Abstract
AIM
To evaluate the increase in corrected distance visual acuity (CDVA) after laser in situ keratomileusis (LASIK) in adults with anisometropic amblyopia.
METHODS
The medical records of consecutive patients diagnosed with anisometropic amblyopia at the time of refractive evaluation who underwent LASIK were retrospectively reviewed. Patients with at least a two-line difference of visual acuity (VA) between the eyes with a spherical refractive error difference of at least 3.00 diopters (D) or an astigmatic difference of at least 2.00D were included. Patients with any other possible reason for amblyopia other than anisometropia or those who had undergone previous amblyopia treatment were excluded. Amblyopic eyes with myopia or myopic astigmatism were considered as group 1, hypermetropia or hypermetropic astigmatism constituted group 2, and mixed astigmatism patients comprised group 3. Uncorrected distance visual acuity (UDVA), subjective manifest refraction, and CDVA were analyzed at 1 week and 1 month, 3, and 6 months.
RESULTS
The study included 57 eyes of 57 patients. There were 33 eyes in group 1, 12 eyes in group 2, and 12 eyes in group 3. The preoperative mean values for spherical equivalent of subjective manifest refraction (SE) in groups 1, 2, and 3 were (-4.66±1.97)D, (4.40±1.00)D, and (0.15±1.05)D, respectively. Mean CDVA improved 0.1 log units (1 line LogMAR) at 6 months (P<0.05). Sixteen eyes (28%) exhibited an improvement in CDVA in week 1. Fourteen eyes (25%) experienced two or more lines of CDVA improvement at month 6. There were no statistically significant differences among the groups in terms of CDVA (P>0.05). Moreover, age, the amount of preoperative refractive error, and the levels of preoperative corrected and UDVA had no effect on postoperative CDVA improvement (P>0.05).
CONCLUSION
Correction of refractive errors with LASIK produced significant CDVA improvement in adult patients with anisometropic amblyopia and no previous amblyopia treatment.
Keywords: amblyopia, laser in situ keratomileusis, refractive surgery, visual acuity
INTRODUCTION
Amblyopia is believed to result from inadequate foveal or peripheral retinal stimulation or abnormal binocular interaction that results in different visual inputs from the foveae[1]. Refractive amblyopia may result from anisometropia.
It is widely accepted that visual plasticity only occurs during critical visual development periods[2]. Accordingly, there is a misconception that amblyopia cannot be treated after childhood and that occlusion therapy must be implemented during the critical period to be effective. However, there are actually three different periods of visual acuity (VA): a period of VA development, a period during which deprivation is effective in producing amblyopia, and a period of recovery during which recovery from amblyopia can be obtained[3]. The critical period corresponds to the period during which deprivation is effective rather than the period during which recovery can be obtained. In fact, particular properties of the visual system have different critical periods, and most of these periods fall somewhere between eye opening and puberty[4]. Importantly, the recovery period is not limited to the critical period(s) and may include adulthood. Studies in the literature report amblyopic eye improvement in an adult patient after visual loss in the non-amblyopic eye[5], [6]. In addition, improved corrected distance visual acuity (CDVA) has been reported after refractive surgery on adult eyes with refractive amblyopia[7]-[10]. These studies are limited in number, and although they agree that there is an improvement, the reported rates of improvement are highly variable.
The purpose of this study was to evaluate CDVA improvement in patients with anisometropic refractive amblyopia to determine the timing and extent of VA improvement. CDVA of non-amblyopic eyes was also analyzed. We also investigated possible relationships with the type of preoperative refractive error, preoperative CDVA and UDVA, preoperative SE of the refractive error, preoperative defocus equivalent (DEQ) of the refractive error, and patient age.
SUBJECTS AND METHODS
Subjects
This retrospective study was approved by the ethics committee of Beyoglu Eye Research and Training Hospital. The medical records of consecutive patients diagnosed with anisometropic refractive amblyopia at the time of refractive evaluation who underwent laser in situ keratomileusis (LASIK) were retrospectively reviewed. Inclusion criteria were simultaneous LASIK surgery on both eyes performed between 2008 and 2011, anisometropic amblyopia in one eye, age ≥20 years old, best corrected VA of at least 20/25 in the non-amblyopic eye, no ocular disease other than the refractive error, normal topographic pattern, and regular retinoscopic reflex. Amblyopia is truly a spectrum of visual loss, ranging from missing a few letters on a line to hand motion vision. In addition to loss of Snellen acuity, amblyopes exhibit loss of sensitivity to contrast over a wide range of spatial frequencies[11], loss of Vernier acuity[12], distortions in stimuli shape[13], an increase in the magnitude of crowding effect[14], and motion deficits[15]. Thus, VA measurement alone is not the best way to investigate visual function loss in these eyes[16]. However, a cut-off value in terms of VA is needed for practical purposes, and an interocular difference of two or more lines in CDVA is a widely used diagnostic criterion for amblyopia[17]. Accordingly, inclusion criteria in this study were at least two lines of difference of CDVA and a spherical refractive error difference of at least 3.00 diopters (D) or astigmatic difference of at least 2.00D between the eyes. Careful history taking is routine in our refractive surgery clinic, and assessment of eye alignment is a part of routine preoperative examinations. Patients with strabismus or previous amblyopia treatment were excluded. In our clinic, refractive surgery patients are routinely seen at postoperative day 1 and month 1, 3, and 6. One of our aims was to analyze the pattern of CDVA increase; only patients who attended all four follow-up visits were therefore included in the study.
