Abstract
Purpose
The cardiopulmonary exercise test (CPET) is an important clinical tool for evaluating exercise capacity and is frequently used to evaluate chronic conditions including congenital heart disease. However, data on the normal CPET values for Korean children and adolescents are lacking. The aim of this study was to provide reference data for CPET variables in children and adolescents.
Methods
From August 2006 to April 2009, 76 healthy children and adolescents underwent the CPET performed using the modified Bruce protocol. Here, we performed a medical record review to obtain data regarding patient' demographics, medical history, and clinical status.
Results
The peak oxygen uptake (VO2Peak) and metabolic equivalent (METMax) were higher in boys than girls. The respiratory minute volume (VE)/CO2 production (VCO2) slope did not significantly differ between boys and girls. The cardiopulmonary exercise test data did not significantly differ between the boys and girls in younger age group (age, 10 to 14 years). However, in older age group (age, 15 to 19 years), the boys had higher VO2Peak and METMax values and lower VE/VCO2 values than the girls.
Conclusion
This study provides reference data for CPET variables in case of children and adolescents and will make it easier to use the CPET for clinical decision-making.
Keywords: Exercise test, Oxygen uptake, Adolescents
Introduction
The cardiopulmonary exercise test (CPET) is an important clinical tool to evaluate exercise capacity and is frequently used to evaluate chronic condition1) including congenital heart disease2,3). A great deal of research has been conducted regarding the utility of CPET, and especially in adults, this test is used as one of the most important indicators of severity and mortality in heart failure cases4-6). It is noninvasive, relatively inexpensive, and provides a wealth of clinically relevant diagnostic and prognostic information. In clinical situation, we frequently use this test for evaluation of exercise intolerance, the effect of specific intervention (i.e., physical training, medication), and change or alteration of exercise capacity in the same subjects7,8). However, variables of this test lack normal values for the adolescence and some reference value might be something old9-12). The aim of this study was to provide a reference data for the variables of CPET in children and adolescents.
Materials and methods
From August 2006 to April 2009, 92 children and adolescents visited our hospital for heart evaluation due to chest pain, dyspnea, syncope and so on (Table 1). We performed physical examination, history taking, chest X-ray, electrocardiography, echocardiography and CPET. Among them, 76 had no specific medical problem and were enrolled in this study. Excluded were patients with asthma, musculoskeletal disability, mental retardation, and infectious disease within 2 weeks. The CPET was performed using modified Bruce protocol on TrueOne 2400 (ParvoMedics, Salt Lake City, UT, USA). All subjects were encouraged to exercise to exhaustion. The medical records of all subjects were reviewed retrospectively to determine their demographics, past medical history and clinical status. The CPET parameters; peak oxygen uptake (VO2Peak), metabolic equivalent (MET) and slope of respiratory minute volume to CO2 (VE/VCO2) production were evaluated and compared between sexes, and two different age groups (younger group, 10 to 14 years vs. older group, ≥15 years). Results are presented as mean±standard deviation and range. Statistical analysis was done by the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). A two sample t-test was used to compare the means of continuous variable with a normal distribution, and Mann-Whiteny U test for continuous variables without normal distribution. A P value less than 0.05 was considered statistically significant. The Institutional Review Board reviewed and approved the study.
Table 1.
