Abstract
AIM
To report on the outcome of Ahmed glaucoma valve (AGV) implantation for the management of neovascular glaucoma (NVG) after 23-gauge vitrectomy for proliferative diabetic retinopathy (PDR).
METHODS
Twelve medically uncontrolled NVG with earlier 23-gauge vitrectomy for PDR underwent AGV implantation. The control of intraocular pressure (IOP), preoperative and postoperative best-corrected visual acuity, the development of intraoperative and postoperative complications were evaluated during the follow-up.
RESULTS
The mean follow-up was 15.4±4.3 months (9-23 months). Mean preoperative IOP was 49.4±5.1mmHg and mean postoperative IOP at the last visit was 17.5±1.6mmHg. The control of IOP was achieved at the final follow-up visits in all patients, however, 8 of 12 patients still needed anti-glaucoma medication (mean number of medications, 0.8±0.7). The visual acuity improved in nine eyes, and the visual acuity unchanged in three eyes at the final follow-up visits. The complications that occurred were minor hyphema in three eyes, choroid detachment in two eyes, and the minor hyphema and choroid detachments were reabsorbed without any surgical intervention.
CONCLUSION
AGV implantation is a safe and effective procedure that enables successful IOP control and vision preservation in the NVG patients with the history of earlier 23-gauge vitrectomy for PDR.
Keywords: Ahmed glaucoma valve implantation, neovascular glaucoma, proliferative diabetic retinopathy, 23-gauge vitrectomy
INTRODUCTION
Neovascular glaucoma (NVG) is one of the common complications of vitrectomy for proliferative diabetic retinopathy (PDR)[1], [2]. For treating NVG, medical management alone is often inadequate for intraocular pressure (IOP) control, which usually necessitates surgical intervention. However, conventional filtration surgery carries a poor prognosis in the eyes with NVG because of enhanced fibrovascular proliferation or bleeding[3]. Glaucoma drainage implants have been used in the treatment of NVG in PDR patients, which has alternate aqueous pathway that promotes bleb formation far from the limbus[4], [5].
Recently, 23-gauge sutureless vitrectomy has be-come more popular than 20-gauge vitrectomy. The small gauge vitrectomy has potential advantages over traditional 20-gauge pars plana vitrectomy, which includes improved healing time, increased postoperative comfort and dimin-ished disturbance of the conjunctiva particularly with regard to trabeculectomy sites[6]. Previous studies have shown the effectiveness of glaucoma drainage implantation for the management of NVG associated with PDR in the 20-gauge vitrectomized eyes[7],[8]. However, little is known about the safety and efficacy of glaucoma drainage implantation for the management of NVG in the eyes with the history of earlier 23-gauge vitrectomy for PDR. The purpose of this retrospective case series was to report on the outcome of Ahmed glaucoma valve (AGV) implantation for the management of the uncontrolled NVG after 23-gauge vitrectomy for PDR.
SUBJECTS AND METHODS
The study was performed in the Department of Ophthalmology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine between January 2010 and July 2012. The research followed the tenets of the Declaration of Helsinki, and approval of the study was obtained from the institutional review board of Ruijin Hospital, Shanghai, China. All patients received a detailed explanation of the study, and provided a written informed consent.
Subjects
All patients were diagnosed as PDR, and had undergone 23-gauge sutureless vitrectomy combined with membrane segmentation or additional procedures (endophotocoagulation, phacoemulsification, and intraocular lens implantation), if needed. In patients with retinal detachment, intraocular tamponade with silicone oil (SO) or C3F8 was used to reattach the retina. The SO was removed at the surgeon's discretion to prevent SO associated complications, and the SO was also removed when the IOP increased owing to the pupillary block that emerged from the SO overfilling.
NVG was diagnosed when an IOP elevation of 22mmHg or more was accompanied by neovascularization of the iris and/or the anterior chamber angle. Twelve patients with PDR developed into NVG after 23-gauge vitrectomy were enrolled in this study, and their IOP was more than 30mmHg despite maximum tolerated oral and topical anti-glaucoma medical therapy.