Refractive error categorization
Patient preoperative refractive errors were categorized into one of the following three groups: Myopia or myopic astigmatism (group 1), hypermetropia or hypermetropic astigmatism (group 2), or mixed astigmatism (group 3). VA deficit and the proportional improvement were calculated as described by MOTAS collaborative group[18].
Methods
In all surgeries, corneal flaps were created with Schwind pendular microkeratome (Schwind-eye-tech solutions GmbH, Kleinostheim, Germany), and excimer laser photoablation was performed with Schwind Amaris 750S (Schwind-eye-tech solutions GmbH, Kleinostheim, Germany) or Schwind Amaris 500E (Schwind-eye-tech solutions GmbH, Kleinostheim, Germany). A standard protocol was carried out for all patients: preoperative pachymetry measurement, flap creation with the microkeratome, static cyclotorsion measurement, lifting of the flap and pachymetry measurement on the stromal bed, excimer laser photoablation, pachymetry measurement on the residual stromal bed, and repositioning of the flap. Online pachymetry measurement and dynamic cyclotorsion control were active during all the treatments. After surgery, patients received a topical antibiotic for 5 days and a topical steroid for 10 days. Artificial tears were prescribed for at least 1 month.
Statistical Analysis
The following categories were reviewed and recorded for each patient at each follow-up visit: age, uncorrected distance visual acuity (UDVA), CDVA, and subjective manifest refraction (SMR). VA was measured with an ETDRS chart and recorded in LogMAR units for subsequent statistical analysis. Each letter was considered to have a value of 0.02 log units. The data were analyzed with Microsoft Excel 2007 (Microsoft Inc., Redmond, WA, USA) and PASW Statistics 18 (SPSS Inc., Chicago, IL, USA). Repeated measures analysis of variance (ANOVA) was used to compare UDVA and CDVA in preoperative and postoperative visits, and post hoc analyses with Bonferroni corrections were performed when statistically significant differences were detected.
RESULTS
Among the 785 cases examined, 57 patients met the inclusion criteria. Of these, 27 (47%) were male and 30 (53%) were female. All patients underwent bilateral LASIK. Table 1 shows the preoperative characteristics of amblyopic eyes and their fellow eyes. There were 33 eyes in group 1, 12 eyes in group 2, and 12 eyes in group 3. Defocus equivalent (DEQ) of the refractive error is a better measure of residual refractive error following refractive procedures for mixed astigmatism cases and was calculated as defined by Holladay et al[19] (Table 1).
Table 1. Preoperative patient charactheristics.
| Parameters | Group 1 (n=33) | Group 2 (n=12) | Group 3 (n=12) |
| Age (a) | 35±9 | 33±7 | 32±5 |
| SE (D) | -4.67±1.90 | 4.40±1.00 | 0.15±1.00 |
| DEQ (D) | 4.67±1.90 | 5.52±1.05 | 4.85±1.28 |
| Astigmatism (D) | -2.50±1.86 | -2.71±1.78 | -3.40±1.84 |
| SE of the fellow eye (D) | -1.89±1.53 | 2.03±1.66 | -0.40±0.22 |
| DEQ of the fellow eye (D) | 1.88±1.56 | 3.34±1.36 | 1.90±1.90 |
n: Number of eyes; SD: Standard deviation; SE: Spherical equivalent of the refractive error; D: Diopters; DEQ: Defocus equivalent of the refractive error.
x±s
Table 2 shows UDVA for pre- and postoperative visits. As expected, there was a statistically significant increase in UDVA after LASIK; however, post hoc analysis did not reveal statistically significant difference between postoperative visits (Table 2).
Table 2. Preoperative and postoperative UDVA.
| Parameters | Mean | Sem. | P (all visits) |
| UDVA preop =A | 1.02 | 0.07 | 1<0.001 |
| UDVA week 1 =B | 0.27 | 0.03 | |
| UDVA month 1=C | 0.24 | 0.03 | |
| UDVA month 3=D | 0.22 | 0.02 | |
| UDVA month 6=E | 0.23 | 0.03 |
Sem: Standard Error of Mean; NS: Nonsignificant; UDVA: Uncorrected distance visual acuity; 1Statistically significant. Repeated Measures (Wilks' Lambda, n=57). Post Hoc Analysis: P(A-B)=0.001, P(A-C)<0.001, P(A-D) <0.001, P(A-E)<0.001, P(B-C)=1 NS, P(B-D)=0.065 NS, P(B-E)=0.346 NS, P(C-D)=0.559 NS, P(C-E)=1 NS, P(D-E)=1 NS.
