Abstract
The nuclear receptor is an emerging therapeutic target in various human diseases. Vitamin D receptor (VDR), a nuclear receptor, mediates the biological functions of vitamin D. Classically, vitamin D is recognized as an essential contributor to mineral and bone homeostasis. Increasing evidence demonstrates that vitamin D is involved in inflammatory responses. Persistent intestinal inflammation is associated with colon cancer. This review focuses on vitamin D and VDR in inflammatory bowel diseases and colon cancer. We place emphasis on the regulatory roles of vitamin D/VDR on in inflammation, enteric bacteria, and tumorigenesis. We summarize the signaling pathways regulated by VDR in intestinal homeostasis. Finally, we discuss the potential application of the insights gleaned from these findings to personalized therapies in chronic inflammation and colon cancer.
Keywords: Colon cancer, inflammation, IBD, nuclear receptor, vitamin D, Vitamin D receptor, gut flora, microbiome, enteric bacteria
Introduction
Vitamin D is known to be involved in calcium and bone development. Since 2000, the public has heard conflicting messages about vitamin D’s other benefits in chronic diseases. In 2010, the Food and Nutrition Board of the Institute of Medicine (IOM) released their Vitamin D Report [1], increasing the daily recommended intake for vitamin D to 600 IU. The IOM concluded that the evidence supports a role for vitamin D and calcium in bone health but not in other health conditions. Despite the IOM’s conclusion, there is widespread enthusiasm regarding the use of vitamin D as an inexpensive and easy supplement for disease prevention and other benefits above and beyond skeletal health.
In this current review, we will focus on recent progress on the role of vitamin D and its vitamin D receptor (VDR) in anti-inflammatory and anti-proliferative actions, especially in inflammatory bowel diseases (IBD) and colon cancer. Emerging evidence supports the critical roles of vitamin D in controlling inflammation and preventing risks for cancer. We will discuss the potential application of the insights gleaned from these research findings to anti-inflammation, anticancer, and personalized medicine.
Vitamin D and Vitamin D receptor
Vitamin D3 is synthesized in human skin with sunlight energy. Vitamin D modulates calcium homeostasis and takes part in the regulation of blood pressure, metabolic syndrome, and inflammation [2•,3]. Most of the biological effects of vitamin D are mediated by VDR. Binding of vitamin D3 to the VDR promotes VDR heterodimerization with the retinoid X receptor and bind cooperatively to vitamin D responsive elements, thus controlling the transcription of target genes. VDR binding sites were significantly enriched near autoimmune and cancer associated genes identified from genome-wide association studies [4••]. Dysfunction of VDR and vitamin D3 deficiency can cause poor bone development and health, as well as increase the risk of many chronic diseases, such as type 1 diabetes, rheumatoid arthritis, infectious diseases, IBD, and cancer [5–6].
In mammals, VDR is highly expressed in metabolic tissues, such as intestine, kidney, skin, and thyroid gland, and moderately expressed in nearly all tissues. There are differences between human VDR and murine VDR. The antimicrobial peptide cathelicidin is a VDR downstream gene. Only human cathelicidin promoter contains an activating VDRE [7]. The human toll-like receptor (TLR)-induced antimicrobial pathway is distinct from the murine pathway-the human pathway is mediated by the activation of the VDR and Cyp27B1 [8••]. Moreover, there are differences between human and mouse VDR protein. The mouse VDR is five amino acids shorter than human VDR. The homology of the DNA-binding domain (DBD) is 100%, but for the ligand-binding domain (LBD) it is 89%. The homology of the internal region between DBD and LBD, including a portion of LBD, is 55%. These may partly explain the limitations of vitamin D associated murine experimental models.
Vitamin D, VDR, and IBD
IBD comprises ulcerative colitis (UC) and Crohn's disease (CD), which cause chronic inflammation in the digestive tract. The etiology of IBD has been described as interactions among environmental, genetic, and immune factors. The combination of these factors can induce inflammation and subsequent development of mucosal lesions and repair. Interestingly, vitamin D and VDR are involved in these factors in the pathogenesis of IBD.
Environment factors
Vitamin D deficiency may contribute to IBD as an environment factor [2•]. At higher latitudes, cutaneous vitamin D3 synthesis is insufficient with lower solar ultraviolet B in winter, and without vitamin D rich diets, leads to seasonal variations in circulating vitamin D3 levels and widespread vitamin D deficiency [9–10]. Prevalence of IBD is higher in the northernmost parts of Europe and America [11]. Patients with IBD have lower serum vitamin D3 levels than healthy controls [12]. The proportion of vitamin D deficiency in children with IBD was higher than that in healthy controls [13].
VDR gene polymorphisms and expression
The IBD-associated genes are on regions of chromosome 12 and 16. VDR gene locates on the chromosome 12. In human, Fok1, Bsm1, Taq1, Apa1, Cdx2, poly (A), and Bgl1, are associated with risk of IBD [14–15]. VDR expression at mRNA and protein levels is significantly decreased in IBD patients [16,17•]. In mouse models, VDR expression is required to control inflammation. VDR−/− mice were more susceptible in dextran sodium sulfate-induced colitis [18,19•].
Immune responses
Vitamin D pathway is involved in innate and adaptive immune pathways. Genes encoding cytokines IL-2, IL-10, and IL-12B are primary target genes of vitamin D [20••,21•]. Vitamin D promotes IL-10 production in human B cells. VDR plays a crucial role in innate immune response, which is the body's first line of defense against bacterial pathogens [22]. VDR is required in the development of CD8aa-expressing T Cells [23••]. Th17 and iTreg cells are direct and indirect targets of vitamin D. The increased expression of Th17 cells in the VDR−/− mice was associated with a reduction in tolerogenic CD103(+) dendritic cells. The increased propensity for development of Th17 cells in VDR−/− mice led to more severe IBD in a mouse model [24].