Methods
A Model FP7 AGV (New World Medical Inc., Rancho Cucamonga, CA, USA) was used for all patients, and the surgery was performed by the same doctor (Zhong YS). The AGVs for 11 patients were implanted on the superotemporal quadrant, and the AGV for one patient was implanted on inferotemporal quadrant because of a superior shallow anterior chamber (broadly peripheral anterior synechia). The AGV implantation was carried out using a superotemporal or inferotemporal fornix-based conjunctival flap, which was created between the superior rectus and the lateral rectus or between the lateral rectus and inferior rectus. A piece of cotton containing 0.02% mitomycin C was placed between the conjunctival flap and sclera 9-12mm posterior to the limbus for 3-5 minutes, then washed thoroughly with balanced salt solution (BSS). Patency of the AGV tube was verified by irrigation with BSS. The AGV plate was fixed to the sclera with 5-0 nylon sutures 8-9mm posterior to the limbus between the rectus muscles. A half-thickness limbus-based scleral flap was then created with a 5mm×5mm rectangle. The anterior chamber was entered through the limbus under the scleral flap with a 23-gauge needle. After the tube was cut to have a bevel-up appearance so that it would not cross the pupil margin in the anterior chamber, the AGV tube was then placed into the anterior chamber parallel to the iris plane through the needle track. Then the scleral flap was sutured to cover the AGV tube. The conjunctiva and Tenon capsule were closed with 8-0 absorption sutures. After AGV implantation, all patients received a standard topical regimen including TobraDex eye drops (0.3% tobramycin + 0.1% dexamethasone, Alcon, Belgium) for 6-8 weeks and 1% atropine sulfate for 1 week-2 weeks.
Data collected included demographic information, the number and type of previous surgery, the interval time between previous surgery and glaucoma valve implantation, the preexistence of glaucoma or neovascularization of the iris and/or the anterior chamber angle before the earlier vitrectomy, preoperative and postoperative best corrected visual acuity (BCVA), and IOP (which was measured with a Goldmann applanation tonometer), number of glaucoma medications, complications and follow-up time. The follow-up period was AGV post-operation 1 day, 1 week, 2 weeks, 1 month, 2 months and 3 months later, and every 3 months thereafter. More frequent examinations were conducted when clinically indicated. Glaucoma medications were prescribed when the postoperative IOP was greater than 21mmHg after massage, and the medications were added or removed according to the IOP change.
Statistical Analysis
Data are expressed as mean±SD, unless otherwise stated. Statistical analyses were performed using the SPSS11.0 Statistical software package. The independent student t-test was used for comparing the IOP and the number of glaucoma medications between the pre-operation and the last visit of post-operation. P<0.05 was considered statistically significant.
RESULTS
All cases were PDR patients and had previously undergone 23-gauge sutureless vitrectomy. All patients had no preexistence of glaucoma or neovascularization of the iris and/or the anterior chamber angle before the earlier vitrectomy. The patients received at least 9 months of follow-up care, and the baseline and clinical characteristics of patients were sum-marized in Table 1.
Table 1. Preoperative and postoperative clinical findings with patient information.