Table 3 shows the mean CDVA of amblyopic eyes pre- and postoperatively. There was a statistically significant increase in CDVA after LASIK (P<0.001). Post hoc analysis did not reveal a significant difference between preoperative visit and postoperative week 1 visit. However, there was a statistically significant improvement of CDVA at postoperative month 1 (P= 0.001), 3 (P<0.001), and 6 (P<0.001) visits when compared to preoperative CDVA (Table 3). We also found a statistically significant improvement between postoperative month 3 and 6 visits (Table 3, P=0.016). However the mean difference between postoperative month 3 and month 6 visits was only 1.5 letters and it was not clinically significant. Preoperative refractive error had no effect on mean postoperative CDVA in amblyopic eyes (Table 4).
Table 3. Preoperative and postoperative CDVA.
| Parameters | Mean | Sem. | P (all visits) |
| CDVA preop =A | 0.23 | 0.01 | 1<0.001 |
| CDVA week 1 =B | 0.21 | 0.02 | |
| CDVA month 1=C | 0.18 | 0.02 | |
| CDVA month 3=D | 0.16 | 0.02 | |
| CDVA month 6=E | 0.13 | 0.02 |
Sem: Standard Error of Mean; NS: Nonsignificant; CDVA: Corrected distance visual acuity; 1Statistically significant; Repeated Measures (Wilks' Lambda), n=57. Post Hoc Analysis: P(A-B)=1 NS, P(A-C)=0.001, P(A-D)<0.001, P(A-E)<0.001, P(B-C)=0.044, P(B-D)<0.001, P(B-E)<0.001, P(C-D)=0.093 NS, P(C-E)<0.001, P(D-E)=0.016.
Table 4. Effect of the preoperative refractive error group on postoperative CDVA.
| CDVA vs. Group (1P=0.204) | Group 1 (n=33) | Group 2 (n=12) | Group 3 (n=12) | P | |
| CDVA week 1 | Mean | 0.19 | 0.25 | 0.20 | 0.60 |
| Sem. | 0.02 | 0.04 | 0.08 | ||
| CDVA month 1 | Mean | 0.15 | 0.25 | 0.18 | 0.14 |
| Sem. | 0.02 | 0.04 | 0.05 | ||
| CDVA month 3 | Mean | 0.14 | 0.20 | 0.15 | 0.48 |
| Sem. | 0.02 | 0.05 | 0.05 | ||
| CDVA month 6 | Mean | 0.11 | 0.18 | 0.15 | 0.29 |
| Sem. | 0.02 | 0.04 | 0.05 | ||
1Repeated Measures(Wilks' Lambda), non-significant; Sem: Standard Error of Mean; CDVA: Corrected distance visual acuity.
Preoperative CDVA, preoperative UDVA, preoperative SE of the refractive error, preoperative DEQ of the refractive error, and patient age were not associated with the increase in CDVA in amblyopic eyes (Table 5). In addition, initial amount of VA deficit and other preoperative factors were not associated with proportional improvement in the VA deficit (Table 6).
Table 5. Effects of preoperative variables on postoperative change of CDVA.
| Parameters | Age | Preop UDVA | Preop CDVA | Preop SE | Preop DEQ | ||
| Group 1 | Change in CDVA | r | 0.17 | -0.06 | -0.24 | -0.02 | 0.02 |
| P | 0.36 | 0.76 | 0.18 | 0.90 | 0.90 | ||
| Group 2 | Change in CDVA | r | 0.15 | 0.37 | -0.3 | 0.06 | 0.02 |
| P | 0.49 | 0.24 | 0.36 | 0.86 | 0.95 | ||
| Group 3 | Change in CDVA | r | 0.11 | -0.62 | 0.03 | -0.40 | -0.41 |
| P | 0.57 | 0.32 | 0.93 | 0.21 | 0.191 | ||
Pearson correlation analysis. CDVA: Corrected distance visual acuity; Preop: Preoperative; SE: spherical equivalent; DEQ: Defocus equivalent.
Table 6. Effects of preoperative variables on proportional improvement of VA deficit.
| Parameters | Age | Preop UDVA | Preop VA deficit | Preop SE | Preop DEQ | ||
| Group 1 | Proportional | r | 0.182 | -0.139 | -0.046 | 0.006 | -0.006 |
| Improvement | P | 0.311 | 0.440 | 0.801 | 0.973 | 0.973 | |
| Group 2 | Proportional | r | 0.168 | -0.178 | 0.227 | -0.223 | -0.353 |
| Improvement | P | 0.359 | 0.580 | 0.478 | 0.486 | 0.260 | |
| Group 3 | Proportional | r | 0.119 | -0.079 | -0.115 | -0.082 | -0.204 |
| Improvement | P | 0.489 | 0.807 | 0.721 | 0.800 | 0.526 | |
| All patients | Proportional | r | 0.311 | -0.065 | -0.038 | -0.131 | -0.081 |
| Improvement | P | 0.199 | 0.629 | 0.780 | 0.332 | 0.550 | |
Pearson correlation analysis. Preop: Preoperative; CDVA: Corrected distance visual acuity; VA: Visual acuity; SE: spherical equivalent; DEQ: Defocus equivalent.
