Abstract
Retinoblastoma (RB) is the most common intraocular cancer of infancy and childhood. This cancer is initiated by mutation on RB1, the tumor suppressor gene that is responsible for the regulation of both cell cycle and gnome stability in retinal cells. Patients with a constitutional mutation on RB1 can be inherited. RB occurs approximately 1 in every 15 000-20 000 live births. The worldwide mortality for this cancer is about 5%-11%. However, this rate rises to about 40%-70% in developing countries due to a delay in diagnosis. A wide variety of options are available for the treatment, but often a combination of therapies is adopted to optimize individualized care.
Keywords: retinoblastoma, retinoblastomal gene, leukocoria, epidemiology, medical management
Introduction
Retinoblastoma(RB) is the most common intraocular cancer of childhood, which was first described by Wardrop in 1809 as a tumor arising from the eye. The worldwide incidence of retinoblastoma is constant at one case per 15 000-20 000 live births, which corresponds to about 9000 new cases every year[1]. This disease is initiated by mutation of RB1, the first described tumor-suppressor gene[2]-[4]. Most patients are born with constitutional loss of one RB1 allele and then lose the other RB1 allele in developing retinal cells initiating development of RB tumors within several years after birth[5].
The most common initial sign of retinoblastoma is an abnormal appearance of the pupil, leukocoria[6]-[8]. This phenotype is first apparent when the tumor is still contained in the eye[9]. The RB remains curable for 3-6 months after the first sign of leucocoria. Signs and symptoms like vision deterioration, a red and irritated eye, faltering growth or delayed development may also be observed during the diagnosis of retinoblastoma.
RB is a treatable cancer, and curable if caught early enough. However, many children stricken with it worldwide die[9], especially in developing countries. Fortunately, positive changes are being made due to the development of genome science and global communications, which allows all children and families affected by RB to have an opportunity for a cure. The first priority of treatment is to preserve the life of the child, and then to preserve the vision as well as to minimize the complications or side effects of the treatment. Straightforward strategies that can greatly improve the chances of survival and quality of life of children with RB should be applied.
The treatment of RB is complex as it involves multiple factors including age of the patient, size and location of the tumor as well as the presence or absence of vitreous and subretinal seeding. A wide variety of options are available for the treatment, but often a combination of therapies is adopted to optimize individualized care[1].
EPIDEMIOLOGY
RB is the most common intraocular cancer in children. This cancer is commonly predisposed by constitutional loss of one RB1 allele and is hereditable. However, approximately 55% of children with RB have the non-heritable form caused by different reasons. In about two thirds of cases, only one eye is affected (unilateral RB) while in the other third, tumors develop in both eyes (bilateral retinoblastoma)[10]. The number and size of tumors in each eye varies. In some cases, the pineal gland is also affected. This is termed trilateral RB. The position, size and quantity of tumors are considered in the ophthalmologic treatment of the disease.
The worldwide incidence of RB is reported to be constant at one case per 15 000-20 000 live births, which corresponds to about more than 1 100 new cases in China every year. There is no evidence for any geographic or population hotspots of this disorder. In the United States, for example, about 300-350 new cases are diagnosed each year. However, the survival rate for affected children varies in different areas due to differences in availability of medical care. About 40%-70% of children with RB die in Asia and Africa. In China, the mortality of retinoblastoma is about 30%-40% while in Europe, Canada, and the USA, this rate is only 3%-5%[1], [11]-[15].
Most children are diagnosed before the age of five. In Canada, for example, the mean age at diagnosis is 27 months (SD 18) for unilateral retinoblastoma and 15 months for bilateral disease[5]. Early diagnosis and intervention is critical to the successful treatment of RB. A delay of more than 6 months from the first clinical sign to diagnosis is associated with 70% mortality recorded in developing countries [5]. In Kenya, where the mean age at diagnosis is 36 months (SD 21.4) for unilateral RB, and 25 months (SD 16.8) for bilateral disease, the mortality of retinoblastoma is 73%[12], which is over 50 times higher than that in Canada (about 1%)[15].
The most common initial sign of RB is leucocoria, which is first apparent when the tumor is still contained within the eye. RB remains curable for 3-6 months after the first sign of leucocoria. However, as leucocoria can also indicate other vision-threatening conditions such as Coats' disease, cataract and toxocariasis, prompt medical attention is needed. Late diagnosis delays treatment and the chances of survival decrease as retinoblastoma spreads from the eye [16].