Patients' baseline clinical characteristics
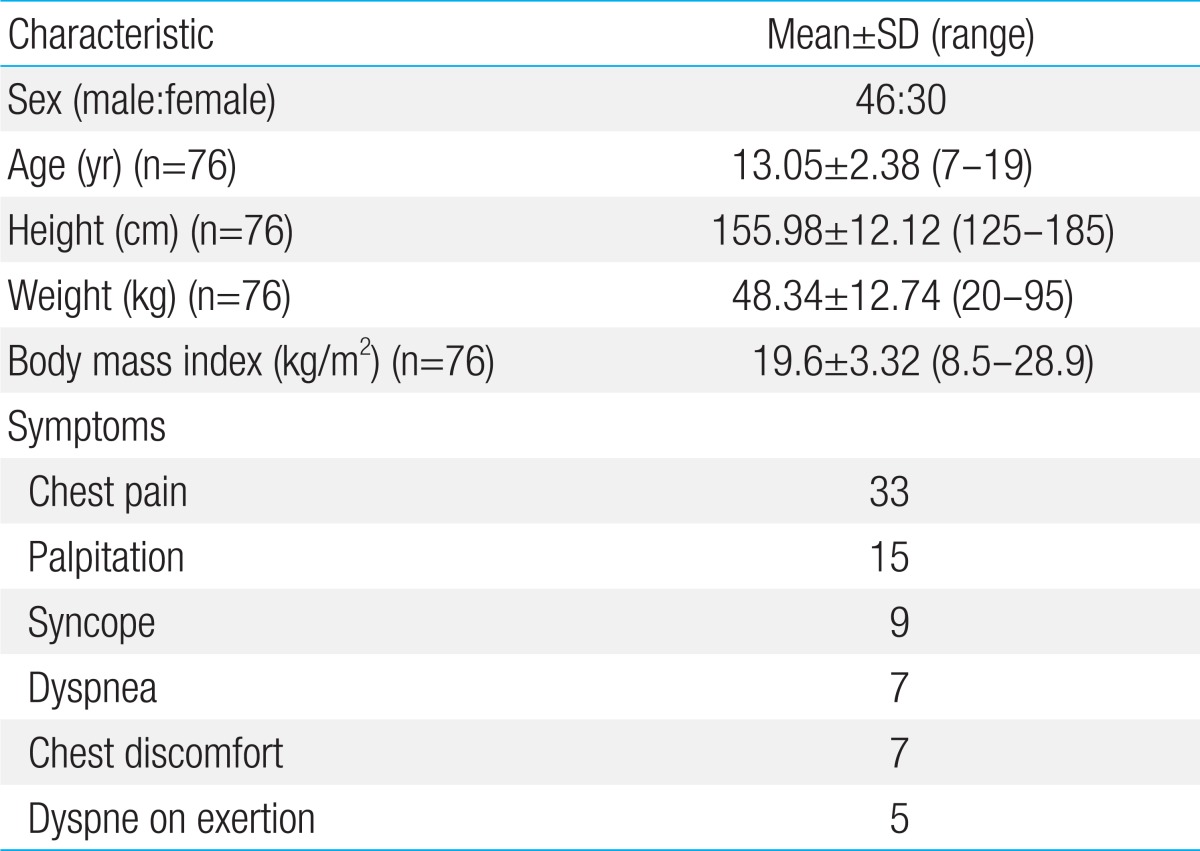
SD, standard deviation.
Results
A total of 76 children and adolescents (46 males, 30 females) were enrolled in this study with an age range of 7-19 years (mean, 13.05±2.38 years). Mean height, body weight and body mass index were 155.98±12.12 cm (range, 128 to 185 cm), 48.34±12.74 kg (range, 20 to 95 kg) and 19.6±3.32 kg/m2 (range, 8.5 to 28.9 kg/m2), respectively (Table 1). All subjects performed the CPET without complication or event and were able to complete the protocol. There were no electrocardiographic abnormalities during exercise test.
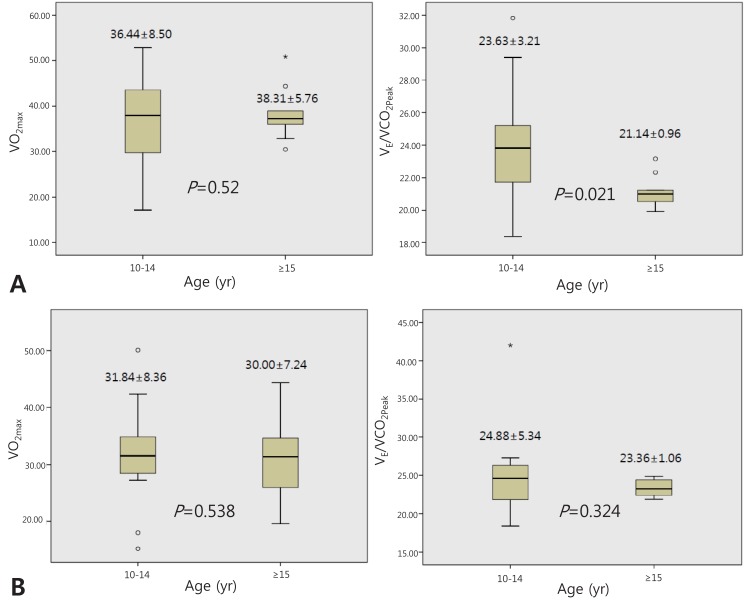
Compared to girls, boys had higher VO2Peak, METMax but similar VE/VCO2 and maximal heart rate (HRMax) (Table 2). However, there was significant difference in age between boys and girls. Thus, we divided study subjects into two groups; younger group (10 to 14 years old) and older group (≥15 years old). In younger group, there was no significant difference in VO2Peak, METMax and VE/VCO2 between boys and girls. On the other hand, in older group, higher VO2Peak, METMax and lower VE/VCO2 were observed in boys (Table 3). According to age, there was no significant change of the VO2Peak between younger group and older group in both boys and girls. However, there was a significant decrease in VE/VCO2 in older boys but not in older girls (Fig. 1).