CDVA of non-amblyopic eyes was also analyzed; however, there was no statistically significant improvement throughout the follow-up period (Table 7).
Table 7. Preoperative and postoperative CDVA (non-amblyopic fellow eyes).
| Parameters | Mean | Sem. | P(all visits) |
| UDVA preop =A | 0.02 | 0.03 | 1 (NS) |
| UDVA week 1 =B | 0.03 | 0.04 | |
| UDVA month 1=C | 0.02 | 0.03 | |
| UDVA month 3=D | 0.03 | 0.03 | |
| UDVA month 6=E | 0.02 | 0.03 |
NS: Nonsignificant; Sem: Standard Error of Mean; CDVA: Corrected distance visual acuity; Repeated Measures (Wilks' Lambda), n=57.
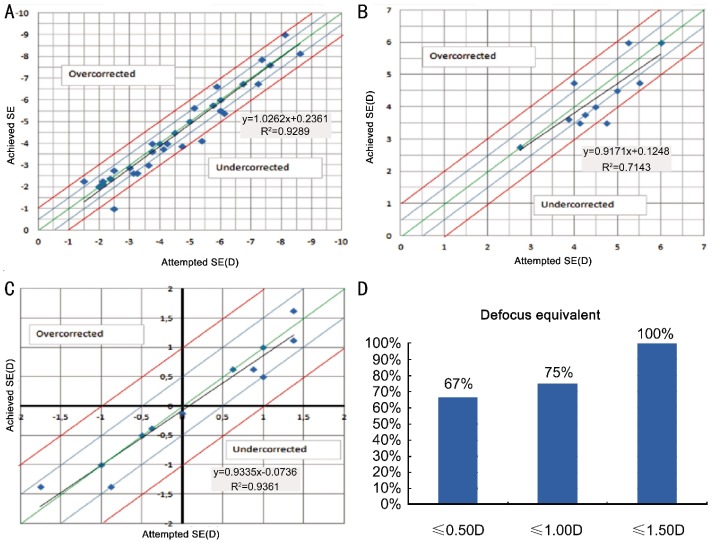
Figures 1A-C show the scatter plots of SE of achieved correction versus SE of intended correction in Groups 1, 2, and 3, respectively. Although the preoperative refractive errors were relatively high, SE of the achieved correction were within 1D of the intended correction in 94% of eyes in Group 1 and 92% of eyes in Group 2, postoperatively (Figure 1A and Figure 1B). In Group 3, the SE of the achieved correction was within ±50D of the intended correction in 100% of eyes (Figure 1C). However, SE is not a good measure in mixed astigmatism cases because the SE of a high refractive error may be very low. DEQ is a better measure for these eyes, and Figure 1D shows postoperative DEQ in Group 3. Overall, 75% of mixed astigmatism cases had postoperative DEQ ≤ 1.00 D. Refractive accuracy was within 1D of the intended correction in 95% of cases when all groups were analyzed together.
Figures 1. The scatter plots of SE of achieved correction versus SE of intended correction in different groups.
A: Group 1; B: Group 2; C: Group 3; D: Postoperative DEQ in Group 3.
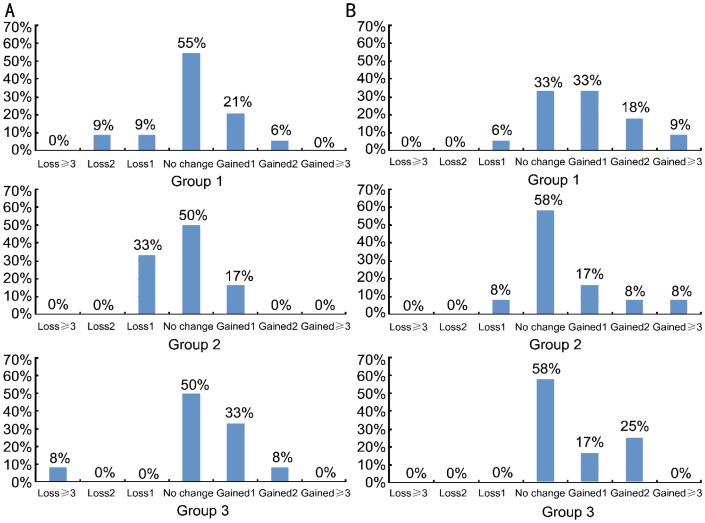
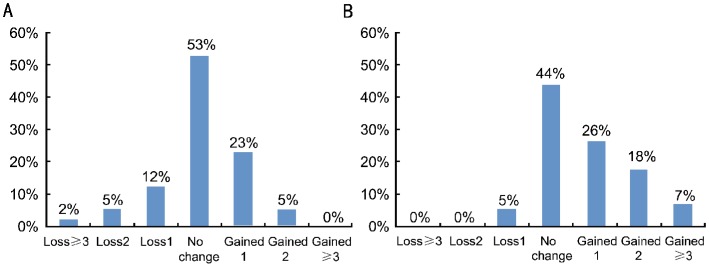
Figures 2A and 2B show the change in CDVA in different groups at week 1 and month 6 visits, respectively. Figures 3A and 3B show the change in CDVA at two different time points when all groups are analyzed together. There was an improvement in CDVA in 16 (28%) patients at the week 1 postoperative visit (Figure 3A). At the same time point, there were also 11 (19%) patients whose visual acuities were either slightly or moderately decreased to counterbalance an improvement in mean CDVA. As a result, statistical analysis comparing mean preoperative and mean week 1 visual acuities did not reveal any statistically significant differences (Table 2).
