Abstract
AIM
To present results of the keratoprosthesis method used at The Filatov Institute of Eye Diseases and Tissue Therapy.
METHODS
A retrospective case series analysis was used to describe the development of new types of keratoprostheses and methods of implantation as well as different ways of leukoma strengthening.
RESULTS
Keratoprosthesis was performed in 1 060 eyes of 1 040 patients with leukomas of different etiology: burns, 725 eyes (68.4%); trauma, 120 eyes (11.3%); keratitis and ocular pemphigoid, 108 eyes (10.2%); and bullous keratopathy, 107 eyes (10.1%). Visual acuity before keratoprosthesis consisted of light perception in 962 eyes (92%), and 98 eyes (8%) had minimal visual acuity (1/200-1/50). Both eyes were blind (visual acuity less than 1/200) in 955 patients (91.8%). The period of blindness varied from 1 to 52 years. As a result of keratoprosthesis, visual acuity of ≥1/200 was restored in 1 023 of 1 060 eyes (96.5%). Visual acuity of 20/200-20/20 was achieved in 716 eyes (67.5%). At the last follow-up visit visual acuity of ≥1/200 was preserved in 806 eyes (76%), visual acuity of 20/200-20/20 was measured in 583 of 1 060 eyes (55%) and good keratoprosthesis fixation in the cornea was achieved in 986 of 1 060 eyes (93%). The minimal follow-up was 12 months (range, 12 months to 37 years, median 5 years).
CONCLUSION
Our techniques of keratoprosthesis effectively restore vision in patients with leukomas that cannot be treated by optical corneal grafting.
Keywords: artificial cornea, corneal grafting, keratoprosthesis, leukoma
INTRODUCTION
The search for a substitute for the natural cornea dates back more than 200 years[1],[2]. Despite of a range keratoprosthesis models have been elaborated and tested[3], very few devices have had successful long-term results and continue in regular clinical use-osteo-odonto-keratoprosthesis (OOKP) and Boston keratoprosthesis[4],[5].
We perform keratoprosthesis to restore vision in patients with complicated leukomas that are unsuitable for optical corneal grafting using original type of keratopsthesis and surgical technique. Complicated leukomas include those that occur due to severe eye burns, corneal traumas, keratouveitis, terminal stages of edematous bullous keratopathy, ocular pemphigoid and trachoma, as well as cases for which multiple corneal graft failures have occurred or human donor corneas are not available for keratoplasty.
The aim of this study was to present short history and main results of application of keratoprosthesis types as well as the keratoprosthesis implantation technique, developed and used at the Filatov Institute since 1966[6]-[15]. The technology was also introduced in China[16], Poland and Bulgaria (unpublished data).
SUBJECTS AND METHODS
Keratoprosthesis studies at the Filatov Institute have been aimed at the development of new keratoprosthesis types, techniques for keratoprosthesis implantation, and methods for strengthening leukomas and keratoprostheses. Additional studies have focused on keratoprosthesis complications and methods for their prevention and treatment, as well as indications for keratoprosthesis.
