGF-β1 level
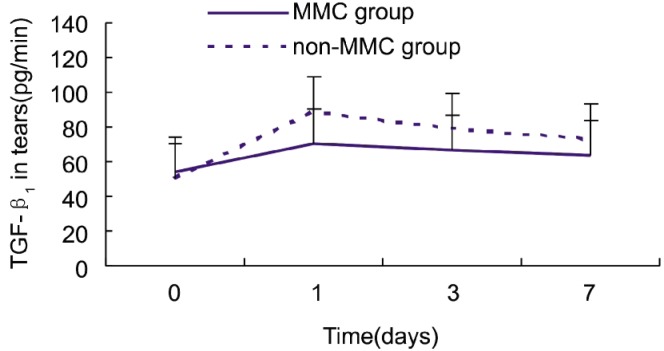
Tear fluid flow (mL/min) into the microcapillary was calculated by dividing the volume of the tear fluid sample by tear fluid collection time. TGF-β1 in the tear fluid was calculated by multiplying the concentration in the sample by the tear fluid flow in the collection microcapillary. Tear fluid TGF-β1 levels were similar in the two groups before surgery (P=0.34), but were significantly higher in the non-MMC group at 1 day, 3, and 7 days postoperatively (P=0.004, 0.008, and 0.012, respectively) (Student's t test; Table 1).
Table 1. Preoperative and postoperative TGF-β1 tear levels.
| MMC group (n=32) | Non-MMC group (n=32) | P | |
| Preoperative | 54.12±12.83 | 50.84±14.46 | 0.34 |
| Postoperative | |||
| Day 1 | 70.34±17.04 | 88.78±30.32 | 0.004 |
| Day 3 | 67.25±14.86 | 79.32±20.22 | 0.008 |
| Day 7 | 63.24±11.87 | 72.68±16.84 | 0.012 |
(x±s, pg/min)
Although TGF-β1 tear levels gradually decreased after the first postoperative day in both groups, the values on postoperative day 7 were still higher than the preoperative values (Figure 1). This result suggests that TGF-β1 may be able to inhibit corneal wound healing.
Figure 1. Tear fluid TGF-β1 levels (pg/min) in the MMC and non-MMC groups.
Haze
No eyes in this study developed a corneal haze worse than score 1 during the follow-up period. In general, corneal haze decreased with time. Corneal haze scores at 1 month after the surgery were significantly higher in the non-MMC group (P=0.03), but were similar to the MMC group at 3 and 6 months after the surgery (P=0.28 and 0.62, respectively) (Pearson Chi-square test, Table 2).
Table 2. Corneal haze scores in the MMC and non-MMC groups.
| Time | Procedure | Haze score |
P | ||
| 0 | 0.5 | 1 | |||
| 1 month | MMC group | 22 | 8 | 2 | 0.03 |
| Non-MMC group | 10 | 12 | 10 | ||
| 3 months | MMC group | 25 | 7 | 0.28 | |
| Non-MMC group | 19 | 9 | 4 | ||
| 6 months | MMC group | 27 | 5 | 0.62 | |
| Non-MMC group | 23 | 7 | 2 | ||
(n=32 each)
DISCUSSION
Corneal haze is the major complication affecting stability and predictability after surface corneal refractive surgery. Haze can also occur after Epi-LASIK, the latest type of surface ablation. MMC was originally used as a systemic chemotherapeutic agent, but topical MMC has also been widely used in ophthalmic indications. MMC is commonly used during glaucoma filtering surgery to prevent scarring and resultant bleb failure and as an adjunctive treatment in pterygium surgery[7]-[9]. Recently, MMC has been advocated as a potential modulator of wound healing after refractive surgery[10]. Here, we attempted to determine if MMC could reduce corneal haze after Epi-LASIK and investigate the possible mechanism.
Haze varies among individuals and is correlated with preoperative diopter, so in this study, MMC was randomly applied in one eye of each patient who underwent Epi-LASIK; the mean preoperative spherical equivalent refraction was not significantly different between groups, which had similar baseline characteristics. Mean epithelial healing time was not statistically significant (P=0.39) between the two groups, suggesting that the use of MMC is safe and that it does not delay epithelialization.