Table 2.
Cardiopulmonary exercise data for boys (n=46) and girls (n=30)
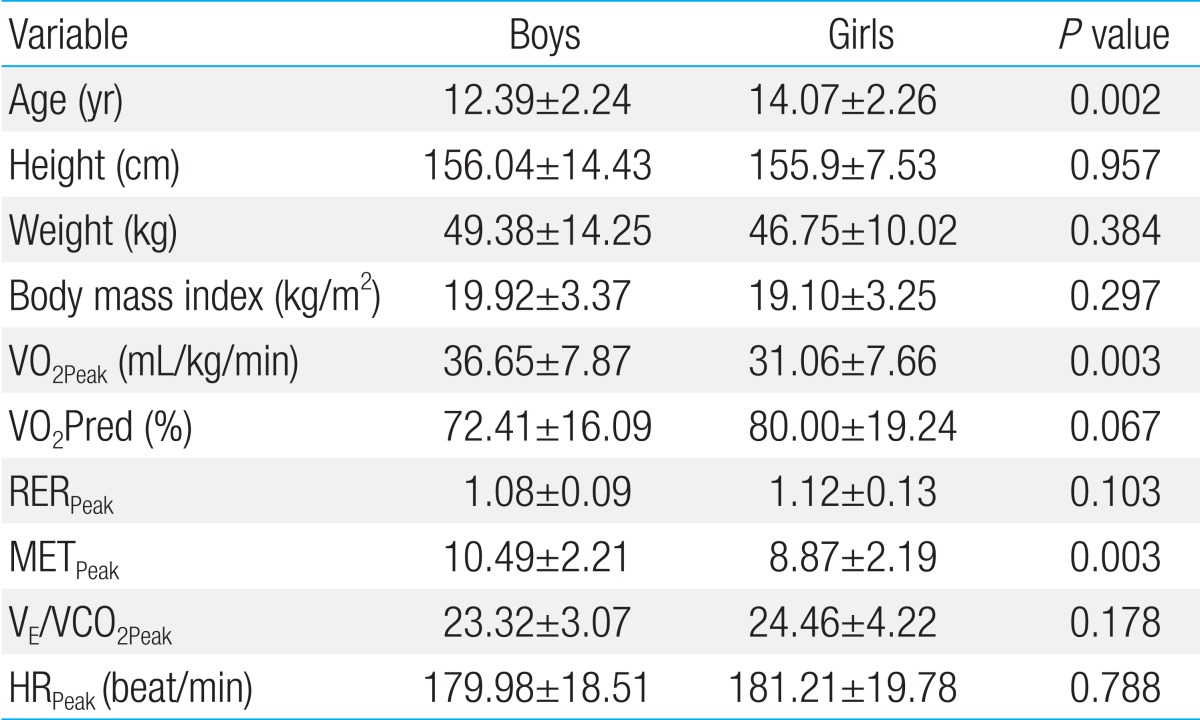
Values are presented as mean±standard deviation.
VO2Peak, peak oxygen uptake; RERPeak, peak respiratory exchange ratio; METPeak, peak metabolic equivalent; VE/VCO2Peak, slope of respiratory minute volume to CO2 production; HRPeak, peak heart rate.
Table 3.