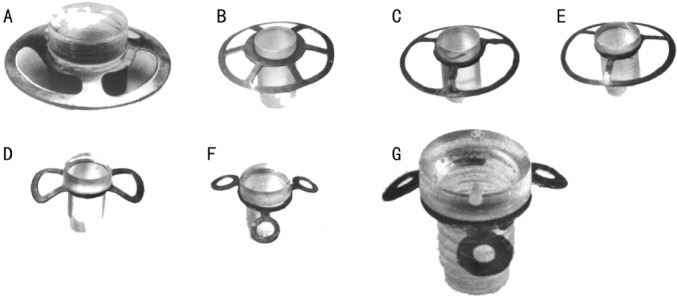
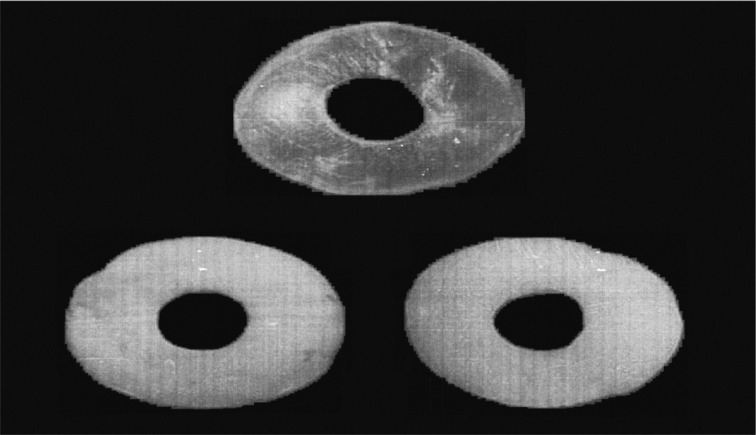
There have been 4 periods of keratoprosthesis use at the Filatov Institute based on development of keratoprosthesis types: 1966-1971, keratoprosthesis using the Puchkovskaya-Golubenko model were applied (40 operations, Figure 1A); 1972-1974, “open-work” keratoprosthesis with the Puchkovskaya-Iakymenko-Golubenko model (70 operations, Figure 1B, C); 1975-1977, “new nondismountable” keratoprostheses using the Iakymenko-Golubenko model (157 operations, Figure 1D-F); and 1978 to the present, “universal dismountable” keratoprosthesis based on the Iakymenko-Golubenko model (793 operations, Figure 1G). All keratoprostheses were made in the Institute’s laboratory and consisted of the optical cylinder, which is made of polymethylmethacrylate, and the support, which is made of tantalum, an inert and highly corrosion resistant metal. The optical cylinder of the entire keratoprosthesis model lineup has not been changed significantly during the years; it had a diameter of 3.3mm in the anterior part and 2.6mm in the posterior part. The height of the anterior part of the optical cylinder may vary from 0.6mm to 2.4mm depending on the thickness of a patient’s cornea. The main adjustments have been made to the support design. Its surface area was finally made as minimal as possible (Figure 1G).
Figure 1. Types of keratoprosthesis developed in the Filatov Institute.
New methods of keratoprosthesis (penetrating, optical-cosmetic, anterior-penetrating, and posterior-penetrating) were developed and used for treating different types of leukomas from 1966 onward (Figures 2 and 3). The construction of a “universal dismountable” keratoprosthesis creates conditions for better implantation of the latter; prevents aseptic necrosis of the cornea, keratoprosthesis extrusion, and aqueous humor filtration along the optic cylinder; and allows keratoprosthesis removal in case of complications. The “universal dismountable” keratoprosthesis also permits using penetrating, optical-cosmetic, anterior-penetrating, or posterior-penetrating methods and shifting anterior-penetrating or posterior-penetrating keratoprosthesis to penetrating or optical-cosmetic (if necessary).
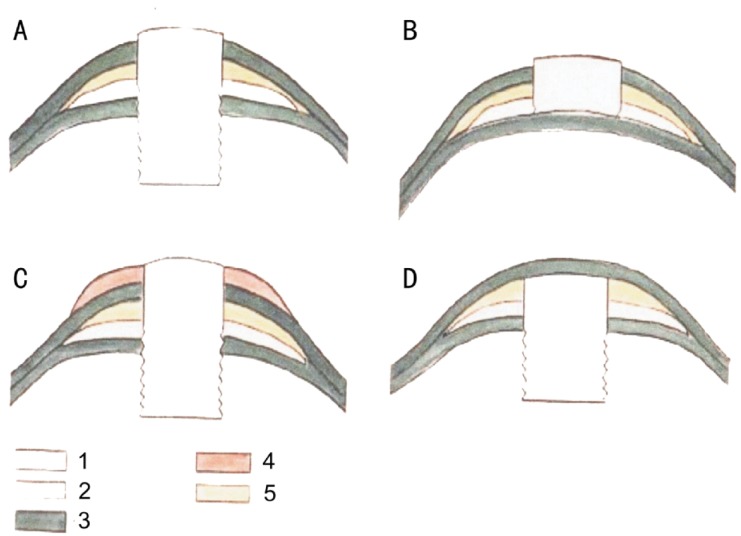
Figure 2. Methods of keratoprosthesis.
A: Penetrating; B: Anterior-penetrating; C: Optical-cosmetic; D: Posterior-penetrating. 1, 2: Keratoprosthesis; 3: Cornea; 4: Cosmetic lens; 5: Intracorneal grafts.