Figure 2. Change in CDVA in different groups.
A: Week 1; B: Month 6.
Figures 3. Change in CDVA in all groups at two different time points.
A: Week 1; B: Month 6.
DISCUSSION
Although studies have evaluated refractive surgery in amblyopic eyes, most of these were performed in pediatric age groups. Others were either performed on a small number of patients or after photorefractive keratectomy (PRK). The healing process after LASIK is shorter compared to PRK, and the improvements in UDVA and CDVA are faster. Therefore, a retrospective analysis of visual acuities of amblyopic adult eyes after LASIK would be more appropriate to show the pattern of improvement in the early postoperative period.
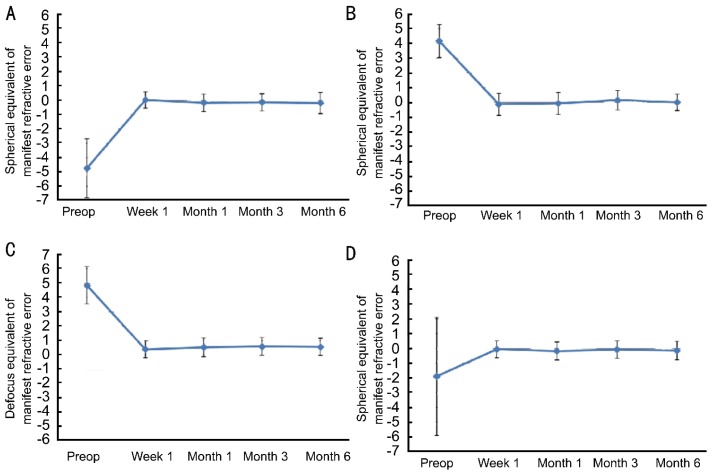
In this study, we retrospectively analyzed refractive results after LASIK in 57 amblyopic eyes. The refractive errors of the patients in the study were heterogeneous (Table 1). However, postoperative refraction was close to emmetropia in all groups (Figure 4). In agreement with the literature, LASIK was found to be highly effective in amblyopic eyes for the surgical correction of amblyogenic refractive errors[7], [9], [20], [21].
Figure 4. Postoperative refraction was stable throughout follow up in all groups.
A, B: Changes in mean SE refraction through the 6-month follow up after LASIK in Groups 1 and 2, respectively; C: Change in DEQ of the refractive error for mixed astigmatism cases (Group 3); D: The mean SE refraction over follow up when all groups were analyzed together.
We observed a statistically significant improvement in CDVA after surgery. The mean improvement was 0.1 log units (P<0.001, Table 2). Each log unit corresponds to a LogMAR line with letter size increasing by a factor of 1.2589 compared to the previous line. Accordingly, a loss of 0.1 log units (one line LogMAR) means that the minimum angle of resolution is multiplied by 1.2589. In contrast, 0.1 log unit (one line LogMAR) improvement in our patients means that the minimum angle of resolution was multiplied by 1/1.2589, resulting in a 21% decrease in the minimum angle of resolution. We found that 15 eyes (26%) gained one line, 10 eyes (18%) gained two lines, and 4 eyes (7%) gained three lines of CDVA. Overall, 14 (25%) of amblyopic eyes gained two or more lines of CDVA at the end of the follow up, and no eyes lost two or more lines. However, there was no improvement in CDVA of non-amblyopic eyes at any time point. There are a limited number of studies in the literature that evaluate CDVA improvement in adult amblyopic eyes after LASIK or PRK. Although they agree that improvement occurs, the rates vary over a wide range. Sakatani et al[9] found an improvement of CDVA in 42.8% of eyes and Dedhia and Behl[7] reported an improvement in 66.7% of patients after LASIK. Roszkowska et al[20] described CDVA improvement in 82.4% of PRK patients; 50%, 21%, and 12% of their patients gained one, two, and three lines, respectively, which is similar to our results. The highest rate of improvement was reported by Cagil et al[21], who performed PRK in adult amblyopic eyes that have not received previous amblyopia treatment. The number and follow-up time (50 eyes, 6 months) was very similar to ours. They reported that more than 25% of the patients gained three or more lines (minimum angle of resolution improvement by a factor of 2 or more), and 6% gained five lines (minimum angle of resolution improvement by a factor of 3). The mean preoperative CDVA of the patients in the studies above are similar to our patient group, and all are above 20/40. Due to floor and ceiling effects for patients with severe amblyopia, those with worse baseline VA may have more room to improve, whereas those with better baseline VA may have less room for improvement. For example 2 lines improvement may represent full (100%) correction of the initial VA deficit in a patient with mild amblyopia, but it would represent less improvement (eg. 50% of the initial VA deficit) in a patient with deeper amblyopia. The statistical analysis of VA in this study showed that the initial VA deficit and postoperative proportional improvement in the VA deficit were not significantly correlated (Table 6). However, negative findings in this study do not mean that there is no association. Spectrum of VA in amblyopia ranges from missing a few letters on a line to hand motion vision and the population in this study does not represent all levels of amblyopia.