RB1 AND THE ETIOLOGY OF RB
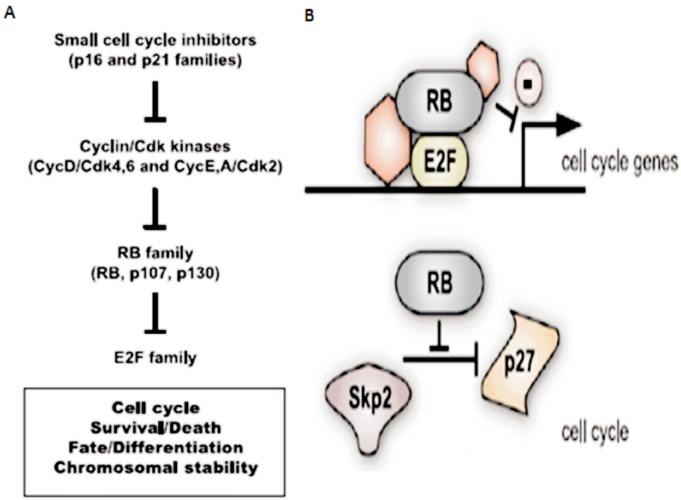
The RB1 gene located on chromosome 13q14 was first described in the 1980s. As the first tumor suppressor gene ever described, RB1 has been studied extensively. This gene is inactivated not only in RB, but also in a wide range of pediatric and adult human cancers. RB1 encodes a 100-kDa nuclear phosphoprotein that has the ability to restrict cell cycle progression at the G1/S transition of the cell cycle by inhibition of E2F transcription factors, which is thought to be essential for its tumor suppression function. When phosphorylated by Cyclin/Cdk (cyclin-dependent kinase) complexes, RB loses its ability to bind to E2F and subsequently initiates proliferation in the cell. Cyclin/Cdk complexes themselves are under the control of small cell cycle inhibitors of the INK4 and CIP/KIP families, including p16Ink4a and p21Cip1[17],[18]. Taken together, this RB pathway is composed of INK4-CIP/KIP cell cycle inhibitors, Cyclin/Cdk complexes, RB and its two family members, p107 and p130 as well as E2F transcription factors (Figure 1A). RB has also been reported to regulate G1/S progression through another pathway by interacting with Cdh1/APC and Skp2 to stabilize P27Kip1 (Figure 1B). Beyond its direct control of the transcription of programs of genes involved in proliferation, RB may also regulate global gene expression by interacting with chromatin remodeling enzymes[19]. Finally, it has been shown that in mouse embryonic fibroblasts lacking the RB genes RB1, p107, and p130 (TKO MEFs), cells proliferate in spite of unrepaired DNA damage. These mitotic cells show chromatid breaks and chromatid cohesion defects that lead to aneuploidy in the descendent cell population, indicating a role for RB1 in G2/M checkpoint and genome stabilization[20].
Figure 1. RB and G1/S regulation[20].
A: Classical pathway of RB and G1/S transition. Small cell cycle inhibitors of the INK4 and CIP/KIP families inhibit the activity of Cyclin/Cdk complexes, which consequently maintain the activity of RB by preventing it from being phosphorylated. Unphosphorylated RB would bind E2F family proteins and block their activities as transfactors; B: Beyond the classical pathway, RB also controls the cell cycle by blocking the degradation of the p27 small cell cycle inhibitor.
Most young children with hereditable RB are diagnosed within several years after birth. This specific age window suggests a model of tumor initiation in which loss of RB function must happen in specific populations of cells that may be transiently present in the developing retina[18]. This is consistent with the fact that many progenitor cell markers are also found in tumors as reviewed by Dyer and Bremner 2005[17]. However, differentiated markers can also be found in some tumor cells[21],[22]. Ajioka et al[23] found that differentiated Rb(-/-); p107(+/-); p130(-/-) horizontal interneurons could re-enter the cell cycle and form metastatic RB, indicating a possible role for differentiated cells in RB initiation. Thus, the identity of the cell of origin of RB is still being debated.
