Dear Sir,
I am Dr. Hande Taylan Sekeroglu, from the Ophthalmology Department of Cukurova University Faculty of Medicine. I want to present a case of recalcitrant ocular cicatricial pemphigoid (OCP) which was coincidently diagnosed with Stevens Johnson syndrome (SJS), and which was successfully treated with intravenous immunoglobulin (IVIg).
OCP being one of the subsets of autoimmune blistering diseases, is also a sight-threatening disease. The diagnosis requires compliance with clinical criteria and typical immunohistopathology confirmed by the biopsy. The uncontrolled slow and chronic inflammation of the eye causes dry eye, subepithelial fibrosis, fornix shortening, lid abnormalities, corneal complications and severe ocular surface damage which eventuates in blindness[1]. The treatment consists of immunosupression and/or immunomodulation and sometimes surgical interventions, of all targeting to control the underlying destructive inflammation.
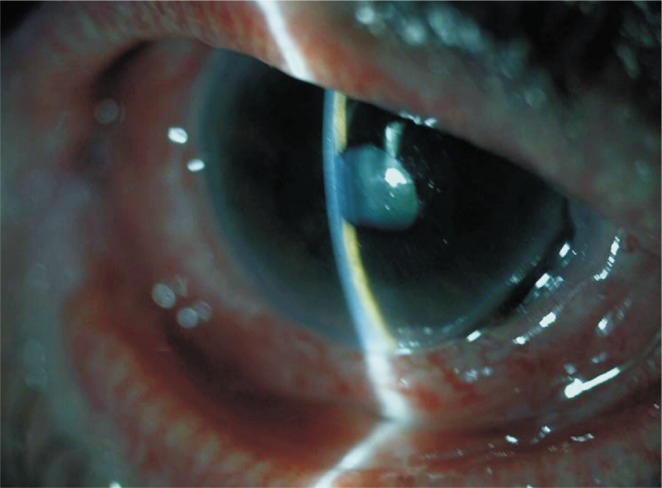
A seventy-six year old woman presented with sudden onset of widespread rashes after taking oral metamizole for headache. She had widespread papules, buccal mucosal and plantar bullae, target lesions on the limbs and diffuse bilateral conjunctival hyperemia. Ophthalmological examination showed bilateral conjunctival hyperemia with prominent inferonasal corneal thinning of the right eye (Figure 1). There was no sign of symblepharon or subepithelial fibrosis. Initial treatment consisted of topical application of 1% dexamethasone (without preservatives) and cyclosporine 0.05% for the right eye and topical lubricants for both eyes. The initial visual acuity was 20/32 bilaterally because of nuclear sclerosis and macular drusens.
Figure 1. Severe inferonasal corneal thinning in the right eye.
The patient started with systemic steroid treatment 1 mg·kg−1·d−1 for SJS. Since the treatment was not sufficient in controlling the disease and ophthalmological status of the patient was started to deteriorate, oral azathioprine 1mg·kg−1·d−1 was added to treatment protocol.
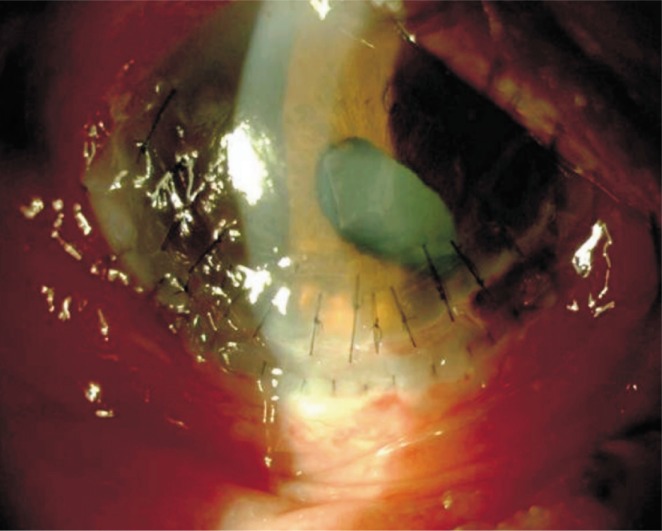
The conjunctival biopsy revealed linear deposition of immunoglobulin G and complement C3 along the epithelial basement membrane zone which confirmed the diagnosis of OCP. Nevertheless, the perilesional skin biopsy showed predominantly lymphocytic infiltration at the dermal-epidermal junction and epidermal necrosis which was compatible with SJS. The systemic skin lesions responded well to systemic steroids and azathioprine, however the corneal thinning continued and caused perforation of the right eye five days after her presentation. A limbal corneal allotransplant was used to maintain anterior chamber stability and to cover the area of perforation (Figure 2). One month after the beginning of the treatment, the patient presented with spontaneous corneal perforation at the inferior quadrant of the right eye which could not be repaired with corneal grafting. The inflammation could not be brought under control and the limbal allotransplant failed, therefore the evisceration of the right eye was inevitable. Systemic steroids tapered and discontinued within one month but azathioprine treatment continued.
Figure 2. Clinical picture of the right eye after successful limbal allotrasplant.