Intestinal Microbiota
Intestinal microbes affect the immune system, supply key nutrients, modulate energy metabolism, stimulate cell growth, repress the growth of harmful microorganisms, and defend against diseases. The target genes of VDR signal include the enzyme Cyp24 and antimicrobial peptides (AMP) [25]. Vitamin D/VDR is responsible for intestinal homeostasis and host protection from bacterial invasion and infection. When the immune system is challenged by pathogens, TGF-beta and IFN-γ are released. Subsequently, VDR is activated to express more cathelicidin and defensin, which are known to regulate the composition of bacterial flora. Additionally, VDR is associated with TLRs, which are expressed to invoke the immune system to recognize bacteria [26•].
VDR−/− mice have increased bacterial loads in the intestine [19•]. Our studies demonstrate that VDR signaling responds to enteric pathogens and commensal bacteria in vivo [27]. Reduced vitamin D/VDR levels, an altered number and diversity of gut bacteria, or both will promote inflammation that might disrupt the mucosal barrier and promote food and other allergen sensitization or abnormal tolerization [28]. It is also possible that vitamin D status in intestine determines how gut flora interacts with the immune system [28].
VDR, NOD2, and autophagy are implicated in the pathogenesis of IBD [29, 16, 30]. Vitamin D3 induces NOD2/CARD15-defensin pathway [31••]. We discussed the link between autophagy and VDR in anti-inflammation in a recent review article [5].
Taken together, synthesis of 1,25(OH)2D3 and VDR status affect the development of colitis. Polymorphisms in the VDR gene and VDR expression are associated with status of IBD. Dysfunction of the vitamin D/VDR signaling is involved in the pathogenesis of IBD. Vitamin D and VDR may affect the composition and functions of gut flora. Hence, restoring vitamin D/VDR signaling may enhance the host’s ability to control inflammation in patients with IBD.
Vitamin D, VDR and colon cancer
Patients with long-term IBD have an increased risk of colorectal carcinoma (CRC)[32–34]. CRC leads to high mortality and accounts for 20% of IBD-related mortality [35]. Epidemiological studies have demonstrated that intensity of local sunlight is inversely correlated with risk of CRC [36]. Adjusted death rates from colon cancer in Caucasian males are nearly three times higher in north eastern states than in sunnier more southerly states in the USA [37]. Inverse associations of CRC risk with dietary vitamin D were found through Meta-analyses. According to a recent review [38], subjects with a serum 25-hydroxyvitamin D level of 33 ng per milliliter or higher had about half the risk of colorectal cancer than those with levels of 12 ng per milliliter or lower [38].
VDR plays an important role in cellular differentiation and inhibition of proliferation. UC with CRC patients showed lower rate of VDR expression than non-colon cancer patients. Moreover, VDR expression was significantly lower in long-term UC patients (more than ten years), who were at high-risk of developing CRC than short-term patients [17•]. High VDR expression has been reported in early colorectal tumor progression, which is considered as a role for vitamin D inhibiting the paracrine/autocrine growth at the early stage of tumor progression [39]. However, VDR expression is lost during tumor dedifferentiation, which correlates with up-regulation of SNAIL1, a transcriptional repressor of VDR [40•]. In addition, BsmI polymorphism of VDR was associated with a lower CRC risk [41–42].
Tumor cells fail to synthesize the active form of vitamin D and respond to VDR-mediated vitamin D effects. Activation of VDR by vitamin D3 can inhibit tumor cell proliferation by inducing differentiation in various cancer cell lines [43]. VDR knock-out mice showed increased sensitivity to carcinogen challenge [44]. In APCmin/+ mouse mode, VDR may play a suppressive role in intestinal tumor growth via inhibition of β-catenin activity [45].
Some bacteria are considered as pathogens associated with colon carcinoma in many case reports, such as Clostridium septicum, Streptococcus infantarius etc [46]. Patients with UC have lower percentages of potentially protective bacterial species than their healthy twins [47]. A disproportionate increase in some mucolytic bacteria, such as Ruminococcus gnavus and Ruminococcus torques, could be found in normal intestinal epithelium of both CD and UC [48]. Imbalance between different species of enteric bacteria is hypothesized to be one of the risk factors for IBD or CRC.
Interestingly, microorganism infection can disable or hijack the VDR signaling. A number of species have been shown to downregulate the activity of VDR [49]. Borrelia burgdorferi can reduce VDR expression by 50 times in monocytes [49]. The caspases are upregulated by Shigella infection that limits the ability of VDR to perform gene transcription [50]. We demonstrated that commensal and pathogenic bacteria directly regulate colonic epithelial VDR expression and location [27]. VDR expression is higher in the proximal colon than in the distal colon, which is correlated with bacterial density and growth [27]. However, the role of vitamin D/VDR in regulating gut flora in tumorigenesis remains unknown.
Vitamin D/VDR regulate response to intestinal immune responses, anti-inflammation, and cancer prevention. VDR dysfunction causes a decline in innate immune function and increases susceptibility to additional infections that contribute to inflammation progression. Hence, it is important to evaluate the associations among VDR, bacterial infection, and colitis-associated colon cancer.
VDR involved signaling pathways in anti-inflammation and anti-proliferation
Inflammatory mediators, such as IL-1, TNF-α, IL-8, nitric oxide, have been shown to be involved in development of inflammation and cancer, especially in the cases of IBD [51••]. Many VDR target genes are involved in dysregulated pathways leading to common human diseases. It is not a surprise that the mechanisms of VDR in IBD and cancer are associated with inflammatory pathways, including NF-kB, JNK, p38, and JAK/STAT (Table 1).
Table 1.