Cardiopulmonary exercise data according to the age groups
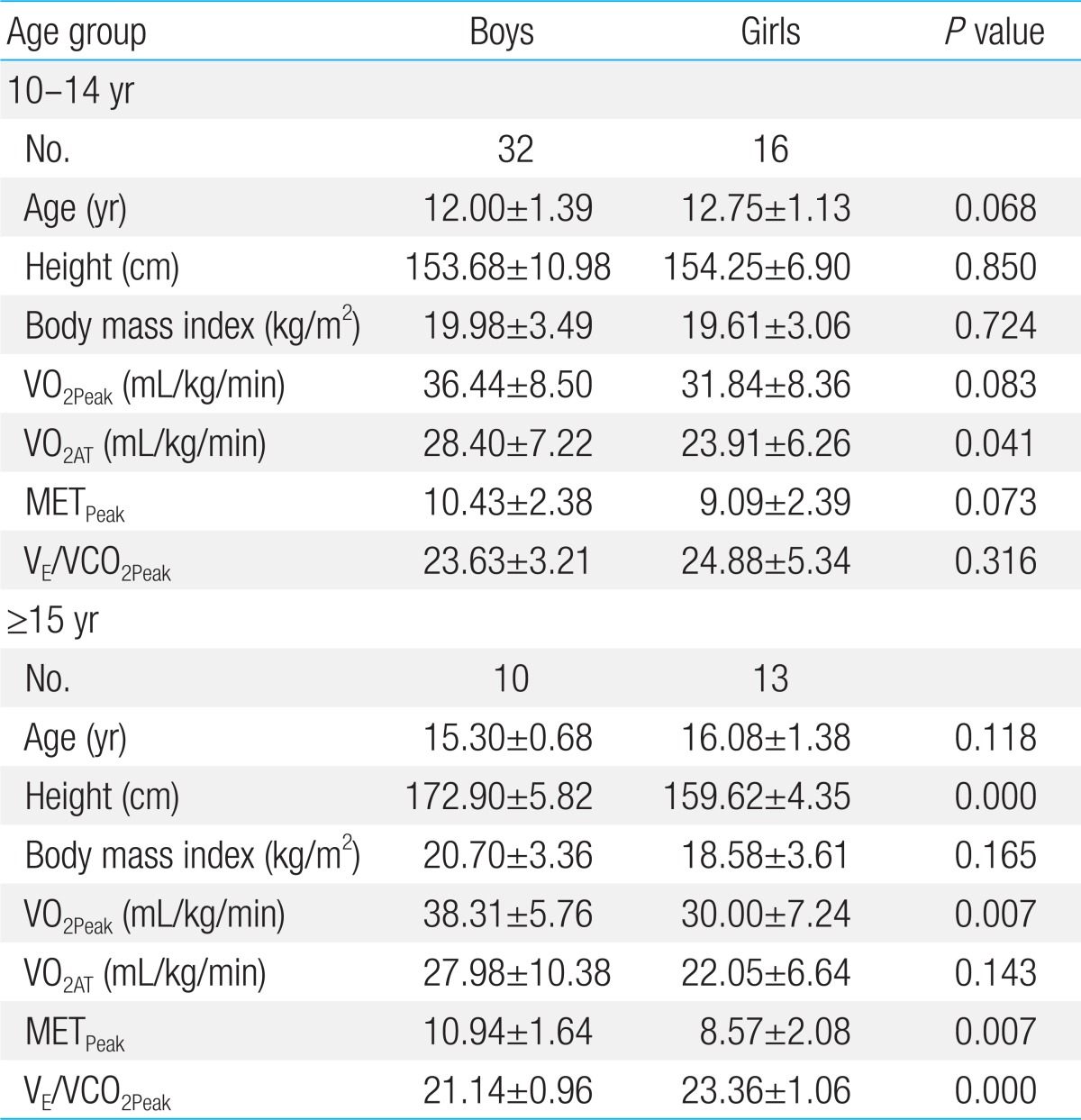
Values are presented as mean±standard deviation.
VO2Peak, peak oxygen uptake; VO2AT, oxygen uptake at anaerobic threshold; METPeak, peak metabolic equivalen; VE/VCO2Peak, slope of respiratory minute volume to CO2 production.
Fig. 1.
(A) Cardiopulmonary exercise data according for boys, according to the age group. (B) Cardiopulmonary exercise data for girls, according to the age group.