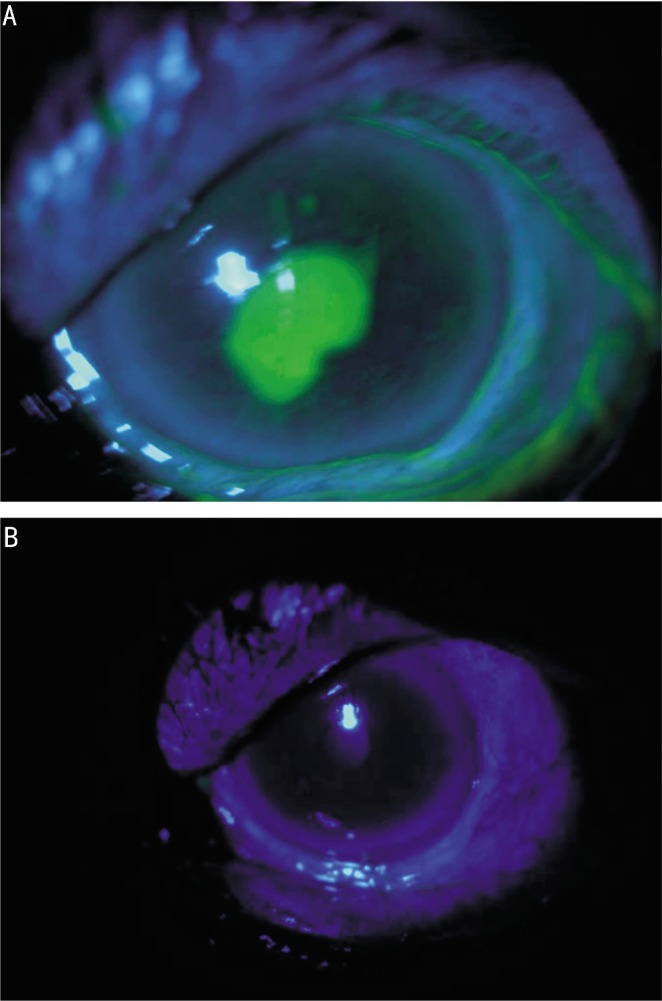
One year after the operation, she presented again with foreign body sensation on her left eye. She had conjunctival hyperemia, progressive inferior corneal thinning, and pericentral corneal staining. Topical treatment including dexamethasone and cyclosporine was restarted in addition to topical preservative-free artificial tears and oral azathioprine. However, inferior corneal thinning associated with severe inflammation of the adjacent conjunctiva continued. IVIg treatment was preferred as an immunomodulatory procedure. The interval between infusions was not equal, they were arranged according to the status of corneal involvement, therefore, IVIg was given within 2 weeks, 4 weeks or 2 months intervals. IVIg 20 mg/d was given within 5 consecutive days during every episode of active corneal thinning under close monitorization of vital signs. The progressive corneal thinning stopped and responded well to treatment during each cycle (Figure 3A,B). The stabilization of the disease was achieved around the eighth cycle. No side effect associated with the treatment was observed during the cycles.
Figure 3. A: Severe pericentral corneal staining of the left eye before IVIg therapy; B: No corneal staining on the left eye at the last day of IVIg cycle associated with symptomatic relief of the patient.
Coexistence of OCP and other autoimmune diseases such as systemic lupus erythematosus and mixed connective tissue disease have been reported previously, and therefore they may share a common specific immune mechanism and immunogenetics[2],[3]. However, coexistence of OCP and SJS is uncommon.
IVIg may be a treatment option for recalcitrant OCP. IVIg may arrest the rapid clinical course and deterioration of vision. However, the treatment is not completely innocent and free of complications, and may cause severe side effects including acute renal failure, hemolysis, neutropenia, anaphylaxis, cerebrovascular accidents and aseptic menengitis[4],[5]. IVIg had a very rapid onset of action and provided rapid attenuation of conjunctival inflammation and retardation of corneal thinning in the present case. Whether several hypotheses have been suggested to explain the mechanism of action of IVIg, the exact mechanism is still unknown.
To the best of our knowledge, this is a very rare case of OCP which was coexistent, even newly diagnosed with simultanenous SJS, and treated successfully with IVIg. One common cause may be the potential trigger of two autoimmune blistering diseases. As a conclusion, in case of an autoimmune blistering disorder, it should be kept in mind that other systemic autoimmune disorders may be coexistent, and may prevent the optimal outcome.
REFERENCES
- 1.Elder MJ, Bernauer W, Leonard J, Dart JKG. Progression of disease in ocular cicatricial pemhigoid. Br J Ophthalmol. 1996;80(4):292–296. doi: 10.1136/bjo.80.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayar M, Wojnarovska F, Yenning V, Taylor CJ. Association of autoimmunity and cicatricial pemphigoid: is there an immunogenetic basis? J Am Acad Dermatol. 1991;25(6 Pt 1):1011–1015. doi: 10.1016/0190-9622(91)70299-h. [DOI] [PubMed] [Google Scholar]
- 3.Malik M, Gürcan HM, Ahmed AR. Coexistence of mucous membrane pemphigoid and connective-tissue disease. Clin Exp Dermatol. 2010;35(2):156–159. doi: 10.1111/j.1365-2230.2009.03222.x. [DOI] [PubMed] [Google Scholar]
- 4.Jolles S, Hughes J, Whittaker S. Dermatological uses of high-dose intravenous immunoglobulin. Arch Dermatol. 1998;134(1):80–86. doi: 10.1001/archderm.134.1.80. [DOI] [PubMed] [Google Scholar]
- 5.Mobini N, Sarela A, Ahmed AR. Intravenous immunoglobulins in the therapy of autoimmune and systemic inflammatory disorders. Ann Allergy Asthma Immunol. 1995;74(2):119–128. [PubMed] [Google Scholar]