Molecular mechanisms of vitamin D/VDR in regulating inflammation and proliferation
| Involved pathways |
In vitro system |
In vivo system |
Summary | Ref. |
|---|---|---|---|---|
| Toll-like receptors | Human monocytes | Vitamin D3 down-regulates intracellular TLR-9 expression and TLR-9-induced IL-6 production. | [79] | |
| MDMs, THP-1 | TLR2/1/CD14 stimulation can activate antibacterial autophagy through VDR signaling activation and cathelicidin induction. | [52••] | ||
| Human peripheral monocytes | TLR2/1-induced IL-15 is required for induction of CYP27b1, VDR, and cathelicidin. | [53•] | ||
| C57BL/6 mouse | Influence of vitamin D3 on TLR-4-L-induced activation of pAPC is dependent on the order of VDR and TLR-4 engagement. | [80] | ||
| NF-κB | Caco2, MEF,HT29C19A | LCA down-regulates NF-κB activity through VDR in colonic cancer cells. | [81] | |
| MEF, Caco2 | C57BL/6 VDR(−/−) mouse | VDR negatively regulates bacterial-stimulated intestinal NF-κB activation and attenuates response to infection. | [27] | |
| HaCaT | Vitamin D3 decreases NF-κB activity by increasing IκBα levels, and it requires VDR for its action on NF-κB activity | [82] | ||
| HKC,clone-8 | mouse | VDR and p65 forms a complex in tubular cells after paricalcitol treatment and paricalcitol inhibits inflammatory infiltration by promoting VDR-mediated sequestration of NF-κB signaling. | [83] | |
| Wnt/β-catenin | HT-29-APC,Caco-2 | Vitamin D3 mediates inhibition of β-catenin transcriptional activity resulting in suppression of β-catenin target genes. Inhibition of β-catenin activity by Vitamin D3 was enhanced by APC. | [84] | |
| Wnt/β-catenin JAK-STAT | HCT116, Hke-3 | Vitamin D3 blocks the activation of STAT1 and the production of IL-1β in macrophages. Vitamin D3 inhibits the ability of macrophages to induce Wnt signaling and to promote proliferation of colon cancer cells. | [64] | |
| WNT/β-catenin p38MAPK | SW480, HT29, Caco-2, MCF-7, IMR90,HaCaT, IEC18,,NIH 3T3 | Vitamin D3 activates the p38MAPK and its target MSK1 gene.Activity of these kinases is required for the inhibition of β-catenin–TCF transcriptional activity. | [85•] | |
| JAK-STAT | EOC-20 | C57BL/6 and SJL/J | Treatment of activated T cells with vitamin D3 inhibits the IL-12-induced tyrosine phosphorylation of Stat3. | [86] |
| JNK p38MAPK | MCF-7, MDA-MB-468 | The p38 and JNK pathways cooperate to trans-activate VDR via c-Jun/AP-1 and sensitize cancer cells to vitamin D3-induced growth inhibition. | [56] | |
| p38 MAPK | HCT116 | MAPK activation selectively induces cell death in K-ras-mutated human colon cancer cells. Levels of VDR protein concentration affect the sensitivity to p38-induced cell death. | [57] | |
| rat | Vitamin D3 activation of p38 MAPK upon aging and abnormal hormone regulation of the c-fos may affect intestinal cell function. | [87] |
Toll-like receptor
Toll-like receptors (TLRs) are members of the pattern recognition receptor (PRR) family that activates numerous signal-transduction pathways. TLRs provide the host with an immediate and rapid defense against invading microbes in immune response and affects the development of colitis-associated colorectal tumors[52••,53•]. TLRs are considered as regulators in vitamin D/VDR signaling. In human, when a pathogen is detected by TLR, gene expressions of VDR and Cyp27B1 are induced. This leads to 1-α-hydroxylation of 25(OH)D, which is taken up from the blood, and subsequent binding of 1,25(OH)2D3 to VDR [8••]. Inhibition of VDR signaling ablates the TLR-induced antimicrobial activity [8••].
NF-κB
An important TLR downstream effector is the NF-κB transcriptional system that is responsible for the development of colitis and inflammation-linked cancers [54]. Binding of vitamin D/VDR yields a transcription factor that represses NF-κB activation and down-regulates adaptive, but enhances innate immune responses. This product also improves redox balance, thus counterbalancing inflammation on multiple levels. VDR blocks NF-κB by binding to it and preventing it from activating other inflammatory molecules. The interaction of VDR and NF-κB can be modulated by bacteria in the colon [27]. VDR knockout mice exhibited a proinflammatory phenotype-indicated by an increased activity of NF-κB and high serum levels of IL-6 even in the absence of infection [27].
JNK/SAPK and p38 MAP kinases
MAPKs are serine/threonine-specific protein kinases that play a key role in transducing extracellular signals to the nucleus. Four different subgroups have been described including ERKs, JNK/SAPK, BMK1, and p38. Activation of JNK pathway is linked to induction of apoptosis and has been suggested to act as a tumor suppressor. Nevertheless, JNK can also lead to increased proliferation and survival responses of some tumors [55]. The direct binding of VDR to c-Jun is described in vitro [56]. Interactions between VDR and JNK/c-Jun pathway contribute to increased VDR activity and subsequent promotion of vitamin D3-induced growth inhibition.
P38 activation induces cell death, which depends on K-ras mutation, in human colon cancer cells. This may occur through AP-1-dependent VDR transsuppression. The sensitivity to p38-induced cell death is determined by the levels of VDR protein concentration in human colon cancer cells [57]. Enhanced VDR expression in K-ras-activated cells inhibits p38 activation-induced cell death [57].