Discussion
The CPET has been used in wide spectrum of clinical applications for evaluation undiagnosed exercise intolerance; for objective determination of functional capacity and impairment; and for evaluation of the effect of a specific intervention. Thereby, the CPET can provide the doctor with relevant information for clinical decision making. However, the exercise test may result in unexpected harmful event including cardiac arrest, severe dyspnea, fatal arrhythmia and cyanosis. Moreover, some subjects may fall by the wayside when the test is getting harder. Especially, in children and adolescents, there are some difficulties; small body size in relation to the equipment; poor peak exercise performance capacity in increasing of work rate; shorter attention span and lower motivation leading to poor compliance. Thus, the test should be performed by experienced staff and the laboratory should be equipped with emergency equipment in comfortable and professional environment.
The CPET involves measurements of variables such as respiratory oxygen uptake (VO2), carbon dioxide production (VCO2), and ventilator efficiency (VE) during a symptom-limited exercise test. VO2 is determined by cardiac output and the difference of oxygen content between arterial and venous blood by the Fick equation. One MET is the resting oxygen uptake in a sitting position and equals 3.5 mL/kg/min13). The maximal oxygen uptake (VO2Max) is the most important variable and represents the exercise capacity of a subject. In healthy subject, a VO2 plateau is found at near maximal exercise. This plateau in VO2 has been traditionally used as the best evidence of VO2Max. However, clinically, the obtainment of clear plateau in VO2 is not easy. Consequently, peak VO2 is often used as an estimate of VO2Max14), and in this study, we used the peak VO2 as substitute for VO2Max. The VO2Max is considered to be the best parameter for exercise capacity and cardiopulmonary fitness15). It is best predictor for survival not only of patients with heart failure5,16) or coronary arteries disease17,18), but also of the general population19). The VE/VCO2 has been alternative prognostic indicator in patients with chronic heart failure13), and VE/VCO2 means how many liters of air are being breathed to eliminate 1 liter of CO2. The abnormally high value of VE/VCO2 is associated with poor outcomes4,20).
In this study, we observed that exercise capacity resented by VO2peak is better in boys than girl. However, in younger group (10 to 14 years), there is no difference in exercise capacity between boys and girls. On the other hand, in older group (≥15 years), the exercise capacity is better in boys. This difference may be caused by different somatic scale, body components (i.e., body fat) and growth pattern11,12). There was no significant difference in exercise capacity according to age in both boys and girls (Fig. 1). Many authors reported on the contribution of biological maturity and body fat to exercise capacity21,22). VO2Max per unit body mass (mL/kg/min) increases slightly with stage of maturity in boys, but tends to decrease with stage of maturity in girls. Cross-sectional studies have shown a clear inverse relationship between aerobic fitness and body fatness in children23). That is, in immature children, there is no significant difference in body mass between boys and girls, resulting in similar VO2Max. However, in mature adolescents, there is significant difference in body mass due to increasing body fat, resulting in significant difference of VO2Max.
The VE/VCO2 has been attributed to maldistribution of pulmonary blood flow with increased physiological dead space ventilation12). The decrease in VE/VCO2 with advancing age has been known by a slightly lower pressure of CO2 set point during exercise in the younger children and higher breathing efficiency in older children12). In this study, the decrease in VE/VCO2 was prominent in boys but not in girls. This also may be due to increased body fat and different growth pattern in girls11,12).