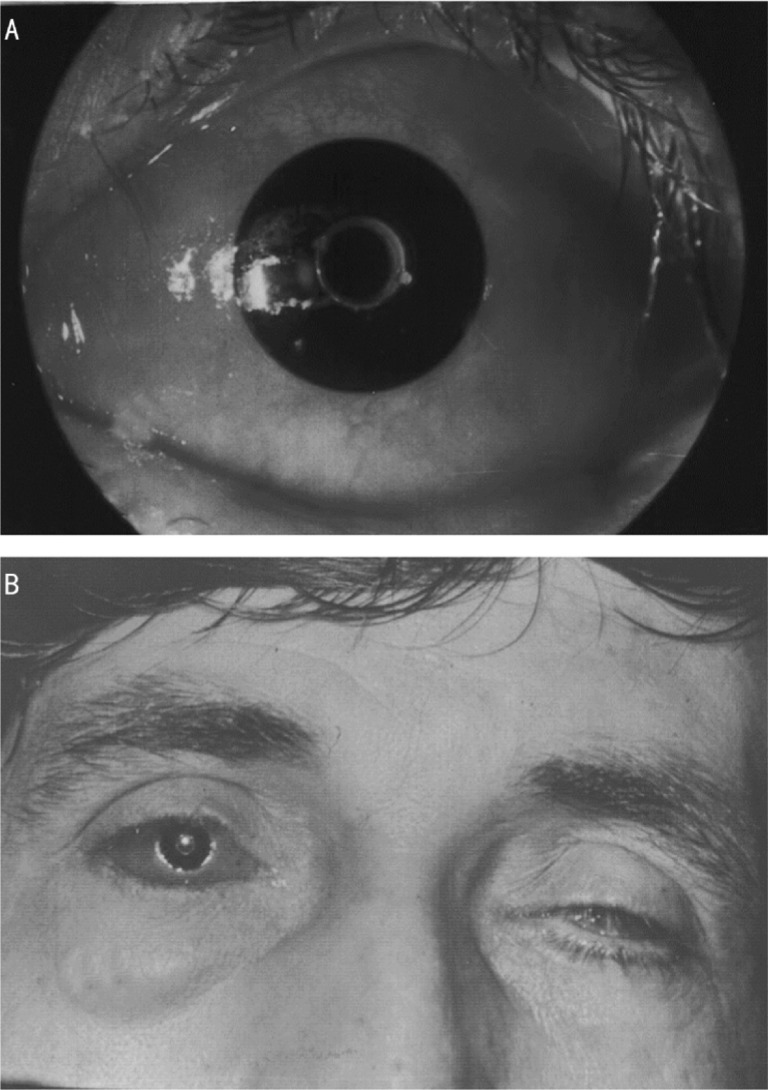
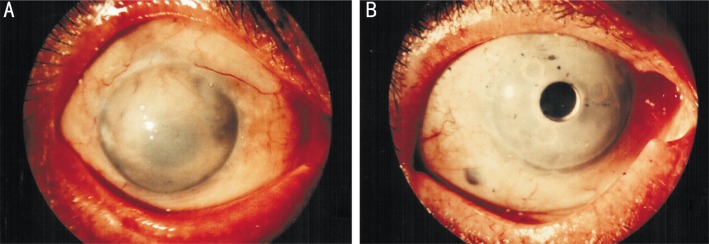
Figure 3. Patient after optical-cosmetic keratoprosthesis (keratoprosthesis with attached cosmetic contact lens).
A: Slit-lamp photo; B: General view.