Wnt/β-catenin
Beta-catenin is a key in intestinal proliferation and tumorigenesis. Beta-catenin activity is inhibited by VDR through VDR/β-catenin interaction. Wnt/β-catenin signaling pathway appears to be an important target of the chemopreventive action of vitamin D3 [40•]. Vitamin D3 prevents β-catenin nuclear translocation, leading to inhibition of TCF-4-responsive genes, such as c-myc [58]. Vitamin D3 inhibits β-catenin transcriptional activity by promoting VDR binding to β-catenin and inducing E-cadherin expression. In VDR−/− mice, TCF-4 is decreased. The vitamin D3/VDR-mediated increase in TCF-4 can restrict colorectal cancer cell growth and may have a protective role in colon cancer as well as Crohn's disease [59•].
JAK-STAT
IL-6 signaling via JAK-STAT has been identified as a crucial pathway in colitis-associated neoplasia [60]. Patients with active UC have significantly more IL-6 and pSTAT-3-positive intestinal epithelial cells than patients with inactive UC [61]. IL-23/Th17 pathway plays a critical role in IBD and defenses against different kinds of bacteria. Vitamin D might be beneficial in reducing the development of tumor by inhibiting the action of STAT3 [62]. Mutations in NOD2, which increase risk of colorectal cancer, result in increased production of IL-1β and greater colonic inflammation [63]. However, 1,25D can inhibit STAT signaling to prevent IL-1β production and inhibit the ability of macrophages to induce Wnt signaling in tumor cells in a VDR-dependent manner [64•].
In summary, VDR/vitamin D is involved in multiple pathways that contribute to inflammation and tumorigenesis (Table 1).
Prevention and personalized medicine of vitamin D/VDR in IBD and colon cancer
Genome-wide association studies have uncovered at least 30 genes related to IBD. It will help scientists develop new diagnostic tools and strategies and target-specific therapeutics. However, there is no report on the statuses of vitamin D, the VDR gene, and the efficacy of IBD therapy.
Identifying the K-ras status is the key for an effective therapeutic strategy for colorectal cancer. In colorectal cancer, VDR overexpression is significantly associated with K-ras and PIK3CA mutations. These data support potential interactions between VDR, ras-MAPK, and PI3K-AKT pathways. This indicates possible influence by K-ras or PIK3CA mutation on therapy or chemoprevention targeting [65].
P53 tumor suppressor has been extensively studied in various cancers. Cancer cells often contain abundant mutant p53. Rectal tumors were associated with high levels of calcium overall and p53 tumor mutations. P53 mutations were significantly associated with dietary calcium intake and certain VDR polymorphisms. This indicated an association among calcium, VDR polymorphism, tumors, as well as p53 mutations [66]. Recently, a study on mutated p53 interaction with VDR also indicate that extra caution should be taken in using Vitamin D or its analogs in individualized cancer therapy [67••]
Recent studies demonstrated the efficacy of a potent VDR agonist for the treatment of IBD [68] [69]. The vitamin D analog TX527 directly targets T cells and imprints them with a specific homing signature favoring migration to sites of inflammation [70••]. Serum vitamin D levels and colorectal cancer has an inverse association. New trials assessing moderate-to-high-dose vitamin D supplementation for cancer prevention are in progress [38]. However, the biggest obstacle to clinical use of vitamin D is its potent hypercalcemic effect. Recent papers have also warned against the use of vitamin D in infectious and autoimmune diseases [71;72]. Additional research is required to quantify proper dosage.
Targeting the microenvironment and blocking inflammatory cytokines may represent a promising therapeutic approach for inflammatory diseases. A number of autoimmune diseases can be reversed by gradually restoring VDR function [72]. Preclinical data demonstrate that VDR agonists and BXL-628 (elocalcitol) targeted bladder cells [73]. A recent study provides the proof of concept for the combination of iron chelators and VDR in the treatment of acute myeloid leukemia [74]. There is a long history of using vitamin D to treat mycobacterial infections [75]. Vitamin D3’s antagonism of M. tuberculosis involves antimicrobial peptides [8••] and autophagy [76••]. Hence, there is potential to develop a strategy against chronic inflammatory diseases by combining VDR’s synergistic effects with other pathways.
Potential strategies for enhancing the anti-inflammatory ability of vitamin D/VDR can be developed at different levels. These include 1) using vitamin D or its analogs to activate vitamin D/VDR binding; 2) using VDR agonists to enhance VDR signaling; 3) blocking inflammatory cytokines; and 4) targeting the microenvironment and correcting the dysfunction of vitamin D/VDR by using alternative methods, such as probiotics to increase VDR expression in intestine [77].
Overall, experimental models and clinical studies show that vitamin D supplementation or VDR agonists produce therapeutic effects. Moreover, genomics data will identify people at increased risk of diseases and better match patients with effective drugs. Combining with discoveries in genetic and immunological research, we will develop personalized medicine for IBD and cancer therapy. Clinical studies on the effects of calcium and vitamin D3 supplementation, VDR agonists on colitis-associated colon cancer characterized by chronic inflammation are therefore warranted.