Our results agree with the literature that corneal refractive surgery in amblyopic adults improves CDVA. However, it is not possible to predict preoperatively whether CDVA will improve. When our patients were divided into three groups (group 1: myopia or myopic astigmatism, group 2: hypermetropia or hypermetropic astigmatism, group 3: mixed astigmatism), there were no significant differences among the groups in terms of CDVA improvement. This finding is in agreement with Cagil et al[21] who reported that neither the type of refractive error nor the degree of amblyopia was associated with CDVA improvement. In contrast to our findings, Sakatani et al[9] reported a statistically significant improvement in the myopic group, but not in the hyperopic or astigmatic groups. However, that study was performed in 21 patients: 11 were myopic, 7 were hyperopic, and 3 had mixed astigmatism, and the small number of patients in the hyperopic and mixed astigmatism groups might account for their conclusion.
The underlying mechanism of improved VA in these patients remains unknown. Minification due to spectacle lenses was suggested as a possible source of visual impairment[8]. Accordingly, the relative magnification of the image and the reduction of visual aberrations after LASIK may be the cause of improvement observed after refractive rehabilitation with a laser. If present, the results of this effect should be evident in early postoperative periods. We believe that this may be at least one of the underlying mechanisms because we have seen some myopic “amblyopic” patients in our clinical practice with improved CDVA as early as 1 day after the surgery. However, the improvement in CDVA in this study was not limited to myopic patients, it was also seen in amblyopic eyes with hypermetropia, hypermetropic astigmatism, and mixed astigmatism. CDVA assessment at day 1 was not examined in our cases because it is not a part of our routine examination procedure. However, we detected CDVA improvement in 16 (28%) patients at the postoperative week 1 visit. At the same time point, there were also 11 (19%) patients whose visual acuities were either slightly or moderately decreased to counterbalance an improvement in mean CDVA. As a result, statistical analysis comparing mean preoperative and mean week 1 VAs did not show a statistically significant difference (Table 2). However, even a few patients with improved CDVA at week 1 (or earlier) is supporting evidence for additional pathology (eg., minification or aberrations induced by spectacle correction), resulting in low preoperative VA. Although the other studies in the literature report efficacy of LASIK or PRK in adult amblyopic eyes, they do not provide information on early postoperative results, so a comparison is not possible. Among 785 LASIK cases, only 57 amblyopic patients who underwent bilateral LASIK were included in the analysis. Because our aim was to observe the pattern of improvement over time and compare the results with the fellow eyes, we excluded patients who did not have VA in all follow-up time points. This may have introduced bias if patients with worse or better VA outcome are more likely to complete all follow-up visits. This was a retrospective study with weaknesses inherent to the design. For example, although ocular alignment assessment is a routine part of our preoperative examination protocol, there may be unidentified cases of monofixation among our patients. In addition, we do not know if the subjects wore refractive correction lenses prior to LASIK. Although amblyopia treatment is a routine question in our forms, the physician may not question it deeply or clearly, or the patient may not remember treatment attempts that took place at an early age. These variables could have impacted the outcome and weakened the results.