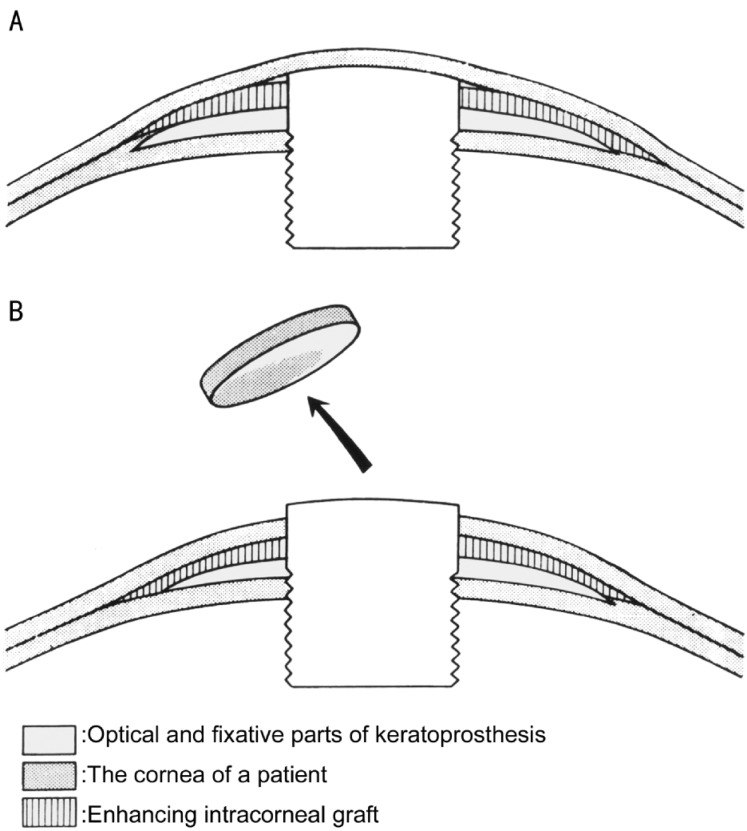
We have further developed and used a “two-stage” method for keratoprosthesis since 1974, which was an important factor in increasing efficacy of the surgery (Figure. 4)[17]. In the first stage, after division of the cornea into anterior and posterior layers at maximal possible depth, only the posterior layers are trephined in the center with 2.5mm trephine where the keratoprosthesis is placed. The keratoprosthesis is then covered with the non-trephined anterior layers of the cornea. If present, lens is extracted at the moment of keratoprosthesis implantation. The extraction is performed either through trephine opening in posterior corneal layers or through additional limbal incision, as previously described[16]. The second stage is performed 3–6 months later, and only the corneal anterior layers over the optical cylinder of keratoprosthesis are trephined with 3.5mm trephine.
Figure 4. Scheme of two-stage method of keratoprosthesis.
A: After the 1st stage; B: After the 2nd stage of the operation
Methods for leukoma strengthening were developed to achieve safe keratoprosthesis fixation[18]. Different keratoplasty methods and tissues are used for this aim. Epicorneal strengthening of the leukoma is fulfilled with donor cornea or with the patient’s oral mucosa 5-6 months before keratoprosthesis (Figure 5).
Figure 5. Scheme of superficial leukoma strengthening.
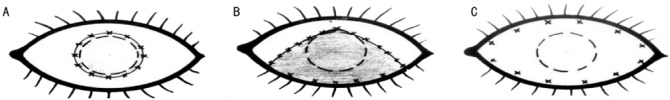
A: Leukoma strengthening with corneal or oral mucosa graft; B: Leukoma strengthening and simultaneous lower eyelid symblepharon removal with oral mucosa graft; C: Leukoma strengthening and simultaneous both eyelids symblepharon removal with oral mucosa graft. Dashed line circle: limbus; continuous line circle and crosses: graft forms and suture placement for episcleral graft fixation.
Intracorneal strengthening of the leukoma is simultaneously performed with keratoprosthesis implantation. Donor lamellar corneal graft (posterior stroma with Descemet’s membrane) or dura mater or the patient’s auricular cartilage are used for this purpose (Figure 6). Grafts for intracorneal strengthening were obtained using 9-10 mm trephines. A trephine with similar diameter of the optical cylinder of the keratoprosthesis, 3.3mm, was used to make opening in the center of the graft. After that, the graft was put on the optical cylinder of the keratoprosthesis either before the keratoprosthesis implantation into the posterior corneal layers opening or immediately after that.
Figure 6. Tissues and graft forms used for intracorneal strengthening of leukoma.
(upper figure) lamellar corneal allograft (posterior stroma and Descemet’s membrane); (bottom left figure) patient’s auricular cartilage; (bottom right figure) dura mater.
Different combinations of the above mentioned methods and tissues were used in majority of cases to get safe keratoprosthesis fixation in leukoma.