Though, loss of RB function does have visible effects in retinal progenitors, it is not sufficient for tumor initiation. For instance, deletion of the mouse Rb gene in the developing mouse retina results in ectopic proliferation and increased cell death in specific cell populations[24], while studies using mouse models showed that Rb+/− animals develop neuroendocrine tumors and die early[25], further deletion of p107 and/or p130 enhances the defects caused by RB deletion and is required to initiate retinoblastoma in mice[22],[24],[26]. Heterozygous deletion of E2F1 prevents the tumor initiation in RB deficient retinal progenitors. Blockade of Cdk2 activity during a short period of time in young mice is also sufficient to prevent retinoblastoma development[27]. Taken together, these experiments indicate that the tumor suppression function of RB is mainly based on its role in G1/S regulation.
Intriguingly, RB+/- mice develop neuroendocrine tumors rather than RB, indicating a role for RB in tumor suppression after G1/S transition. This is consistent with the fact that RB may also interact with several chromatin regulators, such as DNMT1 and Rbp2[28],[29], and regulate the expression of several other histone modifiers including HDAC1. Experiments using a knock-in RB1 allele that harbors a mutation in the LxCxE binding site show that this motif is important for the ability of RB to bind several histone-modifying enzymes, promote histone H4K20 trimethylation (a marker of repressive chromatin) at centromeres, and prevent chromosome fusions that lead to aneuploidy[30]. Further studies indicate that the LxCxE-binding motif in pRb is also important for tumor suppression, as this knock-in allele causes chromosome instability (CIN) and consequently accelerates loss of heterozygosis, tumor formation, and lethality in Trp53-mutant mice[31].
Given the critical role for RB in tumor suppression, it is not surprising that patient carrying a constitutional RB1 mutation has an increased risk of second malignancies of the lung, bladder, bone, soft tissues, skin, and brain throughout life, especially when the children are treated with radiation[32]. In fact, as noted in a study of 1 604 patients, incidence of a second malignancy was recorded at 51% versus 5% for heritable and nonheritable forms of retinoblastoma respectively[33].
MEDICAL MANAGEMENT OF RETINBLASTOMA
Classification of the extent of cancer at presentation should be the first priority. The International Intraocular Retinoblastoma Classification (IIRC) for prediction of outcomes for eyes treated with chemotherapy and focal laser treatment was accepted at the 2003 meeting of the International Society of Genetic Eye Disease and Retinoblastoma[34]. IIRC classification of each eye by extent of intraocular disease at diagnosis, and use of the seventh edition of the American Joint Committee on Cancer and International Union Against Cancer's staging (TNM clinical classification) to assess the whole patient by extent of extraocular disease is generally recommended[5]. In fact, as the gold-standard classification to establish an appropriate care plan for patients with cancer, TNM is used worldwide and recommended by many ophthalmology journals for staging of retinoblastoma[35].
Despite significant advances in treatment options, enucleation is still the first choice for most unilateral patients. In developing countries, eyes of 75% of unilateral retinoblastomas are enucleated as typically the disease is detected at an advanced stage. Care must be taken when eyes with intraocular retinoblastoma are enucleated to prevent the tumor from spreading.
External Beam Radiation Therapy (EBRT) was first used to treat retinoblastoma in the early 1950s[36]. However, for children with a constitutional RB1 mutation, this therapy would heighten lifelong risk of a second cancer[37], [38]. Thus, this therapy is generally replaced by chemotherapy combined with focal laser treatment.
Systemic chemotherapy is widely used for intraocular retinoblastoma. Long-term systemic chemotherapy alone cannot be relied on to control intraocular retinoblastoma, chemotherapy generally requires adjuvant therapies, focal laser treatment or cryotherapy. Close surveillance at frequent examinations under anesthesia is necessary for 2 years or longer after chemotherapy to ensure ablation of all tumor cells[5].
Intra-arterial chemotherapy is to inject chemotherapy directly into the ophthalmic artery thereby potentially avoiding some of the systemic toxicities encountered with intravenous administration of chemotherapeutic agents [39], [40]. However, some reports point out that ophthalmic arterial infusion of melphalan is technically feasible and can result in striking regression of tumor [41],[42]. Further studies are necessary [5].
In 2011, 2 groups of scientists reported a high-dose chemotherapy followed by autologous stem cell rescue to treat systematic RB independently. However, toxicity and follow-up data for a longer period are still needed to judge the efficacy of this therapy.