Conclusion
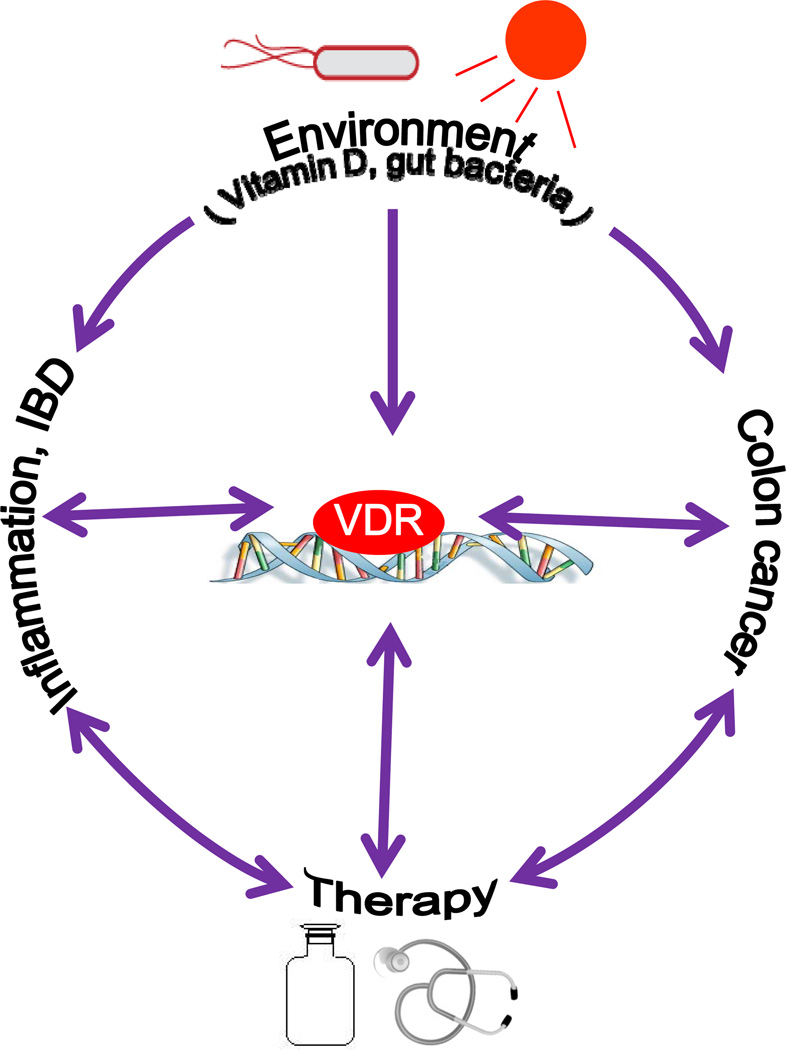
VDR has multiple critical functions in regulating response to intestinal homeostasis, immunity, and cell structure [5]. Vitamin D and gut flora may be critical factors that contribute to the pathogenesis of IBD and colon cancer. Dysregulated VDR signaling may explain the mechanism of chronic inflammation (Fig. 1).
Figure 1.
Vitamin D3/VDR signaling regulates intestinal homeostasis. Vitamin D and gut flora may be critical factors that contribute to the pathogenesis of IBD and colon cancer. Dysregulated VDR signaling may explain the mechanism of chronic inflammation. Because vitamin D3/VDR has critical functions in regulating response to intestinal homeostasis, immunity, and cell structure, manipulating the levels of vitamin D and restoring the function of VDR may represent a new approach to prevention and treatment of IBD and colon cancer.
Although vitamin D has been extensively studied, many critical questions about the biological functions of VDR, especially intestinal VDR, remain unanswered. For basic research, we need to advance our understanding of 1) the molecular mechanism of vitamin D and VDR in synergistic regulation of inflammatory signaling pathways in experimental models; 2) gut flora and activity of the intestinal VDR; and 3) mechanisms by which intestinal VDR is involved in the pathogenesis of chronic inflammation and colon cancer. For clinical research, new trials assessing moderate- to high-dose vitamin D supplementation for chronic diseases are needed. We need personalized info, such as vitamin D status, VDR expression level, k-ras and p53 status, to optimize clinical outcome. Before we obtain certain and consistent data from clinical studies, it is necessary to make individual treatment decisions [78]. Manipulating the levels of serum and local vitamin D and restoring the function of VDR may represent a new approach to prevention and treatment of chronic diseases.
Acknowledge
I thank all present and former members of my group for their contribution to our own work on VDR, inflammation, and colon cancer. I also thank Yuxuan Xia for critical editing of this manuscript.
Funding
This work was supported by the National Institutes of Health (DK075386-0251, R03DK089010-01), the American Cancer Society (RSG-09-075-01-MBC), and the IDEAL award from Empire State Stem Cell Board (N09G-279) to Jun Sun.
Abbreviations
- 1,25(OH)2D3
Vitamin D3, or cholecalciferol
- 25(OH)D
25-hydroxyvitamin D, or calcidiol
- CD
Crohn’s disease
- CDAD
Clostridium difficile-associated disease
- COPD
Chronic obstructive pulmonary disease
- EGFR
Epidermal growth factor receptor
- HD
Human α defensin
- HBD
Human β defensin
- HSP
Heat shock proteins
- IBD
inflammatory bowel disease
- IBS
Irritable bowel syndrome
- IκB
Inhibitor of nuclear factor κB
- IRAK
interleukin-1 receptor-associated kinase
- JAK/STAT
Janus kinase/signal transducers and activators of transcription
- MAPK
mitogen-activated protein kinase
- (ERK)
extracellular signal-regulated protein kinase
- NF
nuclear factor
- NOD
Non-obese diabetic mice
- RXR
Retinoid X receptor
- SAPK/JNK
Stress-activated protein kinase/c-Jun NH2-terminal kinase
- TCR
T cell receptor
- TID
Type I diabetes
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- UC
Ulcerative colitis
- VDR
Vitamin D receptor, Vitamin D2 ergocalcifero
Footnotes
Disclosure
The authors report no conflicts of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guillot X, Semerano L, Saidenberg-Kermanac'h N, et al. Vitamin D and inflammation. Joint Bone Spine. 2010;77:552–557. doi: 10.1016/j.jbspin.2010.09.018. A recet article outlines the the relationship between vitamin D and inflammation.
- 3.Feneis JF, Arora RR. Role of vitamin D in blood pressure homeostasis. Am J Ther. 2010;17:e221–e229. doi: 10.1097/MJT.0b013e3181d16999. [DOI] [PubMed] [Google Scholar]
- 4. Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–1360. doi: 10.1101/gr.107920.110. This study demostrates that VDR occupies 2776 genomic positions and changes 229 genes in expression in response to vitamin D.