Informed consent was obtained from each patient. The study followed the tenets of the Declaration of Helsinki, and institutional review board approval was obtained.
RESULTS
In total, keratoprosthesis was performed by various surgical techniques in 1 060 eyes of 1 040 patients with leukomas of different etiology from 1966 to 2011 (Tables 1, 2).
Table 1. Methods of keratoprosthesis and number of operated eyes.
| Keratoprosthesis method | n |
| Penetrating | 985 |
| Optical-cosmetic | 23 |
| Anterior-penetrating | 22 |
| Posterior-penetrating | 30 |
| Total | 1 060 |
Table 2. Etiology of leukomas and number of operated eyes.
| Etiology of leukoma | n(%) |
| Eye burn | 725 (68.4) |
| Trauma | 120 (11.3) |
| Keratitis, ocular pemphigoid | 108 (10.2) |
| Bullous keratopathy | 107 (10.1) |
| Total | 1 060 (100) |
Different types of corneal grafting had been done in the majority of patients prior to keratoprosthesis, but visual acuity was not improved.
Visual acuity before keratoprosthesis consisted of light perception in 962 eyes (92%) and 98 eyes (8%) had minimal visual acuity (1/200–1/50). Both eyes were blind (visual acuity less than 1/200) in 955 patients (91.8%) (Table 3).The period of blindness varied from 1 to 52 years (median 3 years). The age of patients was 11-82 years (median 39 years). Keratoprosthesis was the only possible method to restore vision in these patients.
Table 3. Visual acuity before keratoprosthesis and at the last follow-up visit.
| Visual acuity | Before | After keratoprosthesis | At the last follow-up visit | Contralateral eye |
| 0 (zero)/anophthalmos | 42 (4) | 462 (44.4) | ||
| Light perception | 962 (92) | 37 (3.5) | 212 (20) | 493 (47.4) |
| 1/200–1/50 | 98 (8) | 130 (12.3) | 106 (10) | 56 (5.4) |
| 6/200–20/200 | 167 (15.7) | 117 (11) | 29 (2.8) | |
| 2/20–10/20 | 376 (35.5) | 286 (27) | ||
| 10/20–20/20 | 350 (33) | 297 (28) | ||
| Total | 1060 | 1060 | 1060 | 1040 |
n(%)
As a result of keratoprosthesis, vision of ≥1/200 was restored in 1 023 of 1 060 eyes (96.5%). Visual acuity of 20/200-20/20 was achieved in 726 eyes (68.5%). At the last follow-up visit, visual acuity of ≥1/200 was preserved in 806 eyes (76%), visual acuity of 20/200-20/20 was measured for 583 of 1 060 eyes (55%), and good keratoprosthesis fixation in the cornea was observed in 986 of 1 060 eyes (93%). The minimal follow-up was 12 months (range 12 months to 37years, median 5 years).
A detailed analysis of complications was performed for 1 020 patients who were operated on in 1972-2010 (Table 4). Forty patients (40 eyes) who were operated on during the first 5 years (1966-1971) were not included in this analysis. The main complication during these years was keratoprosthesis extrusion (rate was more than 30% during the first year after the operation).
Table 4. Types and rates of complications after keratoprosthesis.
| Complications | Keratoprosthesis type |
|||
| Open-work 1972–1974 (70 eyes) | New nondismountable 1975–1977 (157 eyes) | Universal dismountable |
||
| 1978–1994 (543 eyes) | 1995–2010 (250 eyes) | |||
| Aseptic necrosis of cornea | 28.6 | 20.4 | 8.3 | 2.9 |
| Extrusion of keratoprosthesis | 17.1 | 9.5 | 3.5 | 1.5 |
| Phthisis bulbi (uveitis, aqueous humor filtration) | 7.1 | 5.7 | 2.4 | |
| Endophthalmitis | 1.4 | 5.7 | 1.5 | 0.5 |
| Retinal detachment | 1.4 | 2.5 | 1.1 | 0.5 |
| Retrokeratoprosthesis membrane | 45.7 | 14.6 | 13.6 | 16.5 |
| Overgrowth of corneal/conjunctival (oral mucosa) epithelium over keratoprosthesis optic cylinder | 20.0 | 19.7 | 24.9 | 18.3 |
| Secondary glaucoma | 5.7 | 1.3 | 1.3 | 1.5 |
%
The incidence of corneal aseptic necrosis over the keratoprosthesis decreased from 28.6% in 1972-1974 to 2.9% in 1995-2010, and the incidence of keratoprosthesis extrusion decreased from 17.1% in 1971-1974 to 1.5% in 1995-2010. The most frequent post-keratoprosthesis complications were retrokeratoprosthesis membrane formation of different density and obliteration of keratoprosthesis optic cylinder with corneal (conjunctival) epithelium or oral mucosa. However, these complications did not significantly decrease visual acuity in the majority of patients. The epi- and retrokeratoprosthesis membranes were surgically removed if the decrease of visual acuity was significant.