| No. | Gender/age(a) | Eye | Previous surgery | Interval time (month) | Pre-operative |
||
| BCVA | IOP (mmHg) | Meds (n) | |||||
| 1 | F/58 | OS | Vitr+phaco+IOL+SOI+SOR | 7 | 0.2 | 51 | 3 |
| 2 | M/63 | OS | Vitr+phaco+IOL | 11 | FC | 48 | 3 |
| 3 | M/45 | OS | Vitr | 8 | 0.1 | 56 | 3 |
| 4 | F/69 | OD | Vitr+phaco+IOL | 10 | LP | 58 | 3 |
| 5 | F/43 | OD | Vitr | 6 | 0.1 | 46 | 3 |
| 6 | M/48 | OS | Vitr+SOI+SOR | 5 | FC | 43 | 3 |
| 7 | M/70 | OD | Vitr+phaco+IOL | 10 | 0.2 | 48 | 3 |
| 8 | F/81 | OS | Vitr+phaco+IOL | 4 | HM | 53 | 2 |
| 9 | M/56 | OS | Vitr+phaco+IOL | 6 | 0.3 | 40 | 3 |
| 10 | F/40 | OS | Vitr+phaco+IOL | 7 | HM | 52 | 3 |
| 11 | M/68 | OS | Vitr+phaco+SOI+SOR | 7 | LP | 48 | 3 |
| 12 | M/60 | OD | Vitr+phaco+IOL | 9 | 0.01 | 50 | 3 |
| No. | Post-operative |
Follow-up (month) | Complication | ||
| BCVA | IOP (mmHg) | Meds (n) | |||
| 1 | 0.6 | 18 | 1 | 23 | Minor hyphema |
| 2 | FC | 18 | 0 | 21 | Choroid detachment |
| 3 | 0.7 | 16 | 1 | 19 | |
| 4 | 0.08 | 20 | 0 | 17 | |
| 5 | 0.4 | 18 | 2 | 17 | |
| 6 | FC | 17 | 1 | 16 | |
| 7 | 0.6 | 17 | 0 | 14 | Minor hyphema |
| 8 | 0.02 | 19 | 1 | 14 | Choroid detachment |
| 9 | 0.5 | 19 | 1 | 13 | |
| 10 | 0.02 | 18 | 2 | 12 | |
| 11 | LP | 16 | 0 | 10 | Minor hyphema |
| 12 | 0.1 | 14 | 1 | 9 | |
Interval time: Interval time between previous surgery and glaucoma valve implantation; BCVA: Best corrected visual acuity; IOP: Intraocular pressure; Meds: Glaucoma medications; Vitr: Vitrectomy; phaco: Phacoemulsification; IOL: Intraocular lens; SOI: Silicone oil injection; SOR: Silicone oil removal; FC: Finger counting; HM: Hand motions; LP: Light perception.
The mean age of twelve patients (7 male, 5 female) was 58.4±12.6 years (range, 40-81 years). The mean follow-up was 15.4±4.3 months (range, 9-23 months). Seven patients had a previous vitrectomy+phacoemulsification (phaco)+intraocular lens (IOL), one patient had a previous vitrectomy+phaco+IOL+silicone oil injection (SOI)+ silicone oil removal (SOR), one had a previous vitrectomy+SOI+SOR, one had a previous vitrectomy+phaco+ SOI+SOR, and two patients had only a previous vitrectomy. The mean interval time between previous surgery and glaucoma valve implantation was 7.5±2.2 months (range, 4-11 months).
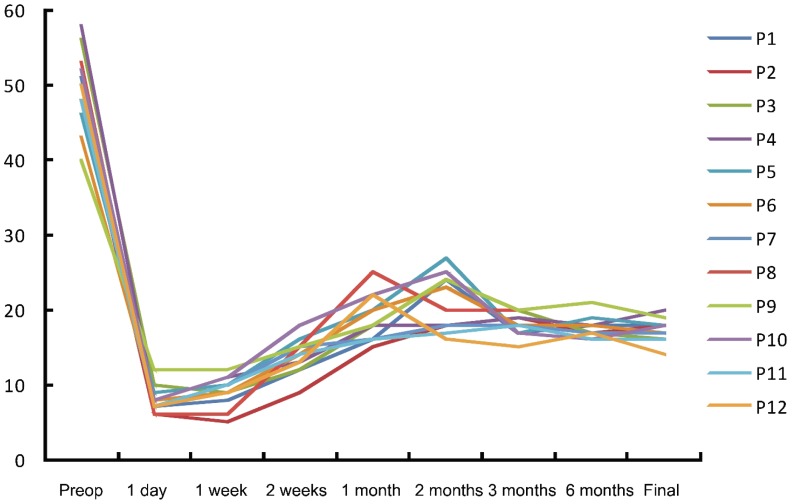
The postoperative IOP in all patients showed significantly reduced. The mean preoperative IOP was 49.4±5.1mmHg and the average postoperative IOP at the last visit was (17.5±1.6)mmHg (P<0.001). Figure 1 showed the profile of IOP change in all patients, and the postoperative IOP in all patients were significantly reduced. Even though the control of IOP (14-20mmHg) was achieved at the final follow-up visits in all patients, 8 of 12 patients still needed anti-glaucoma medication. However, the mean number of topical anti-glaucoma medication (0.8±0.7) was significantly reduced from that of the pre-operation (2.9±0.3) (P<0.001). The visual acuity improved in nine eyes at the final follow-up visits, and the visual acuity unchanged in three eyes with retaining preoperative finger counting or light perception (patient 2, 6, 11) (Table 1).