- 5.Wu S, Sun J. Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection. Discov Med. 2011;11:325–335. [PMC free article] [PubMed] [Google Scholar]
- 6.Holick M. Vitamin D and Health: Evolution, Biologic Functions, and Recommended Dietary Intakes for Vitamin D. Clinical Reviews in Bone and Mineral Metabolism. 2009;7:2–19. [Google Scholar]
- 7.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 8. Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. This is the first report that demonstrates vitamin D/VDR regulation of human TLR-induced antimicrobial pathway.
- 9.White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76:3837–3843. doi: 10.1128/IAI.00353-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 11.Lim WC, Hanauer SB, Li YC. Mechanisms of disease: vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:308–315. doi: 10.1038/ncpgasthep0215. [DOI] [PubMed] [Google Scholar]
- 12.Bours PH, Wielders JP, Vermeijden JR, van de Wiel A. Seasonal variation of serum 25-hydroxyvitamin D levels in adult patients with inflammatory bowel disease. Osteoporos Int. 2010 doi: 10.1007/s00198-010-1484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin AD, Wadhera V, Leach ST, et al. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. 2011;56:830–836. doi: 10.1007/s10620-010-1544-3. [DOI] [PubMed] [Google Scholar]
- 14.Naderi N, Farnood A, Habibi M, et al. Association of vitamin D receptor gene polymorphisms in Iranian patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2008;23:1816–1822. doi: 10.1111/j.1440-1746.2008.05525.x. [DOI] [PubMed] [Google Scholar]
- 15.Pei FH, Wang YJ, Gao SL, et al. Vitamin D receptor gene polymorphism and ulcerative colitis susceptibility in Han Chinese. J Dig Dis. 2011;12:90–98. doi: 10.1111/j.1751-2980.2011.00483.x. [DOI] [PubMed] [Google Scholar]
- 16.Abreu MT, Kantorovich V, Vasiliauskas EA, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn's disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53:1129–1136. doi: 10.1136/gut.2003.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wada K, Tanaka H, Maeda K, et al. Vitamin D receptor expression is associated with colon cancer in ulcerative colitis. Oncol Rep. 2009;22:1021–1025. doi: 10.3892/or_00000530. This work suggestes that correlation seems to exist between the level of VDR expression and carcinogenesis in UC.
- 18.Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–G216. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 19. Lagishetty V, Misharin AV, Liu NQ, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423–2432. doi: 10.1210/en.2010-0089. • This study explores the intestinal bacteria and Vitamin D status in a colitis animal model.
- 20. Heine G, Niesner U, Chang HD, et al. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur J Immunol. 2008;38:2210–2218. doi: 10.1002/eji.200838216. This work demonstrates that vitamin D pathway involves in innate and adaptive immune pathways.
- 21. Matilainen JM, Rasanen A, Gynther P, Vaisanen S. The genes encoding cytokines IL-2, IL-10 and IL-12B are primary 1alpha,25(OH)2D3 target genes. J Steroid Biochem Mol Biol. 2010;121:142–145. doi: 10.1016/j.jsbmb.2010.03.020. This paper explores the relation between vitamin D and cytokines.
- 22.Rasmussen SB, Reinert LS, Paludan SR. Innate recognition of intracellular pathogens: detection and activation of the first line of defense. APMIS. 2009;117:323–337. doi: 10.1111/j.1600-0463.2009.02456.x. [DOI] [PubMed] [Google Scholar]
- 23. Bruce D, Cantorna MT. Intrinsic requirement for the vitamin D receptor in the development of CD8alphaalpha-expressing T cells. J Immunol. 2011;186:2819–2825. doi: 10.4049/jimmunol.1003444. This study suggests the critical role of Vitamin D receptor in development of CD8αα-expressing T cells.
- 24.Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol. 2011;23:519–528. doi: 10.1093/intimm/dxr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlberg C, Dunlop TW. The impact of chromatin organization of vitamin D target genes. Anticancer Res. 2006;26:2637–2645. [PubMed] [Google Scholar]
- 26. Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. This article describes the important role for vitamin D3 in innate immunity.
- 27.Wu S, Liao AP, Xia Y, et al. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010;177:686–697. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss ST. Bacterial components plus vitamin D: the ultimate solution to the asthma (autoimmune disease) epidemic? J Allergy Clin Immunol. 2011;127:1128–1130. doi: 10.1016/j.jaci.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sentongo TA, Semaeo EJ, Stettler N, et al. Vitamin D status in children, adolescents, and young adults with Crohn disease. The American journal of clinical nutrition. 2002;76:1077–1081. doi: 10.1093/ajcn/76.5.1077. [DOI] [PubMed] [Google Scholar]
- 30.Verway M, Behr MA, White JH. Vitamin D, NOD2, autophagy and Crohn's disease. Expert Rev Clin Immunol. 2010;6:505–508. doi: 10.1586/eci.10.31. [DOI] [PubMed] [Google Scholar]
- 31. Wang TT, Dabbas B, Laperriere D, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285:2227–2231. doi: 10.1074/jbc.C109.071225. This paper provides strong molecular links between Vitamin D Receptor and NOD2.