The best results were obtained with a combination of our last type of keratoprosthesis (1978-2010), the two-stage method of keratoprosthesis (1974-2010), and application of different leukoma-strengthening methods, intracorneal or epicorneal (1974-1994). Since 1995 we have been using a combination of epi- and intracorneal leukoma-strengthening methods exclusively. It has led to the most stable keratoprosthesis fixation in patient’s cornea (Table 4).
Terms and number of keratoprosthesis extrusion menace due to aseptic lysis of corneal anterior layers over the keratoprosthesis support, which led to the keratoprosthesis extrusion or forced removal are presented in the Table 5.
Table 5. Terms and number of keratoprosthesis extrusion menace or keratoprosthesis extrusion.
| Keratoprosthesis type | Terms (years after operation) and number of keratoprosthesis extrusion menace / extrusion |
Total number of extrusion menace/ extrusion | Total number of operations | |||
| 1-2 a | 2-5 a | 5-10 a | <10 a | |||
| Open-work | 8/5 | 7/6 | 5/4 | no data | 20 / 15 | 70 |
| New nondismountable | 13/7 | 8/5 | 2/3 | no data | 23 / 15 | 157 |
| Dismountable | 22/13 | 16/6 | 4/2 | 3/1 | 45 / 19 | 543 |
| Dismountable keratoprsothesis with essential epi- and intracorneal strengthening | 4/2 | 2/1 | -/- | 1/1 | 7 / 4 | 250 |
| Total | 47/27 | 33/18 | 11/9 | 4/2 | 95 / 53 | 1020 |
Nevertheless, in 42/95 patients with the keratoprosthesis extrusion menace the keratoprosthesis was saved in the leukoma, if partial corneal lysis over the keratoprosthesis support occurred and patient was referred to our center in time. Following procedures have been used to stabilize the keratoprosthesis in the leukoma: superficial keratoplasty with use of lamellar corneal allograft or patient’s oral mucosa graft (less safe methods), intralamellar keratoplasty with use of lamellar corneal allograft or patient’s auricular cartilage (more safe methods). Choice of the stabilization procedure depended on condition of patient’s cornea. The keratoprosthesis was removed and tectonic corneal grafting was performed in 53/95 patients with keratoprosthesis extrusion menace, if extensive corneal lysis occurred and patient was not referred to our center in time. Some of these patients underwent re-keratoprosthesis later on and lost vision was restored.
If secondary glaucoma was diagnosed both before and after keratoprosthesis, we perform original operation elaborated at the Department–cyclo-gonio-drainage using autoscleral flap[19]. The operation creates new suprachoroidal pathway for aqueous humor drainage. This operation was performed in 24 patients before the keratoprosthesis and in 32 patients after the keratoprosthesis. Normal intraocular pressure in 2-year follow-up was achieved in 49/56 patients (87.5%).
All patients postoperatively received antibacterial and anti-inflammatory therapy during 10-15 days, which included topical and intramuscular antibiotics, topical and intramuscular non-steroidal anti-inflammatory drugs, oral steroids were used, if tolerated. Oral diuretics were used, if ocular hypertension was present after the operation.
Visual field of the patients with keratoprosthesis mainly depended on whether optic nerve and retina disorders were present or not. Many of the patients had optic nerve atrophy and chorioretinal lesions because of underlying disease or secondary glaucoma. If optic nerve and retina were not damaged, visual field equaled 40% of a normal visual field.
Clinical examples are presented in Figures 7, 8.
Figure 7. Patient N.