The improvement in mean CDVA started to be statistically significant at the month 1 visit and continued during the following 5 months. It was not associated with preoperative variables such as patient age, refractive error type, UDVA, CDVA, SE or DEQ. Because the refractive and visual results of LASIK tend to stabilize in the first months, we believe that this continuing improvement even 6 months after surgery reflects the role of neural mechanisms. It is often stated that visual system plasticity is confined to the childhood period; thus, amblyopes cannot be treated beyond a certain age. On the other hand, a careful review of the literature suggests otherwise, and there is accumulating evidence to show that a significant degree of neural plasticity exists in adults, and clinically significant improvement in VA may be achieved in at least some of them[22]-[27]. Simmers and Grey reported a case of a 30-year-old patient with strabismic amblyopia successfully treated with occlusion[28]. Visual function in amblyopic eyes may improve substantially when the fellow eye becomes visually impaired, and age is not a barrier to this phenomenon. Mallah et al[27] reviewed medical records of patients with age-related macular degeneration (AMD) and detected VA improvement in some amblyopic fellow eyes unaffected by AMD. The mean improvement in amblyopic eyes at 12 months was 3.3 lines (LogMAR). Recently a substantial improvement in a wide range of visual functions including VA, positional acuity, and stereopsis were found in adults with amblyopia after a period of playing an action video game[31]. Recent research demonstrates a previously unsuspected high potential for neuronal plasticity in adult human and animal visual systems. Plasticity in “mature” animal visual systems has been elicited by pharmacological treatment with different agents, including fluoxetine, chondroitinase, and valproic acid[30]-[32]. Sale et al[34] showed that environmental enrichment (i.e., increasing the level of environmental stimulation by providing a combination of multi-sensory/cognitive stimulation, increased physical activity, and enhanced social interactions) restored VA in adult amblyopic rats. This effect results from a reduction of inhibitory tone on visual system plasticity. Dark adaptation and caloric restriction are other environmental factors that increased VA in some animals[34], [35]. It was recently reported that a short-term protocol of food restriction is associated with a marked reduction of GABAergic inhibition and increased neural plasticity in the visual system of adult rats, and this renews the capability of recovery from amblyopia in long-term visually deprived animals. These studies clearly show that the mature visual cortex may regain neural plasticity under specific pharmacological and/or environmental conditions. Although treating amblyopia is theoretically possible by triggering plasticity in the adult human visual cortex, the exact environmental and cellular factors underlying neuronal plasticity are currently unknown.
In conclusion, although the visual potential of the adult amblyopic eye is usually disregarded, evidence in the literature demonstrates that VA may improve with refractive correction, occlusion, or vision loss of the non-amblyopic eye. In this study, we found that 25% of the amblyopic eyes showed two or more lines improvement in CDVA after successful refractive correction with LASIK. Although some patients experienced this improvement as early as the first week, statistically significant improvement in mean CDVA began at the first month and continued for the next 5 months. The mechanism of improvement in VA in these adults is unknown and may be a combination of different mechanisms.
REFERENCES
- 1.Lempert P. Retinal area and optic disc rim area in amblyopic, fellow, and normal hyperopic eyes: a hypothesis for decreased acuity in amblyopia. Ophthalmology. 2008;115(12):2259–2261. doi: 10.1016/j.ophtha.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Parks MM. Treatment of the sensorial adaptations and amblyopia. In: Duane TD, editor. Clinical ophthalmology. 1. Vol. 11. Hagerstown, MD: Harper and Row; 1989. pp. 1–14. [Google Scholar]
- 3.Daw NW. Critical Periods and Amblyopia. Arch Ophthalmol. 1998;116(4):502–505. doi: 10.1001/archopht.116.4.502. [DOI] [PubMed] [Google Scholar]
- 4.Daw NW. 2nd ed. New York, NY: Springer; 2006. Visual Development; pp. 169–200. [Google Scholar]
- 5.Lengyel D, Valmaggia C. Visual improvement of an amblyopic eye in an adult patient after visual loss in the non-amblyopic eye. Klin Monatsbl Augenheilkd. 2006;223(15):462–464. doi: 10.1055/s-2006-926596. [DOI] [PubMed] [Google Scholar]
- 6.Wilson ME. Adult amblyopia reversed by contralateral cataract formation. J Pediatr Ophthalmol Strabismus. 1992;29(2):100–102. doi: 10.3928/0191-3913-19920301-09. [DOI] [PubMed] [Google Scholar]
- 7.Dedhia NC, Behl S. Laser in situ keratomileusis for anisometropic amblyopia. J Refract Surg. 2000;16:264–267. doi: 10.3928/1081-597X-20000302-15. [DOI] [PubMed] [Google Scholar]
- 8.Barequet IS, Wygnanski-Jaffe T, Hirsh A. Laser in situ keratomileusis improves visual acuity in some adult eyes with amblyopia. J Refract Surg. 2004;20(1):25–28. doi: 10.3928/1081-597X-20040101-05. [DOI] [PubMed] [Google Scholar]