SUMMARY
Retinoblastoma occurs approximately 1 in every 15 000-20 000 live births and is known as the most common intraocular cancer of infancy and childhood. It can occur in one eye (unilateral) or in both eyes (bilateral). This tumor is usually confined to the eye, and is treatable or even curable if found early enough. However, late diagnosis delays treatment and retinoblastomas might spread from the eye increasing the risk of mortality.
As the first described tumor suppressor gene, RB1 is believed to be essential for the development of retinal cells. The protein RB, which is encoded by the RB1 gene, is known to take part in three pathways. Firstly, it cooperates with INK4-CIP/KIP cell cycle inhibitors, Cyclin/Cdk complexes, p107 and p130 as well as E2F transcription factors (Figure 1A) to regulate G1/S transition during mitosis. Secondly, this protein may also regulate G1/S progression by interacting with Cdh1/APC and Skp2 to stabilize P27Kip1 (Figure 1B). And finally, RB can interact with several chromatin regulators to maintain gnome stability. Loss of function of RB1 initiates retinoma and causes genome instability in retinal cells, but is insufficient to cause retinoblastoma. The genome instability would subsequently increase the chance for mutations on other genes and finally cause retinoblastoma.
A wide variety of options are available for the treatment, including enucleation, External Beam Radiation Therapy (EBRT), systemic chemotherapy, intra-arterial chemotherapy and several other approaches under trial currently. The treatment of retinoblastoma is complex. Treatment involves multiple factors including age of the patient, size and location of the tumor as well as the presence or absence of vitreous and subretinal seeding. A combination of therapies is usually adopted to optimize individualized care.
References
- 1.Kivela T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93(9):1129–1131. doi: 10.1136/bjo.2008.150292. [DOI] [PubMed] [Google Scholar]
- 2.Comings DE. A general theory of carcinogenesis. Proc Natl Acad Sci USA. 1973;70(12):3324–3328. doi: 10.1073/pnas.70.12.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friend SH, Bernards R, Rogelj S, Weinberg RA, Rapaport JM, Albert DM, Dryja TP. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- 4.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimaras H, Kimani K, Dimba EA, Gronsdahl P, White A, Chan HS, Gallie BL. Retinoblastoma. Lancet. 2012;379(9824):1436–1446. doi: 10.1016/S0140-6736(11)61137-9. [DOI] [PubMed] [Google Scholar]
- 6.Menon BS, Alagaratnam J, Juraida E, Mohamed M, Ibrahim H, Naing NN. Late presentation of retinoblastoma in Malaysia. Pediatr Blood Cancer. 2009;52(2):215–217. doi: 10.1002/pbc.21791. [DOI] [PubMed] [Google Scholar]
- 7.Bowman RJ, Mafwiri M, Luthert P, Luande J, Wood M. Outcome of retinoblastoma in east Africa. Pediatr Blood Cancer. 2008;50(1):160–162. doi: 10.1002/pbc.21080. [DOI] [PubMed] [Google Scholar]
- 8.Abramson DH, Frank CM, Susman M, Whalen MP, Dunkel IJ, Boyd NW., 3rd Presenting signs of retinoblastoma. J Pediatr. 1998;132(3 Pt 1):505–508. doi: 10.1016/s0022-3476(98)70028-9. [DOI] [PubMed] [Google Scholar]
- 9.Yun J, Li Y, Xu CT, Pan BR. Epidemiology and Rb1 gene of retinoblastoma. Int J Ophthalmol. 2011;4(1):103–109. doi: 10.3980/j.issn.2222-3959.2011.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacCarthy A, Birch JM, Draper GJ, Hungerford JL, Kingston JE, Kroll ME, Onadim Z, Stiller CA, Vincent TJ, Murphy MF. Retinoblastoma in Great Britain 1963-2002. Br J Ophthalmol. 2009;93(1):33–37. doi: 10.1136/bjo.2008.139618. [DOI] [PubMed] [Google Scholar]