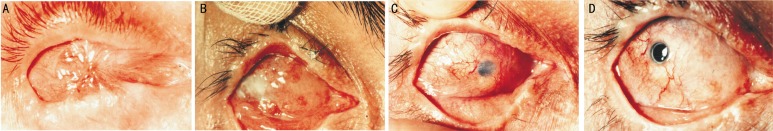
A: Preoperative status of both eyes 2 years after alkali burn; visual acuity consisted of light perception; B: After symblepharon removal with simultaneous epicorneal strengthening of the leukoma with oral mucosa on the right eye; C: After the first stage of keratoprosthesis with simultaneous intracorneal strengthening of the leukoma with auricular cartilage autograft; D: After the second stage of keratoprosthesis. Visual acuity was 20/20. Follow-up period was 32 years.
Figure 8. Patient Yu.
A: Preoperative status, terminal stage of bullous keratopathy, aphakia, previously operated on secondary glaucoma; B: After the keratoprosthesis with simultaneous intracorneal strengthening of the leukoma with lamellar corneal allograft. Visual acuity was 12/20. Follow-up period was 16 years.
DISCUSSION
Recent advances in tissue engineering have now made it possible to produce non-immunogenic natural corneal substitutes from highly purified collagen, which seamlessly integrate into host cornea[20],[21]. These materials should become the future of artificial corneas. On the other hand, advances in corneal grafting techniques and ocular surface reconstruction have broadened the indications and improved results of corneal grafting in severely damaged corneas[22]. But still, there is a big number of leukomas, where corneal grafting fails and keratoprosthesis remains the last real option for these patients. The osteo-odonto-keratoprosthesis for the severe dry eye and the Boston type 1 keratoprosthesis for the “wet blinking eye” are the operations of choice in this field[3].
Long-term retention rate and visual outcome of keratoprosthesis technology developed at the Filatov Institute are similar to that in the above mentioned methods. We suppose that due to range of developed leukoma strengthening methods (epicorneal, intracorneal or combination of both) our keratoprosthesis method is indicated for both dry and wet eyes. Concomitant lid malpositions (symblepharon, entropion, ectropion) are removed during epicorneal leukoma strengthening. Thin (thickness less than 500µm) and/or insufficiently vascularised leukoma are the most challenging situations for this method. If cornea is thin, the division of the latter in two layers at maximal possible depth can be a hard task and risk of Descemet’s membrane perforation exists. But even if perforation occurs, it can be sutured, and keratoprosthesis can be successfully implanted. If there is lack of leukoma vascularisation, epicorneal strengthening may fail, thus safety of keratoprosthesis fixation in corneal layers decreases, because intracorneal strengthening alone remains an option to get safe keratoprosthesis fixation. These leukomas will probably better fit the Boston keratoprosthesis. However, thin and non-vascularised leukomas are not absolute contraindications for our keratoprosthesis method.
Nevertheless, keratoprosthesis with the use of technologies developed at the Filatov Institute is an effective method to restore vision in patients with complicated leukomas unsuitable for optical corneal grafting. The results were obtained because of continuing improvement of keratoprosthesis design and use of a two-stage method paired with different methods of leukoma strengthening.
Acknowledgments
The author would like to thank Dr Oleksiy Buznyk MD, PhD, The Filatov Institute of Eye Diseases and Tissue Therapy, who has made personal contribution to this manuscript.