- 9.Sakatani K, Jabbur NS, O'Brien TP. Improvement in best corrected visual acuity in amblyopic adult eyes after laser in situ keratomileusis. J Cataract Refract Surg. 2004;30(12):2517–2521. doi: 10.1016/j.jcrs.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Lanza M, Rosa N, Capasso L, Iaccarino G, Rossi S, Romano A. Can we utilize photorefractive keratectomy to improve visual acuity in adult amblyopic eyes? Ophthalmology. 2005;112(10):1684–1691. doi: 10.1016/j.ophtha.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Bradley A, Freeman RD. Contrast sensitivity in anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1981;21(3):467–476. [PubMed] [Google Scholar]
- 12.Levi DM, Klein S. Hyperacuity and amblyopia. Nature. 1982;298(5871):268–270. doi: 10.1038/298268a0. [DOI] [PubMed] [Google Scholar]
- 13.Hess RF, Campbell FW, Greenhalgh T. On the nature of the neural abnormality in human amblyopia: neural aberrations and neural sensitivity loss. Pfluger's Arch. 1978;377(3):201–207. doi: 10.1007/BF00584273. [DOI] [PubMed] [Google Scholar]
- 14.Stuart JA, Burian HM. A study of separation difficulty. Am J Ophthalmol. 1962;53:471–477. [PubMed] [Google Scholar]
- 15.Hess RF, DeMannis R, Bex PJ. A reduced motion after-effect in strabismus amblyopia. Vision Res. 1997;37(10):1303–1311. doi: 10.1016/s0042-6989(96)00277-5. [DOI] [PubMed] [Google Scholar]
- 16.Lynee Kiorpes. Sensory processing: animal models of amblyopia. In: Moseley M, Fielder AR, editors. Amblyopia: a multidisciplinary approach. Oxford, UK: Butterworth-Heinemann; 2002. [Google Scholar]
- 17.American Academy of Ophthalmology Pediatric Ophthalmology/Strabismus Panel . San Francisco, CA: American Academy of Ophthalmology; 2012. Preferred practice Pattern Guidelines. Amblyopia. Available at: www. aao.org/ppp. [Google Scholar]
- 18.Stewart CE, Moseley MJ, Stephens DA, Fielder AR on behalf of the MOTAS Cooperative. Treatment Dose–Response in Amblyopia Therapy: The Monitored Occlusion Treatment of Amblyopia Study (MOTAS) Invest Ophthalmol Vis Sci. 2004;45(9):3048–3054. doi: 10.1167/iovs.04-0250. [DOI] [PubMed] [Google Scholar]
- 19.Holladay JT, Lynn MJ, Waring GO, Gemmill M, Keehn CG, Fielding B. The relationship of visual acuity, refractive error, and pupil size after radial keratotomy. Arch Ophthalmol. 1991;109(1):70–76. doi: 10.1001/archopht.1991.01080010072036. [DOI] [PubMed] [Google Scholar]
- 20.Roszkowska AM, Biondi S, Chisari G, Messina A, Ferreri FMB, Meduri A. Visual outcome after excimer laser refractive surgery in adult patients with amblyopia. Eur J Ophthalmol. 2006;16(2):214–218. doi: 10.1177/112067210601600204. [DOI] [PubMed] [Google Scholar]
- 21.Cagil N, Ugurlu N, Cakmak HB, Kocamis SI, Turak D, Simsek S. Photorefractive keratectomy in treatment of refractive amblyopia in the adult population. J Cataract Refract Surg. 2011;37(12):2167–2174. doi: 10.1016/j.jcrs.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 22.Vuori E, Tervo TMT, Holopainen MVA, Holopainen JM. Improvement of visual acuity following refractive surgery for myopia and myopic anisometropia. J Refract Surg. 2007;23(5):447–455. doi: 10.3928/1081-597X-20070501-05. [DOI] [PubMed] [Google Scholar]
- 23.Levi DM, Li RW. Improving the performance of the amblyopic visual system. Philos Trans R Soc Lond B. 2009;364(1515):399–407. doi: 10.1098/rstb.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proc Natl Acad Sci USA. 1996;93(13):6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levi DM, Polat U, Hu Y-S. Improvement in Vernier acuity in adults with amblyopia; practice makes better. Invest Ophthalmol Vis Sci. 1997;38(8):1493–1510. [PubMed] [Google Scholar]
- 26.Chino YM, Kaas JH, Smith EL, III, Langston AL, Cheng H. Rapid reorganization of cortical maps in adult cats following restricted deafferentation in retina. Vision Res. 1992;32(5):789–796. doi: 10.1016/0042-6989(92)90021-a. [DOI] [PubMed] [Google Scholar]
- 27.El Mallah MK, Chakravarthy U, Hart PM. Amblyopia: is visual loss permanent? Br J Ophthalmol. 2000;84(9):952–956. doi: 10.1136/bjo.84.9.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmers AJ, Gray LS. Improvement of visual function in an adult amblyope. Optom Vis Sci. 1999;76:82–87. doi: 10.1097/00006324-199902000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Li RW, Ngo C, Nguyen J, Levi DM. Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biol. 2011 Aug;9(8):e1001135. doi: 10.1371/journal.pbio.1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vetencourt JFM, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320(5874):385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 31.Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci USA. 2006;103(22):8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silingardi D, Scali M, Belluomini G, Pizzorusso T. Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur J Neurosci. 2010;31(12):2185–2192. doi: 10.1111/j.1460-9568.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- 33.Sale A, Vetencourt MJF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10(6):679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- 34.He HY, Ray B, Dennis K, Quinlan EM. Experience- dependent recoveryofvision following chronic deprivation amblyopia. Nat Neurosci. 2007;10(9):1134–1136. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
- 35.Spolidoro M, Baroncelli L, Putignano E, Vetencourt MJF, Viegi A, Maffei L. Food restriction enhances visual cortex plasticity in adulthood. Nat Commun. 2011;2:320–327. doi: 10.1038/ncomms1323. [DOI] [PubMed] [Google Scholar]