In conclusion, there are few normal reference data of CPET in healthy children and adolescents. This study was performed to provide normal reference data of Korean children and adolescents and we expect this study will be helpful in understanding and estimating the exercise capacity of children and adolescents in clinical setting. In applying to practice, we consider the differences of sex; the stage of maturity; obesity. But there are some limitations. First, number of subjects are small, especially in younger than 10 years old. Second, we have no hematologic data (i.e., hemoglobin, lipid, cardiac marker). Third, we could not reflect the sexual maturation (i.e., Tanner stage).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Wasserman K, Hansen JE, Sue DY, Casaburi R, Whipp BJ. Principles of exercise testing and interpretation. 3rd ed. Baltimore: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- 2.Takken T, Blank AC, Hulzebos EH, van Brussel M, Groen WG, Helders PJ. Cardiopulmonary exercise testing in congenital heart disease: equipment and test protocols. Neth Heart J. 2009;17:339–344. doi: 10.1007/BF03086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connuck DM. The role of exercise stress testing in pediatric patients with heart disease. Prog Pediatr Cardiol. 2005;20:45–52. [Google Scholar]
- 4.Chua TP, Ponikowski P, Harrington D, Anker SD, Webb-Peploe K, Clark AL, et al. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1997;29:1585–1590. doi: 10.1016/s0735-1097(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 5.Myers J, Gullestad L, Vagelos R, Do D, Bellin D, Ross H, et al. Clinical, hemodynamic, and cardiopulmonary exercise test determinants of survival in patients referred for evaluation of heart failure. Ann Intern Med. 1998;129:286–293. doi: 10.7326/0003-4819-129-4-199808150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Myers J, Arena R, Dewey F, Bensimhon D, Abella J, Hsu L, et al. A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. Am Heart J. 2008;156:1177–1183. doi: 10.1016/j.ahj.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes SM, McElhinney DB, Khairy P, Graham DA, Landzberg MJ, Rhodes J. Serial cardiopulmonary exercise testing in patients with previous Fontan surgery. Pediatr Cardiol. 2010;31:175–180. doi: 10.1007/s00246-009-9580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kipps AK, Graham DA, Harrild DM, Lewis E, Powell AJ, Rhodes J. Longitudinal exercise capacity of patients with repaired tetralogy of fallot. Am J Cardiol. 2011;108:99–105. doi: 10.1016/j.amjcard.2011.02.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol. 1984;56:628–634. doi: 10.1152/jappl.1984.56.3.628. [DOI] [PubMed] [Google Scholar]
- 10.Washington RL, van Gundy JC, Cohen C, Sondheimer HM, Wolfe RR. Normal aerobic and anaerobic exercise data for North American school-age children. J Pediatr. 1988;112:223–233. doi: 10.1016/s0022-3476(88)80059-3. [DOI] [PubMed] [Google Scholar]
- 11.Gulmans VA, de Meer K, Binkhorst RA, Helders PJ, Saris WH. Reference values for maximum work capacity in relation to body composition in healthy Dutch children. Eur Respir J. 1997;10:94–97. [PubMed] [Google Scholar]
- 12.Ten Harkel AD, Takken T, Van Osch-Gevers M, Helbing WA. Normal values for cardiopulmonary exercise testing in children. Eur J Cardiovasc Prev Rehabil. 2011;18:48–54. doi: 10.1097/HJR.0b013e32833cca4d. [DOI] [PubMed] [Google Scholar]
- 13.Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Heart. 2007;93:1285–1292. doi: 10.1136/hrt.2007.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks GA. Anaerobic threshold: review of the concept and directions for future research. Med Sci Sports Exerc. 1985;17:22–34. [PubMed] [Google Scholar]
- 15.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002;106:1883–1892. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 16.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–332. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 17.Vanhees L, Fagard R, Thijs L, Staessen J, Amery A. Prognostic significance of peak exercise capacity in patients with coronary artery disease. J Am Coll Cardiol. 1994;23:358–363. doi: 10.1016/0735-1097(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 18.Lipinski M, Froelicher V, Atwood E, Tseitlin A, Franklin B, Osterberg L, et al. Comparison of treadmill scores with physician estimates of diagnosis and prognosis in patients with coronary artery disease. Am Heart J. 2002;143:650–658. doi: 10.1067/mhj.2002.120967. [DOI] [PubMed] [Google Scholar]
- 19.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 20.Kleber FX, Vietzke G, Wernecke KD, Bauer U, Opitz C, Wensel R, et al. Impairment of ventilatory efficiency in heart failure: prognostic impact. Circulation. 2000;101:2803–2809. doi: 10.1161/01.cir.101.24.2803. [DOI] [PubMed] [Google Scholar]
- 21.Hitchen PJ, Jones MA, Stratton G. Maturity and sex effects on selected physical fitness parameters in children of high school age. J Sports Sci. 1999;17:18–19. [Google Scholar]
- 22.Mueller WH, Harrist RB, Doyle SR, Ayars CL, Labarthe DR. Body measurement variability, fatness, and fat-free mass in children 8, 11, and 14 years of age: Project HeartBeat. Am J Hum Biol. 1999;11:69–78. doi: 10.1002/(SICI)1520-6300(1999)11:1<69::AID-AJHB7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 23.Gutin B, Manos TM. Physical activity in the prevention of childhood obesity. Ann N Y Acad Sci. 1993;699:115–126. doi: 10.1111/j.1749-6632.1993.tb18843.x. [DOI] [PubMed] [Google Scholar]