REFERENCES
- 1.Pellier de Quengsy G. Vol. 1. Paris: Didot&Mequignon; 1789–1790. Course of operations in the surgery of the eyes; p. 91. [Google Scholar]
- 2.Nussbaum JN. Cornea artificialis. Deutsch Klein. 1853;5:367. [Google Scholar]
- 3.Gomaa A, Comyn O, Liu C. Keratoprosthesis in clinical practice: a review. Clin Experiment Ophthalmol. 2010;38(2):211–224. doi: 10.1111/j.1442-9071.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Paul B, Tandon R, Lee E, Fong K, Mavrikakis I, Herold J, Thorp S, Brittain P, Francis I, Ferrett C, Hull C, Lloyd A, Green D, Franklin V, Tighe B, Fukuda M, Hamada S. The osteo-odonto-keratoprosthesis (OOKP) Semin Ophthalmol. 2005;20(2):113–128. doi: 10.1080/08820530590931386. [DOI] [PubMed] [Google Scholar]
- 5.Aquavella JV, Qian Y, McCormick GJ, Palakuru JR. Keratoprosthesis: the Dohlman-Doane device. Am J Ophthalmol. 2005;140(6):1032–1038. doi: 10.1016/j.ajo.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 6.Puchkovskaya NA, Yakimenko SA. Optical keratoprosthesing. Zdorov’e: Kiev. 1986:35–182. [Google Scholar]
- 7.Yakimenko SA. Keratoprosthesis in treatment of terminal stages of edematous bullous keratopathy. Annals del Instituto Barraquer. 2001;30(1–2):87–88. [Google Scholar]
- 8.Yakimenko SA. Surgical treatment of secondary glaucoma in patients with keratoprosthesis. Annals del Instituto Barraquer. 2002;31(2):163. [Google Scholar]
- 9.Iakymenko SA. Keratoprosthesis: 40-years experience of study and application in the Filatov Institute (analysis of 1000 operations) In: Lang GK, editor. World Ophthalmology Congress abstracts. Berlin: MediaService GmbH; 2010. p. 41. [Google Scholar]
- 10.Puchkovskaia NA, Iakimenko SA, Golubenko EA. Personal experience with keratoprosthesis. Oftalmol Zh. 1975;30(7):490–495. [PubMed] [Google Scholar]
- 11.Puchkovskaia NA, Iakimenko SA. Penetrating keratoprosthesis insertion in leucoma using improved models of keratoprostheses and surgical technique of their insertion. Klinika Oczna. 1981;83(2):105–107. [PubMed] [Google Scholar]
- 12.Puchkovskaya NA, Muchnik SR, Golubenko EA. Corneal alloplasty. Oftalmol Zh. 1970;25(4):247–252. [PubMed] [Google Scholar]
- 13.Iakimenko SA. Methods for optical keratoprosthesis, the indications, potentials and results of their use. Oftalmol Zh. 1985;(3):134–137. [PubMed] [Google Scholar]
- 14.Iakimenko SA. Optic penetrating keratoprosthesis using new models of corneal prostheses. Oftalmol Zh. 1981;36(2):102–104. [PubMed] [Google Scholar]
- 15.Yakimenko S. Results of PMMA/Titanium keratoprosthesis in 502 eyes. Refract Corneal Surg. 1993;9:197–198. [Google Scholar]
- 16.Pan HW, Iakymenko S, Xu JT, Hou GH, Sun BJ, Zheng AN. Implantation of Iakymenko keratoprosthesis in patients with severe ocular injury. Int J Ophthalmol. 2012;5(2):167–171. doi: 10.3980/j.issn.2222-3959.2012.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puchkovskaya NA, Yakimenko SA. Two-staged method of keratoprosthesis. Oftalmol Zh. 1978;33:498–500. [Google Scholar]
- 18.Yakimenko SA. Methods for strengthening leukoma during keratoprosthesis and research of their effectiveness in follow-up. Oftalmol Zh. 1984;39:406–410. [PubMed] [Google Scholar]
- 19.Puchkovskaya NA, Yakimenko SA, Nepomyaschaya VM. Moscow: Meditsina Publishers; 2001. Eye burns; pp. 166–170. [Google Scholar]
- 20.McLaughlin CR, Fagerholm P, Muzakare L, Lagali N, Forrester JV, Kuffova L, Rafat MA, Liu Y, Shinozaki N, Vascotto SG, Munger R, Griffith M. Regeneration of corneal cells and nerves in an implanted collagen corneal substitute. Cornea. 2008;27(5):580–589. doi: 10.1097/ICO.0b013e3181658408. [DOI] [PubMed] [Google Scholar]
- 21.Fagerholm P, Lagali NS, Merrett K, Jackson WB, Munger R, Liu Y, Polarek JW, Söderqvist M, Griffith M. A biosynthetic alternative to human donor tissue for inducing corneal regeneration: 24-month follow-up of a phase 1 clinical study. Sci Transl Med. 2010;2(46):46ra61. doi: 10.1126/scitranslmed.3001022. [DOI] [PubMed] [Google Scholar]
- 22.Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379(9827):1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]