- 11.MacCarthy A, Draper GJ, Steliarova-Foucher E, Kingston JE. Retinoblastoma incidence and survival in European children (1978-1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2092–2102. doi: 10.1016/j.ejca.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Nyamori JM, Kimani K, Njuguna MW, Dimaras H. The incidence and distribution of retinoblastoma in Kenya. Br J Ophthalmol. 2012;96(1):141–143. doi: 10.1136/bjophthalmol-2011-300739. [DOI] [PubMed] [Google Scholar]
- 13.Leal-Leal C, Flores-Rojo M, Medina-Sansón A, Cerecedo-Díaz F, Sánchez-Félix S, González-Ramella O, Pérez-Pérez F, Gómez-Martínez R, Quero-Hernández A, Altamirano-Alvarez E, Alejo-González F, Figueroa-Carbajal J, Ellis-Irigoyen A, Tejocote-Romero I, Cervantes-Paz R, Pantoja-Guillén F, Vega-Vega L, Carrete-Ramírez F. A multicentre report from the Mexican Retinoblastoma Group. Br J Ophthalmol. 2004;88(8):1074–1077. doi: 10.1136/bjo.2003.035642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues KE, Latorre Mdo R, de Camargo B. Delayed diagnosis in retinoblastoma. J Pediatr (Rio J) 2004;80(6):511–516. [PubMed] [Google Scholar]
- 15.Canadian Retinoblastoma Society National Retinoblastoma Strategy Canadian guidelines for care: stratégie thérapeutique du rétinoblastome guide clinique canadien. Can J Ophthalmol. 2009;44(suppl 2):S1–88. doi: 10.3129/i09-194. [DOI] [PubMed] [Google Scholar]
- 16.Leander C, Fu LC, Peña A, Howard SC, Rodriguez-Galindo C, Wilimas JA, Ribeiro RC, Haik B. Impact of an education program on late diagnosis of retinoblastoma in Honduras. Pediatr Blood Cancer. 2007;49(6):817–819. doi: 10.1002/pbc.21052. [DOI] [PubMed] [Google Scholar]
- 17.Dyer MA, Bremner R. The search for the retinoblastoma cell of origin. Nat Rev Cancer. 2005;5(2):91–101. doi: 10.1038/nrc1545. [DOI] [PubMed] [Google Scholar]
- 18.Sage J. The retinoblastoma tumor suppressor and stem cell biology. Genes Dev. 2012;26(13):1409–1420. doi: 10.1101/gad.193730.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flowers S, Beck GR, Jr, Moran E. Transcriptional activation by pRB and its coordination with SWI/SNF recruitment. Cancer Res. 2010;70(21):8282–8287. doi: 10.1158/0008-5472.CAN-10-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Harn T, Foijer F, van Vugt M, Banerjee R, Yang F, Oostra A, Joenje H, te Riele H. Loss of Rb proteins causes genomic instability in the absence of mitogenic signaling. Genes Dev. 2010;24(13):1377–1388. doi: 10.1101/gad.580710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu XL, Fang Y, Lee TC, Forrest D, Gregory-Evans C, Almeida D, Liu A, Jhanwar SC, Abramson DH, Cobrinik D. Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specific MDM2 signaling. Cell. 2009;137(6):1018–1031. doi: 10.1016/j.cell.2009.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEvoy J, Flores-Otero J, Zhang J, Nemeth K, Brennan R, Bradley C, Krafcik F, Rodriguez-Galindo C, Wilson M, Xiong S, Lozano G, Sage J, Fu L, Louhibi L, Trimarchi J, Pani A, Smeyne R, Johnson D, Dyer MA. Coexpression of normally incompatible developmental pathways in retinoblastoma genesis. Cancer Cell. 2011;20(2):260–275. doi: 10.1016/j.ccr.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajioka I, Martins RA, Bayazitov IT, Donovan S, Johnson DA, Frase S, Cicero SA, Boyd K, Zakharenko SS, Dyer MA. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131(2):378–390. doi: 10.1016/j.cell.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen D, Livne-Bar I, Vanderluit JL, Slack RS, Agochiya M, Bremner R. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell. 2004;5(6):539–551. doi: 10.1016/j.ccr.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 25.Hu N, Gutsmann A, Herbert DC, Bradley A, Lee WH, Lee EY. Heterozygous Rb-1 delta 20/+ mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene. 1994;9(4):1021–1027. [PubMed] [Google Scholar]