Figure 1. IOP changes in all patients at the preoperation (preop) and postoperation.
No unmanageable intraoperative or postoperative complications developed in these patients. Minor hyphema occurred at the intraoperation in three eyes, which recovered spontaneously at the postoperative 2-3 days. Choroid detachment occurred in two eyes at the postoperative early phase, and the choroid detachments were reabsorbed without any surgical intervention. More interesting, no shallow anterior chamber was found in the eyes with choroid detachment. No tube obstruction, plate exposure, conjunctival erosion and endophthalmitis were found in these patients.
DISCUSSION
Vitrectomy has long been the mainstay of surgical treatment for the blinding complications of advanced PDR such as persistent vitreous hemorrhage, tractional retinal detachment, and fibrovascular proliferation involving the macula[9]-[12]. However, vitrectomized eyes often show postoperative progression of neovascularization in the anterior segment, which results in NVG[13]. The incidence of postoperative NVG has been reported to range from 2% to 18%[14]-[18]. Goto et al[13] reported that the probability of NVG occurrence at 6 and 12 months after vitrectomy was 6.0% and 7.1% respectively, and the mean period between vitrectomy and development of NVG was 151 days (range, 50-530 days). The present study showed that the mean interval time between previous vitrectomy and AGV implantation was (7.5±2.2) months, which was basically consistent with the period between vitrectomy and development of NVG reported by Goto et al[13].
Earlier studies revealed that NVG occurrence in PDR patients after vitrectomy was significantly associated with combined lens extraction or preoperative aphakia[15], [19], [20] and residual retinal detachment after vitrectomy[20], [21]. However, the current procedure for vitrectomy of PDR eyes rarely leads to postoperative phakic eyes because lens sparing causes postoperative cataract formation[22], [23], and inadequate vitrectomy is associated with a higher rate of subsequent operations[24], [25]. Recent combined lens extraction with vitrectomy has been performed by phacoemulsification with an intraocular lens implantation[13]. Nine of 12 eyes were also performed 23-gauge vitrectomy combined with phacoemulsification in our study.
The glaucoma drainage implantation is gaining wide acceptance as a primary procedure for the patients with NVG. Variable success rates for IOP control have been reported. Netland et al[26] reported surgical success rates of 73.1% at 1 year and 61.9% at 2 years after AVG implantation. Yalvac et al[5] reported 63.2%and 56.2% at 1 year and 2 years after AGV implantation, respectively. After AGV implantation in NVG with earlier vitrectomy, Park et al[7] reported the cumulative probabilities of success were 89.9% at 1 year, 74.8% at 2 years, and 62.5% at 3 years for the NVG patients with the history of earlier 20-gauge vitrectomy in PDR. However, there is almost no report in literature on the result of AGV implantation in the NVG patients with the history of earlier 23-gauge vitrectomy for PDR. The present study was conducted to evaluate the clinical outcomes of the AGV implantation in the NVG patients with the history of earlier 23-gauge vitrectomy for PDR. Our results showed that the control of IOP was achieved at the final follow-up visits in all patients, but the majority of patients (8 of 12 patients) still needed anti-glaucoma medication. However, the mean number of topical anti-glaucoma medication was significantly reduced from that of the pre-operation, meanwhile, the visual acuity improved in the majority of patients. No serious postoperative com-plications were noted in any of the 12 patients during the follow-up period.