- 32.Lukas M. Inflammatory bowel disease as a risk factor for colorectal cancer. Dig Dis. 2010;28:619–624. doi: 10.1159/000320276. [DOI] [PubMed] [Google Scholar]
- 33.Shaukat A, Virnig DJ, Salfiti NI, et al. Is Inflammatory Bowel Disease an Important Risk Factor Among Older Persons with Colorectal Cancer in the United States? A Population-Based Case-Control Study. Dig Dis Sci. 2011 doi: 10.1007/s10620-011-1632-z. [DOI] [PubMed] [Google Scholar]
- 34.Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–2737. [PubMed] [Google Scholar]
- 35.Basseri RJ, Basseri B, Papadakis KA. Dysplasia and cancer in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2011;5:59–66. doi: 10.1586/egh.10.77. [DOI] [PubMed] [Google Scholar]
- 36.Thorne J, Campbell MJ. The vitamin D receptor in cancer. Proc Nutr Soc. 2008;67:115–127. doi: 10.1017/S0029665108006964. [DOI] [PubMed] [Google Scholar]
- 37.Slattery ML, Neuhausen SL, Hoffman M, et al. Dietary calcium, vitamin D, VDR genotypes and colorectal cancer. Int J Cancer. 2004;111:750–756. doi: 10.1002/ijc.20330. [DOI] [PubMed] [Google Scholar]
- 38.Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer--ready for prime time? N Engl J Med. 2011;364:1385–1387. doi: 10.1056/NEJMp1102022. [DOI] [PubMed] [Google Scholar]
- 39.Bouillon R, Carmeli G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pendas-Franco N, Aguilera O, Pereira F, et al. Vitamin D and Wnt/beta-catenin pathway in colon cancer: role and regulation of DICKKOPF genes. Anticancer Res. 2008;28:2613–2623. This paper show that Vitamin D3 exerts a complex set of regulatory actions leading to the inhibition of the Wnt/beta-catenin pathway in colon cancer.
- 41.Touvier M, Chan DS, Lau R, et al. Meta-analyses of vitamin d intake, 25-hydroxyvitamin d status, vitamin d receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20:1003–1016. doi: 10.1158/1055-9965.EPI-10-1141. [DOI] [PubMed] [Google Scholar]
- 42.Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis. 2009;30:1170–1180. doi: 10.1093/carcin/bgp103. [DOI] [PubMed] [Google Scholar]
- 43.Samuel S, Sitrin MD. Vitamin D's role in cell proliferation and differentiation. Nutr Rev. 2008;66:S116–S124. doi: 10.1111/j.1753-4887.2008.00094.x. [DOI] [PubMed] [Google Scholar]
- 44.Zinser GM, Welsh J. Effect of Vitamin D3 receptor ablation on murine mammary gland development and tumorigenesis. J Steroid Biochem Mol Biol. 2004;89–90:433–436. doi: 10.1016/j.jsbmb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 45.Zheng W, Wong KE, Zhang Z, et al. Inactivation of the vitamin D receptor in APC(min/+) mice reveals a critical role for the vitamin D receptor in intestinal tumor growth. Int J Cancer. 2011 doi: 10.1002/ijc.25992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirza NN, McCloud JM, Cheetham MJ. Clostridium septicum sepsis and colorectal cancer - a reminder. World J Surg Oncol. 2009;7:73. doi: 10.1186/1477-7819-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lepage P, Hasler R, Spehlmann ME, et al. Twin Study Indicates Loss of Interaction Between Microbiota and Mucosa of Patients With Ulcerative Colitis. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Png CW, Linden SK, Gilshenan KS, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 49.Salazar JC, Duhnam-Ems S, La Vake C, et al. Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-beta. PLoS Pathog. 2009;5:e1000444. doi: 10.1371/journal.ppat.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki T, Franchi L, Toma C, et al. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 2007;3:e111. doi: 10.1371/journal.ppat.0030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tlaskalova-Hogenova H, Stepankova R, Kozakova H, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110–120. doi: 10.1038/cmi.2010.67. This work demonstrates direct involvement of components of the microbiota in inflammatory bowel disease and colorectal carcinoma.
- 52. Shin DM, Yuk JM, Lee HM, et al. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2010;12:1648–1665. doi: 10.1111/j.1462-5822.2010.01497.x. This paper describes antimycobacterial host defence through a functional link of TLR2/1/CD14 and VDR to the induction of autophagy.
- 53. Krutzik SR, Hewison M, Liu PT, et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–7120. doi: 10.4049/jimmunol.181.10.7115. This article show that TLR2/1-induced IL-15 depends on VDR.
- 54.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 55.Ke H, Harris R, Coloff JL, et al. The c-Jun NH2-terminal kinase 2 plays a dominant role in human epidermal neoplasia. Cancer Res. 2010;70:3080–3088. doi: 10.1158/0008-5472.CAN-09-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qi X, Pramanik R, Wang J, et al. The p38 and JNK pathways cooperate to trans-activate vitamin D receptor via c-Jun/AP-1 and sensitize human breast cancer cells to vitamin D(3)-induced growth inhibition. J Biol Chem. 2002;277:25884–25892. doi: 10.1074/jbc.M203039200. [DOI] [PubMed] [Google Scholar]
- 57.Qi X, Tang J, Pramanik R, et al. p38 MAPK activation selectively induces cell death in K-ras-mutated human colon cancer cells through regulation of vitamin D receptor. J Biol Chem. 2004;279:22138–22144. doi: 10.1074/jbc.M313964200. [DOI] [PubMed] [Google Scholar]
- 58.Sansom OJ, Meniel VS, Muncan V, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 59. Beildeck ME, Islam M, Shah S, et al. Control of TCF-4 expression by VDR and vitamin D in the mouse mammary gland and colorectal cancer cell lines. PLoS One. 2009;4:e7872. doi: 10.1371/journal.pone.0007872. This paper suggests that vitamin D3/VDR has a protective role in colon cancer as well a s in Crohn's disease.