We found that tear fluid TGF-β1 levels were lower in the MMC group at early postoperative time points. Although the reason is not clear, we hypothesize that MMC may be able to inhibit TGF-β1 production. MMC is an antibiotic derived from Streptomyces caespitosus[11]. Its alkylating properties enable it to inhibit DNA synthesis by cross-linking adenosine and guanine nucleotides[11], [12]. At high concentrations, RNA and protein synthesis can also be affected[11], [12]. So, MMC primarily affects rapidly proliferating cells[5], including activated keratocytes, and induces keratocyte apoptosis in the anterior stroma[13]. Stromal myofibroblasts are thought to be a significant contributor to corneal haze. Corneal myofibroblasts produce atypical extracellular material and have been shown to cause greater light scatter and haze [14], [15]. Cytokine penetration facilitates the development of myofibroblasts[16], which are derived from keratocytes under the influence of TGF-β[17]. Previous studies have demonstrated that TGF-β1 is a key cytokine regulator of myofibroblast differentiation[18]-[20]. TGF-β1 is a multifunctional cytokine involved in the regulation of keratocyte activation; myofibroblast transformation, proliferation, and chemotaxis; and wound healing after refractive surgery. TGF-β1 may be released from regenerating corneal epithelial and stromal cells in the ablated area following excimer laser ablation and can be found in tear fluid[21]-[23]. MMC-induced keratocyte apoptosis could decrease the amount of TGF-β1 released, causing a corresponding reduction in the amount found in the tear fluid. These characteristics account for MMC's ability to prevent haze, which may be mediated through TGF-β1 suppression.
The corneal haze score was lower in the MMC group 1 month after surgery. A previous study reported that TGF-β1 was positively related with corneal scar formation[24], which led us to believe that the difference in the haze score between the groups could be related to amount of TGF-β1 released. Angunawela and Marshall [25] published results showing that TGF-β1 inhibition could prevent haze after refractive surgery. However, corneal haze decreased in both groups over time, and there was no statistically significant difference between the two groups at 3 and 6 months after the operation, suggesting that the effects of MMC are short lived.
In conclusion, our findings suggest that use of MMC following Epi-LASIK is associated with a lower grade of corneal haze in the early postoperative period, and this effect may be mediated through TGF-β1 suppression. We did not observe any long-term effects of MMC on haze after Epi-LASIK. Further investigation, including pharmacological and molecular biological studies, will provide more information regarding MMC's mechanism.
Footnotes
Foundation item: Medical Scientific Foundation of Guangdong Province, China (No. B2010249)
REFERENCES
- 1.Trattler WB, Barnes SD. Current trends in advanced surface ablation. Curr Opin Ophthalmol. 2008;19(4):330–334. doi: 10.1097/ICU.0b013e3283034210. [DOI] [PubMed] [Google Scholar]
- 2.Pallikaris IG, Katsanevaki VJ, Kalyvianaki MI, Naoumidi II. Advances in subepithelial excimer refractive surgery techniques: Epi-LASIK. Curr Opin Ophthalmol. 2003;14(4):207–212. doi: 10.1097/00055735-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Katsanevaki VJ, Naoumidi II, Kalyvianaki MI, Pallikaris G. Epi-LASIK: histological findings of separated epithelial sheets 24 hours after treatment. J Refract Surg. 2006;22(2):151–154. doi: 10.3928/1081-597X-20060201-12. [DOI] [PubMed] [Google Scholar]
- 4.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19(1):113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 5.Carones F, Vigo L, Scandola E, Vacchini L. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy. J Cataract Refract Surg. 2002;28(12):2088–2095. doi: 10.1016/s0886-3350(02)01701-7. [DOI] [PubMed] [Google Scholar]
- 6.Fantes FE, Hanna KD, Waring GO, III, Pouliquen Y, Thompson KP, Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990;108(5):665–675. doi: 10.1001/archopht.1990.01070070051034. [DOI] [PubMed] [Google Scholar]
- 7.Palmer SS. Mitomycin as adjunct chemotherapy with trabeculectomy. Ophthalmology. 1991;98(3):317–321. doi: 10.1016/s0161-6420(91)32293-0. [DOI] [PubMed] [Google Scholar]