Limitations of the present study include its retrospective nature, lack of a control series, small sample size, and lim-ited follow-up period. A direct case-controlled study and further prospective randomized trials with a longer follow-up will be required to evaluate the safety and efficacy of AGV implantation in the NVG patients with the history of earlier 23-gauge vitrectomy for PDR.
In summary, AGV implantation is a safe and effective procedure that enables successful IOP control and vision preservation in the NVG patients with the history of earlier 23-gauge vitrectomy for PDR.
Footnotes
Foundation item: Shanghai Leading Academic Discipline Project, China (No.S30205)
References
- 1.Diabetic Retinopathy Vitrectomy Study Research Group Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Four-year results of a randomized trial: Diabetic Retinopathy Vitrectomy study report 5. Arch Ophthalmol. 1990;108(7):958–9642. doi: 10.1001/archopht.1990.01070090060040. [DOI] [PubMed] [Google Scholar]
- 2.Shazly TA, Latina MA. Neovascular glaucoma: etiology, diagnosis and prognosis. Semin Ophthalmol. 2009;24(2):113–121. doi: 10.1080/08820530902800801. [DOI] [PubMed] [Google Scholar]
- 3.Allen RC, Bellows AR, Hutchinson BT, Murphy SD. Filtration surgery in the treatment of neovascular glaucoma. Ophthalmology. 1982;89(10):1181–1187. doi: 10.1016/s0161-6420(82)34672-2. [DOI] [PubMed] [Google Scholar]
- 4.Hong CH, Arosemena A, Zurakowski D, Ayyala RS. Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol. 2005;50(1):48–60. doi: 10.1016/j.survophthal.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Yalvac IS, Eksioglu U, Satana B, Duman S. Long-term results of Ahmed valve and Molteno implant in neovascular glaucoma. Eye. 2007;21(1):65–70. doi: 10.1038/sj.eye.6702125. [DOI] [PubMed] [Google Scholar]
- 6.Wimpissinger B, Kellner L, Brannath W, Krepler K, Stolba U, Mihalics C, Binder S. 23-gauge versus 20-gauge system for pars planavitrectomy: a prospective randomized clinical trial. Br J Ophthalmol. 2008;92(11):1483–1487. doi: 10.1136/bjo.2008.140509. [DOI] [PubMed] [Google Scholar]
- 7.Park UC, Park KH, Kim DM, Yu HG. Ahmed glaucoma valve implantation for neovascular glaucoma after vitrectomy for proliferative diabetic retinopathy. J Glaucoma. 2011;20(7):433–438. doi: 10.1097/IJG.0b013e3181f3eb06. [DOI] [PubMed] [Google Scholar]
- 8.Scott IU, Alexandrakis G, Flynn HW, Jr, Smiddy WE, Murray TG, Schiffman J, Gedde SJ, Budenz DL, Fantes F, Parrish RK. Combined pars plana vitrectomy and glaucoma drainage implant placement for refractory glaucoma. Am J Ophthalmol. 2000;129(3):334–341. doi: 10.1016/s0002-9394(99)00363-3. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298(8):902–916. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- 10.Scanlon PH. Why do patients still require surgery for the late complications of proliferative diabetic retinopathy? Eye. 2010;24(3):435–440. doi: 10.1038/eye.2009.320. [DOI] [PubMed] [Google Scholar]
- 11.Newman DK. Surgical management of the late complications of proliferative diabetic retinopathy. Eye. 2010;24(3):441–449. doi: 10.1038/eye.2009.325. [DOI] [PubMed] [Google Scholar]