- 26.MacPherson D, Sage J, Kim T, Ho D, McLaughlin ME, Jacks T. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 2004;18(14):1681–1694. doi: 10.1101/gad.1203304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sangwan M, McCurdy SR, Livne-Bar I, Ahmad M, Wrana JL, Chen D, Bremner R. Established and new mouse models reveal E2f1 and Cdk2 dependency of retinoblastoma, and expose effective strategies to block tumor initiation. Oncogene. 2012;31(48):5019–5028. doi: 10.1038/onc.2011.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson KD, Ait-Si-Ali S, Yokochi T, Wade PA, Jones PL, Wolffe AP. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat Genet. 2000;25(3):338–342. doi: 10.1038/77124. [DOI] [PubMed] [Google Scholar]
- 29.Christensen J, Agger K, Cloos PA, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell. 2007;128(6):1063–1076. doi: 10.1016/j.cell.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Isaac CE, Francis SM, Martens AL, Julian LM, Seifried LA, Erdmann N, Binné UK, Harrington L, Sicinski P, Bérubé NG, Dyson NJ, Dick FA. The retinoblastoma protein regulates pericentric heterochromatin. Mol Cell Biol. 2006;26(9):3659–3671. doi: 10.1128/MCB.26.9.3659-3671.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coschi CH, Martens AL, Ritchie K, Francis SM, Chakrabarti S, Berube NG, Dick FA. Mitotic chromosome condensation mediated by the retinoblastoma protein is tumor-suppressive. Genes Dev. 2010;24(13):1351–1363. doi: 10.1101/gad.1917610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eng C, Li FP, Abramson DH, Ellsworth RM, Wong FL, Goldman MB, Seddon J, Tarbell N, Boice JD., Jr Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst. 1993;85(14):1121–1128. doi: 10.1093/jnci/85.14.1121. [DOI] [PubMed] [Google Scholar]
- 33.Wong FL, Boice JD, Jr, Abramson DH, Tarone RE, Kleinerman RA, Stovall M, Goldman MB, Seddon JM, Tarbell N, Fraumeni JF, Jr, Li FP. Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA. 1997;278(15):1262–1267. doi: 10.1001/jama.278.15.1262. [DOI] [PubMed] [Google Scholar]
- 34.Linn Murphree A. Intraocular retinoblastoma: the case for a new group classifi cation. Ophthalmol Clin North Am. 2005;18(1):41–53. doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Kivela T, Singh A. Information for authors project. 2011 http:// eyecancerbig.com/EyeCaBIG/Journals_and_Societies.html. [Google Scholar]
- 36.Stallard HB. Irradiation of retinoblastoma. Lancet. 1952;1(6717):1046–1049. doi: 10.1016/s0140-6736(52)90697-1. [DOI] [PubMed] [Google Scholar]
- 37.Kleinerman RA, Tucker MA, Tarone RE, Abramson DH, Seddon JM, Stovall M, Li FP, Fraumeni JF., Jr Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol. 2005;23(10):2272–2279. doi: 10.1200/JCO.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 38.Fletcher O, Easton D, Anderson K, Gilham C, Jay M, Peto J. Lifetime risks of common cancers among retinoblastoma survivors. J Natl Cancer Inst. 2004;96(5):357–363. doi: 10.1093/jnci/djh058. [DOI] [PubMed] [Google Scholar]
- 39.Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intraarterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011;129(6):731–737. doi: 10.1001/archophthalmol.2011.5. [DOI] [PubMed] [Google Scholar]
- 40.Vajzovic LM, Murray TG, Aziz-Sultan MA, Schefler AC, Wolfe SQ, Hess D, Fernandes CE, Dubovy SR. Supraselective intra-arterial chemotherapy: evaluation of treatment-related complications in advanced retinoblastoma. Clin Ophthalmol. 2011;5:171–176. doi: 10.2147/OPTH.S12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. Ophthalmology. 2008;115(8):1398–1404. doi: 10.1016/j.ophtha.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 42.Abramson DH, Dunkel IJ, Brodie SE, Marr B, Gobin YP. Bilateral superselective ophthalmic artery chemotherapy for bilateral retinoblastoma: tandem therapy. Arch Ophthalmol. 2010;128(3):370–372. doi: 10.1001/archophthalmol.2010.7. [DOI] [PubMed] [Google Scholar]