- 8.Mahar PS, Nwokora GE. Role of mitomycin C in pterygium surgery. Br J Ophthalmol. 1993;77(7):433–435. doi: 10.1136/bjo.77.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraut A, Drnovsek-Olup B. Instillation of mitomycin C after recurrent pterygium surgery. Eur J Ophthalmol. 1996;6(3):264–267. doi: 10.1177/112067219600600307. [DOI] [PubMed] [Google Scholar]
- 10.Talamo JH, Gollamudi S, Green WR, De La Cruz Z, Filatov V, Stark WJ. Modulation of corneal wound healing after excimer laser keratomileusis using topical mitomycin C and steroids. Arch Ophthalmol. 1991;109(8):1141–1146. doi: 10.1001/archopht.1991.01080080101040. [DOI] [PubMed] [Google Scholar]
- 11.Galm U, Hager MH, Van Lanen SG, Ju J, Thorson JS, Shen B. Antitumor antibiotics: bleomycin, enediynes, and mitomycin. Chem Rev. 2005;105(2):739–758. doi: 10.1021/cr030117g. [DOI] [PubMed] [Google Scholar]
- 12.Fujita T, Tamura T, Yamada H, Yamamoto A, Muranishi S. Pharmacokinetics of mitomycin C (MMC) after intraperitoneal administration of MMC-gelatin gel and its antitumor effects against sarcoma-180 bearing mice. J Drug Target. 1997;4(5):289–296. doi: 10.3109/10611869708995844. [DOI] [PubMed] [Google Scholar]
- 13.Gambato C, Ghirlando A, Moretto E, Busato F, Midena E. Mitomycin C modulation of corneal wound healing after photorefractive keratectomy in highly myopic eyes. Ophthalmology. 2005;112(2):208–218. doi: 10.1016/j.ophtha.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Latvala T, Tervo K, Mustonen R, Tervo T. Expression of cellular fibronectin and tenascin in the rabbit cornea after excimer laser photorefractive keratectomy: a 12 month study. Br J Ophthalmol. 1995;79(1):65–69. doi: 10.1136/bjo.79.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jester JV, Budge A, Fisher S, Huang J. Corneal keratocytes: phenotypic and species differences in abundant protein expression and in vitro light-scattering. Invest Ophthalmol Vis Sci. 2005;46(7):2369–2378. doi: 10.1167/iovs.04-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pallikaris IG, Naoumidi II, Kalyvianaki MI, Katsanevaki VJ. Epi-LASIK: comparative histological evaluation of mechanical and alcohol-assisted epithelial separation. J Cataract Refract Surg. 2003;29(8):1496–1501. doi: 10.1016/s0886-3350(03)00348-1. [DOI] [PubMed] [Google Scholar]
- 17.Barbosa FL, Chaurasia S, Cutler A, Asosingh K, Kaur H, Medeiros FW, Agrawal V, Wilson SE. Corneal myofibroblast generation from bone marrow- devided cells. Exp eye Res. 2010;91(1):92–96. doi: 10.1016/j.exer.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrington LM, Albon J, Anderson I, Kamma C, Boulton M. Differential regulation of key stages in early corneal wound healing by TGF-β isoforms and their inhibitors. Invest Ophthalmol Vis Sci. 2006;47(5):1886–1894. doi: 10.1167/iovs.05-0635. [DOI] [PubMed] [Google Scholar]
- 19.Wilson SE, Mohan RR, Ambrosio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20(5):625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 20.Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-β1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257(5):180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin HC, Marshall J. Growth factors in corneal wound healing following refractive surgery: a review. Acta Ophthalmol Scand. 2002;80(3):238–247. doi: 10.1034/j.1600-0420.2002.800303.x. [DOI] [PubMed] [Google Scholar]
- 22.Mita T, Yamashita H, Kaji Y, Obata H, Yamada H, Kato M, Hanyu A, Suzuki M, Tobari I. Effects of transforming growth factor-β on corneal epithelial and stromal cell function in rat wound healing model after excimer laser keratectromy. Graefe's Arc Clin Exp Ophthalmol. 1998;236(11):834–843. doi: 10.1007/s004170050168. [DOI] [PubMed] [Google Scholar]
- 23.Vesaluoma M, Teppo AM, Gronhagen-Riska C, Tervo T. Release of TGF-β1 and VEGF in tears following photorefractive keratectomy. Curr Eye Res. 1997;16(1):19–25. doi: 10.1076/ceyr.16.1.19.5119. [DOI] [PubMed] [Google Scholar]
- 24.Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77(5):581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 25.Angunawela RI, Marshall J. Inhibition of transforming growth factor-beta1 and its effects on human corneal fibroblasts by mannose-6-phosphate Potential for preventing haze after refractive surgery. J Cataract Refract Surg. 2010;36(1):121–126. doi: 10.1016/j.jcrs.2009.07.042. [DOI] [PubMed] [Google Scholar]