- 12.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 13.Goto A, Inatani M, Inoue T, Awai-Kasaoka N, Takihara Y, Ito Y, Fukushima M, Tanihara H. Frequency and risk factors for neovascular glaucoma after vitrectomy in eyes with proliferative diabetic retinopathy. J Glaucoma. 2012 doi: 10.1097/IJG.0b013e31824d514a. Epub ahead of print, PMID:22407392. [DOI] [PubMed] [Google Scholar]
- 14.Blankenship GW, Machemer R. Long-term diabetic vitrectomy results. Report of 10 year follow-up. Ophthalmology. 1985;92(4):503–506. doi: 10.1016/s0161-6420(85)34015-0. [DOI] [PubMed] [Google Scholar]
- 15.Summanen P. Neovascular glaucoma following vitrectomy for diabetic eye disease. Acta Ophthalmol. 1988;66(1):110–116. doi: 10.1111/j.1755-3768.1988.tb08544.x. [DOI] [PubMed] [Google Scholar]
- 16.Oldendoerp J, Spitznas M. Factors influencing the results of vitreous surgery in diabetic retinopathy. I. Iris rubeosis and/or active neovascularization at the fundus. Graefes Arch Clin Exp Ophthalmol. 1989;227(1):1–8. doi: 10.1007/BF02169815. [DOI] [PubMed] [Google Scholar]
- 17.Sima P, Zoran T. Long-term results of vitreous surgery for proliferative diabetic retinopathy. Doc Ophthalmol. 1994;87(3):223–232. doi: 10.1007/BF01203852. [DOI] [PubMed] [Google Scholar]
- 18.Kumagai K, Furukawa M, Ogino N, Larson E, Iwaki M, Tachi N. Long-term follow-up of vitrectomy for diffuse nontractional diabetic macular edema. Retina. 2009;29(4):464–472. doi: 10.1097/IAE.0b013e31819c632f. [DOI] [PubMed] [Google Scholar]
- 19.Blankenship GW. The lens influence on diabetic vitrectomy results. Report of a prospective randomized study. Arch Ophthalmol. 1980;98(12):2196–2198. doi: 10.1001/archopht.1980.01020041048008. [DOI] [PubMed] [Google Scholar]
- 20.Scuderi JJ, Blumenkranz MS, Blankenship G. Regression of diabetic rubeosis iridis following successful surgical reattachment of the retina by vitrectomy. Retina. 1982;2(4):193–196. [PubMed] [Google Scholar]
- 21.Rice TA, Michels RG, Rice EF. Vitrectomy for diabetic traction retinal detachment involving the macula. Am J Ophthalmol. 1983;95(1):22–33. doi: 10.1016/0002-9394(83)90330-6. [DOI] [PubMed] [Google Scholar]
- 22.Novak MA, Rice TA, Michels RG, Auer C. The crystalline lens after vitrectomy for diabetic retinopathy. Ophthalmology. 1984;91(12):1480–1484. doi: 10.1016/s0161-6420(84)34100-8. [DOI] [PubMed] [Google Scholar]
- 23.Hutton WL, Pesicka GA, Fuller DG. Cataract extraction in the diabetic eye after vitrectomy. Am J Ophthalmol. 1987;104(1):1–4. doi: 10.1016/0002-9394(87)90284-4. [DOI] [PubMed] [Google Scholar]
- 24.Kadonosono K, Matsumoto S, Uchio E, Sugita M, Akura J, Ohno S. Iris neovascularization after vitrectomy combined with phacoemulsification and intraocular lens implantation for proliferative diabetic retinopathy. Ophthalmic Surg Lasers. 2001;32(1):19–24. [PubMed] [Google Scholar]
- 25.Schiff WM, Barile GR, Hwang JC, Tseng JJ, Cekiç O, Del Priore LV, Chang S. Diabetic vitrectomy: influence of lens status upon anatomic and visual outcomes. Ophthalmology. 2007;114(3):544–550. doi: 10.1016/j.ophtha.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Netland PA, Ishida K, Boyle JW. The Ahmed glaucoma valve in patients with and without neovascular glaucoma. J Glaucoma. 2010;19(9):581–586. doi: 10.1097/IJG.0b013e3181ca7f7f. [DOI] [PubMed] [Google Scholar]