- 60.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22:83–89. doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 61.Li Y, de Haar C, Chen M, et al. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59:227–235. doi: 10.1136/gut.2009.184176. [DOI] [PubMed] [Google Scholar]
- 62.Grant WB. Vitamin D may reduce prostate cancer metastasis by several mechanisms including blocking Stat3. Am J Pathol. 2008;173:1589–1590. doi: 10.2353/ajpath.2008.080579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maeda S, Hsu LC, Liu H, et al. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 64. Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene. 2009;28:3892–3902. doi: 10.1038/onc.2009.247. •• This study demonstrates the cross-talks between macrophages and epithelial cells through Wnt and Vitamin D singling pathways.
- 65.Kure S, Nosho K, Baba Y, et al. Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2765–2772. doi: 10.1158/1055-9965.EPI-09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slattery ML, Wolff RK, Herrick JS, et al. Calcium, vitamin D, VDR genotypes, and epigenetic and genetic changes in rectal tumors. Nutr Cancer. 2010;62:436–442. doi: 10.1080/01635580903441204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stambolsky P, Tabach Y, Fontemaggi G, et al. Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell. 2010;17:273–285. doi: 10.1016/j.ccr.2009.11.025. ••This study is notable for findings the interaction between mutated p53 and VDR.
- 68.Laverny G, Penna G, Vetrano S, et al. Efficacy of a potent and safe vitamin D receptor agonist for the treatment of inflammatory bowel disease. Immunol Lett. 2010;131:49–58. doi: 10.1016/j.imlet.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial: vitamin D3 treatment in Crohn's disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010;32:377–383. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 70. Baeke F, Korf H, Overbergh L, et al. The vitamin D analog, TX527, promotes a human CD4+CD25highCD127low regulatory T cell profile and induces a migratory signature specific for homing to sites of inflammation. J Immunol. 2011;186:132–142. doi: 10.4049/jimmunol.1000695. A immunologic t study demonstrating the importance of Vitamin D and T cell functions.
- 71.Albert PJ, Proal AD, Marshall TG. Vitamin D: the alternative hypothesis. Autoimmun Rev. 2009;8:639–644. doi: 10.1016/j.autrev.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 72.Waterhouse JC, Perez TH, Albert PJ. Reversing bacteria-induced vitamin D receptor dysfunction is key to autoimmune disease. Ann N Y Acad Sci. 2009;1173:757–765. doi: 10.1111/j.1749-6632.2009.04637.x. [DOI] [PubMed] [Google Scholar]
- 73.Penna G, Fibbi B, Amuchastegui S, et al. The vitamin D receptor agonist elocalcitol inhibits IL-8-dependent benign prostatic hyperplasia stromal cell proliferation and inflammatory response by targeting the RhoA/Rho kinase and NF-kappaB pathways. Prostate. 2009;69:480–493. doi: 10.1002/pros.20896. [DOI] [PubMed] [Google Scholar]
- 74.Callens C, Coulon S, Naudin J, et al. Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J Exp Med. 2010;207:731–750. doi: 10.1084/jem.20091488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu PT, Modlin RL. Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol. 2008;20:371–376. doi: 10.1016/j.coi.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 76. Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. • An interesting study dissects the differential regulation of macrophages in phagocytic and antimicrobial functions.
- 77.Yoon S, Sun J. Intestinal Microflora, Probiotics and Anti-inflammation. Gastroenterology Research and Practice. 2011 [Google Scholar]
- 78.Sun J. Vitamin D and mucosal immune function. Curr Opin Gastroenterol. 2010;26:591–595. doi: 10.1097/MOG.0b013e32833d4b9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dickie LJ, Church LD, Coulthard LR, et al. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology (Oxford) 2010;49:1466–1471. doi: 10.1093/rheumatology/keq124. [DOI] [PubMed] [Google Scholar]
- 80.Gambhir V, Kim J, Siddiqui S, et al. Influence of 1,25-dihydroxy vitamin D3 on TLR4-induced activation of antigen presenting cells is dependent on the order of receptor engagement. Immunobiology. 2011 doi: 10.1016/j.imbio.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 81.Sun J, Mustafi R, Cerda S, et al. Lithocholic acid down-regulation of NF-kappaB activity through vitamin D receptor in colonic cancer cells. J Steroid Biochem Mol Biol. 2008;111:37–40. doi: 10.1016/j.jsbmb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Janjetovic Z, Zmijewski MA, Tuckey RC, et al. 20-Hydroxycholecalciferol, product of vitamin D3 hydroxylation by P450scc, decreases NF-kappaB activity by increasing IkappaB alpha levels in human keratinocytes. PLoS One. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tan X, Wen X, Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-kappaB signaling. J Am Soc Nephrol. 2008;19:1741–1752. doi: 10.1681/ASN.2007060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Egan JB, Thompson PA, Vitanov MV, et al. Vitamin D receptor ligands, adenomatous polyposis coli, and the vitamin D receptor FokI polymorphism collectively modulate beta-catenin activity in colon cancer cells. Mol Carcinog. 2010;49:337–352. doi: 10.1002/mc.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ordonez-Moran P, Larriba MJ, Palmer HG, et al. RhoA-ROCK and p38MAPK-MSK1 mediate vitamin D effects on gene expression, phenotype, and Wnt pathway in colon cancer cells. J Cell Biol. 2008;183:697–710. doi: 10.1083/jcb.200803020. This article describes the dual action of VDR as a transcription factor and a nongenomic activator of RhoA-ROCK and p38MAPK-MSK1 in vitro.
- 86.Muthian G, Raikwar HP, Rajasingh J, Bright JJ. 1,25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res. 2006;83:1299–1309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- 87.Pardo VG, Facchinetti MM, Curino A, et al. Age-related alteration of 1alpha,25(OH)2-vitamin D3-dependent activation of p38 MAPK in rat intestinal cells. Biogerontology. 2007;8:13–24. doi: 10.1007/s10522-006-9031-0. [DOI] [PubMed] [Google Scholar]